Published online Jul 28, 2010. doi: 10.3748/wjg.v16.i28.3541
Revised: May 19, 2010
Accepted: May 26, 2010
Published online: July 28, 2010
AIM: To study the role of hepcidin in hereditary hyperferritinemia cataract syndrome (HHCS).
METHODS: Six patