Published online Jul 28, 2010. doi: 10.3748/wjg.v16.i28.3546
Revised: February 4, 2010
Accepted: February 11, 2010
Published online: July 28, 2010
AIM: To investigate the effectiveness of Clostridium novyi (C. novyi)-NT spores for the treatment of established subcutaneous pancreatic tumor in the syngeneic, immunocompetent Panc02/C57Bl/6 model.
METHODS: C. novyi-NT spores were applied intravenously to animals carrying established pancreatic tumors of three different sizes. Systemic immune responses in peripheral blood and spleen were examined by flow cytometry. Supplementary, cytotoxic activity of lymphocytes against syngeneic tumor targets was analyzed.
RESULTS: Application of spores identified, that (1) small tumors (< 150 mm3) were completely unaffected (n = 10); (2) very large tumors (> 450 mm3) responded with substantial necrosis followed by shrinkage and significant lethality most likely due to tumor lysis syndrome (n = 6); and (3) an optimal treatment window exists for tumors of approximately 250 mm3 (n = 21). In this latter group, all tumor-bearing animals had complete tumor regression and remained free of tumor recurrence. In subsequent tumor rechallenge experiments a significant delay in tumor growth compared to the initial tumor cell inoculation was observed (tumor volume at day 28: 197.8 ± 87.3 mm3vs 500.1 ± 50.9 mm3, P < 0.05). These effects were accompanied by systemic activation of immune response mechanisms predominantly mediated by the innate arm of the immune system.
CONCLUSION: The observed complete tumor regression is encouraging and shows that immunotherapy with C. novyi-NT is an interesting strategy for the treatment of pancreatic carcinomas of defined sizes.
- Citation: Maletzki C, Gock M, Klier U, Klar E, Linnebacher M. Bacteriolytic therapy of experimental pancreatic carcinoma. World J Gastroenterol 2010; 16(28): 3546-3552
- URL: https://www.wjgnet.com/1007-9327/full/v16/i28/3546.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i28.3546
Spontaneous regression of tumors in patients with concurrent bacterial infections was described for the first time over 300 years ago[1]. Two centuries later, it was William Coley who developed pioneering work on a treatment strategy using bacteria. He applied-with remarkable results-a combination of Streptococcus pyogenes and Serratia marcescens to patients suffering from inoperable tumors[2,3]. With the emergence of modern chemotherapy, bacteriological therapy of cancer fell more and more into oblivion. However, in 1976 Morales et al[4] reintroduced the principle into the clinic with the development of a treatment regimen for superficial bladder cancer using bacillus Calmette-Guerin. Remarkably, this therapy is still in use today.
It is well known that hypoxic zones in poorly vascularised or necrotic tumors are a major handicap in cancer therapy preventing effective delivery of therapeutic medications to tumor cells or limiting radiation efficacy. On the other hand, these zones of severe hypoxia offer the opportunity for tumor-specific colonization with anaerobic bacteria along with subsequent tumor destruction[5]. Anaerobic bacteria are strictly limited to growth in oxygen-deprived areas and have several advantages compared to viruses such as (1) ease of production and storage stability; (2) no interference with the genome of the recipient; and (3) the possibility of complete eradication of bacteria with antibiotics[5]. In the 1960’s several reports described the lytical effects of specific clostridial strains targeting tumors by colonization of hypoxic tumor areas[6,7]. In the past few years, several Clostridium species have been studied for their antitumor potential[8]. In 2001, the Vogelstein group investigated a variety of anaerobic bacteria, including several Clostridium strains[9]. They found Clostridium novyi to be the best at colonizing tumors. A detoxified strain of C. novyi [C. novyi non-toxic (NT)] was shown to germinate and grow within the avascular regions of xenografted human cancer and to destroy surrounding tumor cells in immunodeficient mice. Similar results were obtained for the treatment of experimental murine cancer in syngeneic, immunocompetent animals. They showed complete tumor regression in up to 30% of treated animals with renal and colorectal tumors[10]. Successfully treated animals even rejected rechallenged tumors and remained disease free. The authors presumed a substantial participation of immune mechanisms in order to explain their results[10].
So far, there are no experimental data regarding the antitumoral effects of C. novyi-NT against pancreatic carcinomas. Our study therefore examines the efficacy of C. novyi-NT treatment in a syngeneic pancreatic carcinoma mouse model. These experiments demonstrated that bacteriolytic therapy is effective in eradication of pancreatic tumors, although strictly dependent on the initial tumor size. We found that (1) small tumors (< 150 mm3) were completely unaffected; (2) very large tumors (> 450 mm3) responded with substantial necrosis followed by shrinkage accompanied by significant lethality; and (3) an optimal treatment window exists for tumors of approximately 250 mm3 finally resulting in long-term tumor-free survival.
Experiments were performed on female 8-10-wk-old C57Bl/6N mice (Charles River, Fa. Wiga, Sulzfeld, Germany) weighing 18-20 g. All animals were fed standard laboratory chow and given free access to water. Experiments were performed in accordance with the German legislation on protection of animals and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council; NIH Guide, vol.25, no.28, 1996).
The pancreatic tumor model using Panc02 cells was originally described in 1984 by Corbett et al[11]. Panc02 cells were cultivated in Dulbecco’s modified Eagle’s medium/Ham’s F12 medium, supplemented with 10% FCS and 200 mmol/L Glutamine and were incubated at 37°C in an atmosphere of 5% CO2 under humidity of 95%. All media and supplements were from PAA unless stated otherwise (Cölbe, Germany).
Clostridium novyi-NT spores were kindly provided by Bert Vogelstein (Oncology Center, Johns Hopkins University, Baltimore, USA). Obtained spores were first cultivated on reinforced clostridial medium (Difco, Le Pont de Claix, France). About three to five days after cultivation in an anaerobic atmosphere, colonies of vegetative bacteria were transferred into cooked meat medium (Difco) for sporulation. Spores settled in the cooked meat particle layer and were purified from contaminating vegetative forms on a discontinuous Percoll gradient (70%, 55%). Aliquots of 7.5 × 107 spores were preserved in phosphate buffered saline (PBS) until injection.
Under brief ether anaesthesia 1 × 106 Panc02 cells were injected subcutaneously (s.c.) into the right hind leg. Tumor growth was routinely controlled at least twice a week and tumor volume was estimated according to the formula: V = width2× length × 0.52.
Three groups with different tumor volumes were defined (150 mm3, n = 10, 250 mm3, n = 21 and 450 mm3; n = 6). After reaching the predetermined tumor volumes, 7.5 × 107 spores were injected intravenously (i.v.) via tail vein injection in 50 μL PBS. As controls, one tumor-carrying group and one group without tumor received PBS (vehicle) alone.
Animals were euthanized at day 28 post-treatment or when they became moribund due to extensive tumor growth. Subsequently, complete tumor, spleen, mesenteric lymph nodes, femur bone marrow and blood samples were collected for further analysis.
After successful treatment (defined as macroscopic tumor disappearance), respective animals (n = 5) received a second dose of 1 × 106 Panc02 tumor cells into the left hind leg contralateral to the initial tumor cell injection site. Tumor volumes were evaluated twice a week as described above.
Flow cytometry was performed with leukocytes from peripheral blood, spleen and bone marrow. Leukocytes from spleens were isolated after homogenization and subsequent lysis of erythrocytes in lysis buffer (0.17 mol/L Tris, 0.16 mol/L NH4Cl). Purified bone marrow cells were obtained after washing in PBS.
Whole blood cells and isolated leukocytes from spleens and bone marrow were labelled using the following fluorescein-isothiocyanate (FITC)- and phycoerythrin (PE)-conjugated rat anti-mouse monoclonal antibodies (mAbs): CD3epsilon FITC (1 μg, ImmunoTools, Friesoythe, Germany), CD11b FITC, CD11c FITC, CD19 FITC, CD4 PE, CD8 PE, CD62L PE, Gr1 PE (1 μg, Miltenyi Biotec, Bergisch-Gladbach, Germany) and NK1.1 FITC (0.5 μg BD Pharmingen) followed by lysis of erythrocytes in the case of whole blood cells (FACS Lysing Solution, BD Pharmingen, Heidelberg, Germany). Negative controls consisted of lymphocytes stained with the appropriate isotypes (BD Pharmingen). Samples were analyzed on a FACSCalibur Cytometer (BD Pharmingen). Data analysis was performed using CellQuest software (BD Pharmingen).
Lytic activity of lymphocytes (spleen) from treated animals against target cells (Panc02, CMT-93, and MC3T3-E1) was determined by flow cytometry. Prior to co-culture, target cells were labelled with CFDA-SE (carboxyfluorescein diacetate succinimidyl ester, final concentration: 2 μmol/L). Target cells without effector cells were used as negative controls. Following co-incubation for five hours at an effector to target cell ratio of 30:1 and 10:1, propidium iodide (PI) was added to measure death of target cells based on carboxylfluorescein diacetate/propidium iodide (CFDA/PI) double positive cells. Cytotoxicity was calculated according to the following formula: % cytotoxicity = experimental release-spontaneous release of target cells.
All values are expressed as mean ± SE. After proving the assumption of normality, differences between tumor control and treated animals were determined by using the unpaired Student’s t-test. If normality failed, the nonparametric Mann-Whitney U-Test was applied. The tests were performed by using Sigma-Stat 3.0 (Jandel Corporation, San Rafael, CA, USA). The criterion for significance was set to P < 0.05.
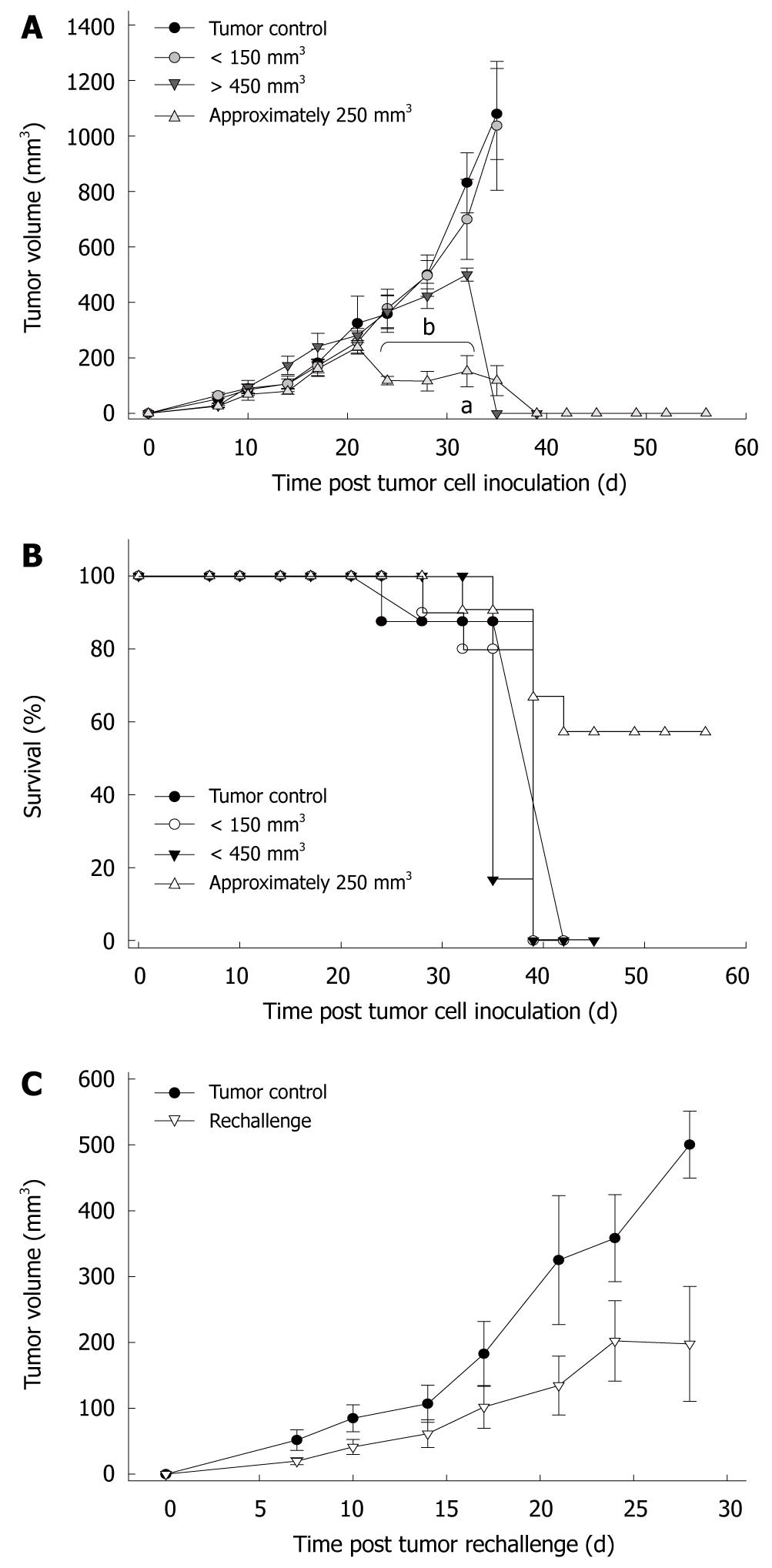
Earlier experiments described complete eradication of tumors subsequent to intravenous injection of C. novyi spores. However, there is a lack of experimental data for the treatment of pancreatic carcinomas. This has prompted the investigation of whether bacteriolytic therapy is also applicable for this tumor entity. Different sizes of the tumor were chosen to establish an effective treatment regimen (Figure 1A).
In the first series of experiments, animals with small tumors below 150 mm3 were treated. Macroscopically, tumor growth was completely unaffected, with tumor sizes remaining comparable to controls until the endpoint (day 21: 1036.6 ± 232.9 mm3vs 1079.1 ± 164.3 mm3). This was most likely due to the absence of intratumoral hypoxia and necrotic areas, thereby prohibiting germination of bacteria within the tumor. To overcome this, animals with large tumors (> 450 mm3) were employed. All tumors responded with substantial necrosis and subsequent shrinkage within a few hours (P < 0.05 vs tumor control).
Another group consisted of animals carrying tumors of about 250 mm3. This tumor size was found to be optimal for C. novyi treatment. Tumors displayed rapid necrosis within 48 h and macroscopically disappeared 10-14 d later (P < 0.05 vs tumor control). There was no tumor regrowth until the experimental endpoint (day 31), indicative of complete tumor regression.
Toxicity of C. novyi-NT spores is thought to be dependent on germination of bacteria within hypoxic regions of tumors. Accordingly, tumor-free control animals only receiving a single intravenous injection of spores displayed no clinical symptoms such as weight loss, lethargy or anorexia. Macroscopic examination of the inner organs revealed no abnormalities. Toxicity was also low in tumor-carrying animals where germination did not occur (Figure 1B). However, toxicity appeared to be greater when larger tumors were employed. We observed up to 40% lethality in mice carrying tumors of about 250 mm3. Death was observed predominantly in the post-acute phase of infection between days 10-18. In this time, mice were cured of their tumors. Toxicity is thus related to germination of the bacteria within tumors. This finding is reinforced by the massive lethality post-treatment of larger tumors. All animals (n = 6) died within 24 h after spore administration.
Next, we analyzed to what extent the bacteriolytic therapy not only led to tumor regression but also induced protective immunologic memory towards a re-exposure to Panc02 tumor cells. To perform these rechallenge experiments, successfully cured animals (approximately 250 mm3) received a tumorigenic dose of Panc02 cells into the contralateral flank. Slowly growing tumors (197.8 ± 87.3 mm3, P < 0.05 vs tumor control) developed in 4/5 mice four weeks after tumor inoculation, with one animal remaining tumor-free until the end of the experiment (Figure 1C). However, when compared with untreated, naïve mice (500.1 ± 50.3 mm3), tumor development was significantly sustained (Figure 1C). Taken together, this indicates, that C. novyi therapy mediates potent antitumor reactivity in vivo. However, complete protective immunity was obtained in only 20% of the animals.
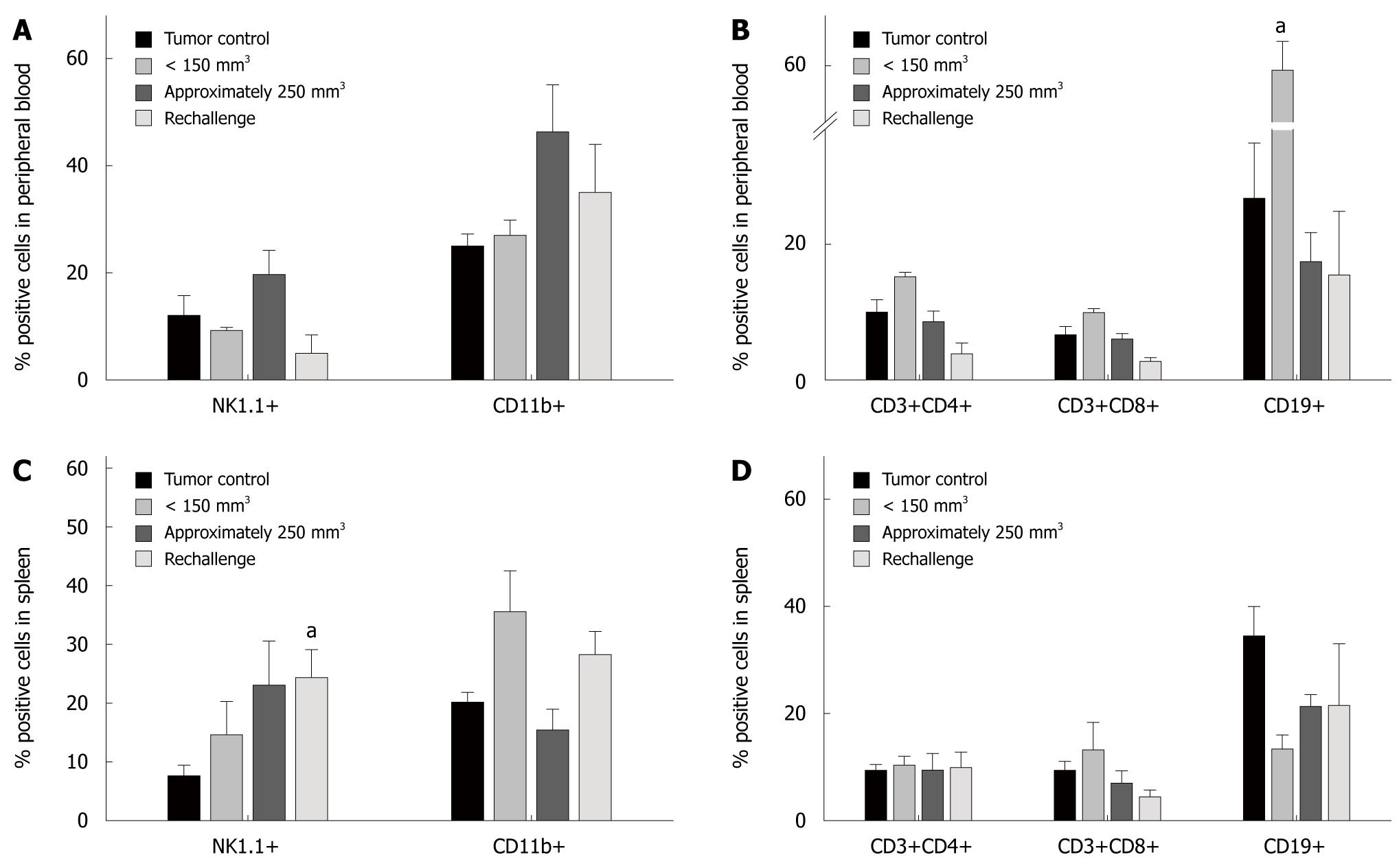
Regression of tumors subsequent to C. novyi-NT treatment is thought to be, at least in part, due to the stimulation of immune response mechanisms. We therefore examined the influence of spore application on immune cells. Flow cytometric analysis of circulating leukocytes identified increased numbers of monocytes, granulocytes (CD11b+) and NK cells (NK1.1+) after treatment (250 mm3 tumors), while levels of T (CD3+/CD4+, CD3+/CD8+) and B cells (CD19+) were similar to controls (Figure 2A and B). Therapy of small tumors (< 150 mm3), however, resulted in considerably elevated levels of CD19+ B cells (P < 0.05 vs tumor control), but again, numbers of T cells remained unchanged. Likewise, monocytes and NK cells showed no alteration (Figure 2B).
In spleens, C. noyvi mediated increases in NK cells (Figure 2C). Elevation of this cell population was highest after treatment of 250 mm3 tumors. As well as in peripheral blood, levels of T and B cells were not affected by the application of bacteria (Figure 2D). This was also seen post-bacterial exposure in small tumors. Here, numbers of NK1.1 and CD11b positive cells were only slightly induced, while CD19 positive B cells were found to be down-regulated. Other analyzed cell populations, such as CD11c+ dendritic cells and Gr1+ granulocytes were also not induced (data not shown).
To sum up these findings, bacteriolytic treatment predominantly induced the innate arm of the immune system.
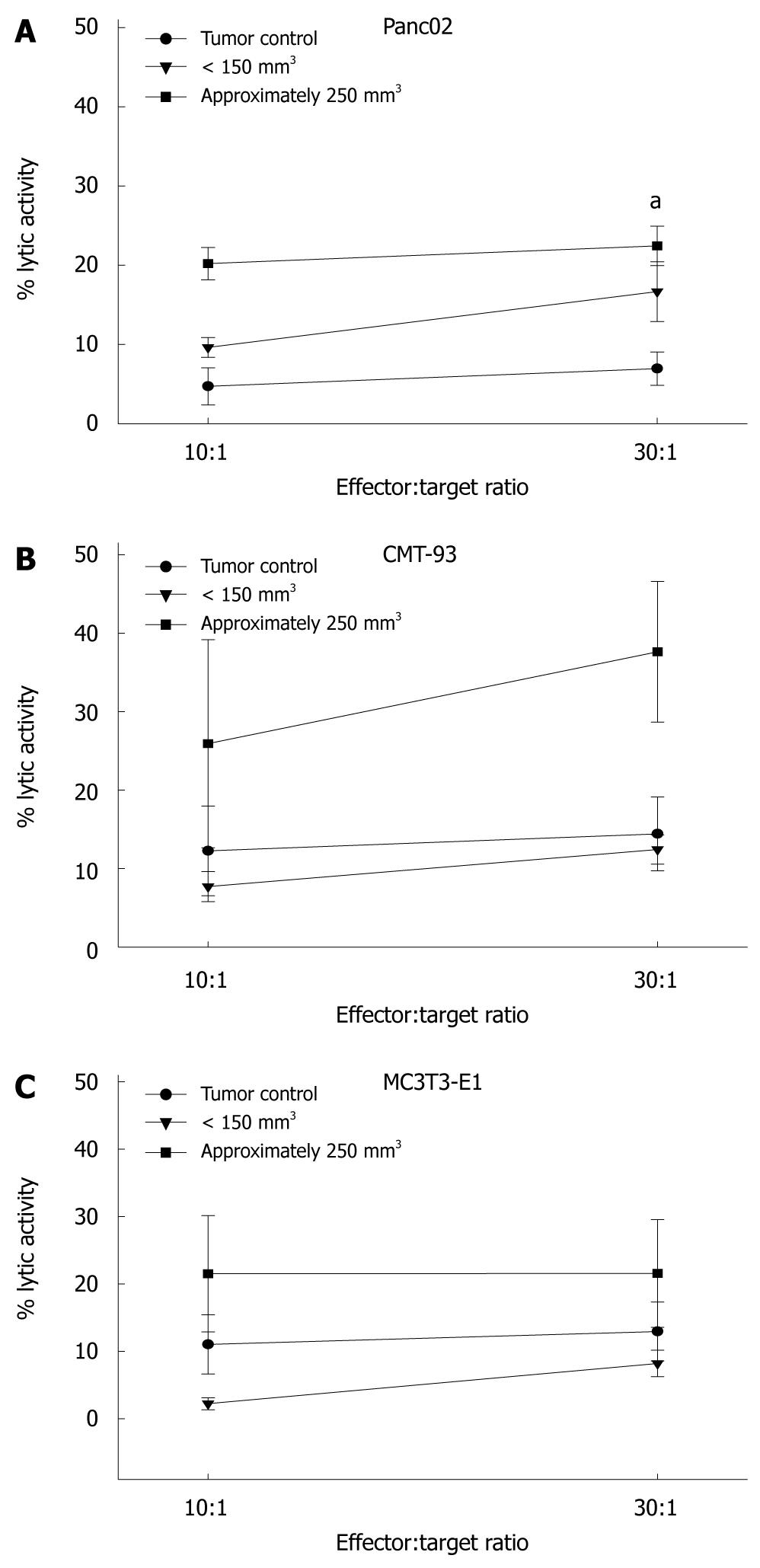
We next examined whether immune cells isolated from treated animals were able to react against tumor cells. In a functional cytotoxicity assay, splenocytes were used as effectors. Panc02 tumor cells were lysed by immune cells from successfully treated mice. Similar results were obtained with the syngeneic colorectal cell line CMT-93 (Figure 3). However, this reaction was not restricted to tumor cells. Reactivity of splenocytes to the non-cancerous MC3T3-E1 fibroblasts was also observed, indicative of non-specific stimulation of immune cells. Thus, these effects were more likely mediated by activated NK cells than by tumor-antigen specific T cells.
The cure of cancer by severe bacterial infections was reported by W.B. Coley over a century ago. These historical observations have provided a basis for active microbial immunotherapy, which has been extensively studied in recent years. In 2001, the Vogelstein group demonstrated in pioneering work that spores of C. novyi-NT are very efficient in eradicating established solid tumors[9]. These effects could be boosted when combined with standard chemotherapy[9], resulting in long-term protective immunologic memory. Similarly, other bacteria such as Salmonella and Mycobacteria have been shown to stimulate an inflammatory response with neutrophil-directed cytokines leading to a potent cellular antitumoral immune response[12-14].
Among the more frequent tumors, pancreatic cancer has an outstandingly poor prognosis. Recent treatment regimens have so far failed to substantially improve the clinical outcome of this tumor entity. Moreover, immunotherapeutic approaches aiming to specifically stimulate the host’s immune system against tumor cells are rare in pancreatic malignancies. Here, we investigated the effectiveness of Clostridium spores for the treatment of established Panc02 tumors in the syngeneic, immunocompetent C57Bl/6 model. We found that (1) small tumors (< 150 mm3) were completely unaffected; (2) very large tumors (> 450 mm3) responded with substantial necrosis followed by shrinkage but accompanied by significant lethality (80%); and (3) an optimal treatment window exists for tumors of approximately 250 mm3. These findings are, in part, consistent with previous studies in other tumor entities, where therapeutic effectiveness and toxicity were also found to be related to the size of the tumor. However, lethality was predominantly observed after combined bacteriolytic therapy, but not after administration of spores or chemotherapeutics alone even when large tumors were employed[9]. In 2005, Diaz et al[15] described the relationship between toxicity, spore dose and tumor size. They found an increased mortality at higher spore doses injected into Balb/c mice carrying large tumors and concluded a germinating-dependent toxic effect of C. novyi. In our study, we also observed massive toxicity only after treatment of mice with large tumor burdens. On the other hand, mortality was absent in animals carrying no tumors or only small tumors, where anoxic areas allowing for bacterial germination were missing. It is therefore conceivable that toxicity was related to germination of the bacteria within tumors. This germination occurred rapidly in larger Panc02 tumors which contained extended areas of necrosis resulting from inadequate oxygenation and nutrition. As a consequence, massive destruction of neoplastic cells occurred, accompanied by the release of intracellular ions and metabolic by-products into the systemic circulation that may lead to metabolic complications and death. This phenomenon is recognized in the clinic as “tumor lysis syndrome” usually occurring in patients with bulky, rapidly proliferating tumors responding to treatment. Clinically, the syndrome is characterized by the development of hyperuricaemia, hyperkalaemia, hyperphosphataemia, hypocalcaemia, and acute renal failure[16]. Nevertheless, the tumor lysis syndrome appeared not to be the lethal cause in our experimental setting since clinical parameters, such as levels of serum uric acid and calcium were not altered (data not shown).
Another important fact of bacterial therapy is the activation of immune response mechanisms. It has been reported that C. noyvi infection is associated with inflammation and subsequent development of a potent immune response. Consistent with these data, we also observed raised numbers of circulating NK cells, granulocytes and monocytes even four weeks post-infection. Of note, this immune stimulation was paralleled by additional signs of systemic inflammation like reactive splenomegaly. In subsequent rechallenge experiments, a growth retardation of tumors was observed. However, in a functional in vitro cytotoxicity assay, these effects were found not to be tumor specific as reactivity was also observed towards non-malignant syngeneic cells like fibroblasts. Thus, in our study, the bacterial treatment predominantly activated the innate arm of the immune system. Most likely, NK cells are the main cytotoxic cell type involved in recognition of Panc02 tumor cells. These observations are to some extent contrary to those described by Agrawal and coworkers, who achieved prevention of tumor growth subsequent to adoptive transfer of CD8+ lymphocytes from tumor-carrying mice cured by C. novyi-NT application.
Recently, we provided convincing data that experimental pancreatic tumors can be successfully treated with the facultative anaerobic bacterium S. pyogenes. Living bacteria as well as bacterial lysates mediated regression of established tumors in the very same syngeneic mouse model[17,18]. Compared to these results, we found here that despite their ability to induce substantial tumor necrosis even after application of only small numbers into large tumors, C. noyvi spores are substantially more toxic, and most importantly from the immunological point of view, are a less potent inducer of tumor-reactive T cells.
Pancreatic carcinoma has an outstandingly poor prognosis. This has prompted research on alternative treatment approaches that may complement conventional anticancer strategies. In this context, the therapeutic application of bacteria is equally simple and promising.
Immunotherapy has repeatedly been shown to have a very strong antitumoral potential. Most groups focus on tumor antigens together with dendritic cell vaccination. Recently, several groups have developed novel antitumoral approaches using tumor-therapeutic bacterial strains.
Vogelstein and coworkers proved that anaerobic bacteria such as Clostridium noyvi (C. noyvi)-NT selectively germinate and grow within hypoxic regions of tumors. However, they always combined the i.v. administration of lethal toxin-free spores of Clostridium novyi with standard chemotherapy. This is the first report on the therapeutic effectiveness of Clostridium novyi-spores as a single agent for the treatment of pancreatic carcinoma. Additionally, the study showed that this effect is due to an immunological response dominated by the innate immune system.
The observed complete tumor regression is encouraging and shows that immunotherapy with Clostridium novyi-spores is an interesting strategy for the treatment of pancreatic carcinomas of defined sizes.
The study investigates a novel approach for pancreatic cancer treatment through applying anaerobic bacteria. The idea behind this approach is that anaerobic bacteria which are strictly limited to growth in oxygen-deprived areas would colonize in tumors which have hypoxic areas but not in normal organs. The study investigates the effectiveness of C. novyi-NT spores on the established tumor in a subcutaneous model of pancreatic cancer. The authors show that the effects of C. novyi-NT on the pancreatic tumor depend on the size of the tumor.
Peer reviewer: Anna S Gukovskaya, Professor, VA Greater Los Angeles Health Care System, University of California, Los Angeles, 11301 Wilshire Blvd, Los Angeles, CA 91301, United States
S- Editor Wang YR L- Editor Webster JR E- Editor Ma WH
| 1. | Nowotny N. Antitumour effects of endotoxins. Handbook of endotoxin, cellular biology of endotoxin. New York: Elsevier Science Inc 1985; 389. |
| 2. | Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. 1893. Clin Orthop Relat Res. 1991;3-11. |
| 3. | Wiemann B, Starnes CO. Coley’s toxins, tumor necrosis factor and cancer research: a historical perspective. Pharmacol Ther. 1994;64:529-564. |
| 4. | Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976;116:180-183. |
| 5. | Kizaka-Kondoh S, Inoue M, Harada H, Hiraoka M. Tumor hypoxia: a target for selective cancer therapy. Cancer Sci. 2003;94:1021-1028. |
| 6. | Engelbart K, Gericke D. Oncolysis by clostridia. V. Transplanted tumors of the hamster. Cancer Res. 1964;24:239-242. |
| 7. | Moese JR, Moese G. Oncolysis by clostridia. I. Activity of clostridium butyricum (m-55) and other nonpathogenic clostridia against the ehrlich carcinoma. Cancer Res. 1964;24:212-216. |
| 8. | Heppner F, Möse JR. The liquefaction (oncolysis) of malignant gliomas by a non pathogenic Clostridium. Acta Neurochir (Wien). 1978;42:123-125. |
| 9. | Dang LH, Bettegowda C, Huso DL, Kinzler KW, Vogelstein B. Combination bacteriolytic therapy for the treatment of experimental tumors. Proc Natl Acad Sci USA. 2001;98:15155-15160. |
| 10. | Agrawal N, Bettegowda C, Cheong I, Geschwind JF, Drake CG, Hipkiss EL, Tatsumi M, Dang LH, Diaz LA Jr, Pomper M. Bacteriolytic therapy can generate a potent immune response against experimental tumors. Proc Natl Acad Sci USA. 2004;101:15172-15177. |
| 11. | Corbett TH, Roberts BJ, Leopold WR, Peckham JC, Wilkoff LJ, Griswold DP Jr, Schabel FM Jr. Induction and chemotherapeutic response of two transplantable ductal adenocarcinomas of the pancreas in C57BL/6 mice. Cancer Res. 1984;44:717-726. |
| 12. | Simons MP, O’Donnell MA, Griffith TS. Role of neutrophils in BCG immunotherapy for bladder cancer. Urol Oncol. 2008;26:341-345. |
| 13. | Lee CH, Wu CL, Shiau AL. Toll-like receptor 4 mediates an antitumor host response induced by Salmonella choleraesuis. Clin Cancer Res. 2008;14:1905-1912. |
| 14. | Hayashi K, Zhao M, Yamauchi K, Yamamoto N, Tsuchiya H, Tomita K, Hoffman RM. Cancer metastasis directly eradicated by targeted therapy with a modified Salmonella typhimurium. J Cell Biochem. 2009;106:992-998. |
| 15. | Diaz LA Jr, Cheong I, Foss CA, Zhang X, Peters BA, Agrawal N, Bettegowda C, Karim B, Liu G, Khan K. Pharmacologic and toxicologic evaluation of C. novyi-NT spores. Toxicol Sci. 2005;88:562-575. |
| 16. | Mott FE, Esana A, Chakmakjian C, Herrington JD. Tumor lysis syndrome in solid tumors. Support Cancer Ther. 2005;2:188-191. |
| 17. | Maletzki C, Linnebacher M, Kreikemeyer B, Emmrich J. Pancreatic cancer regression by intratumoural injection of live Streptococcus pyogenes in a syngeneic mouse model. Gut. 2008;57:483-491. |
| 18. | Linnebacher M, Maletzki C, Emmrich J, Kreikemeyer B. Lysates of S. pyogenes serotype M49 induce pancreatic tumor growth delay by specific and unspecific antitumor immune responses. J Immunother. 2008;31:704-713. |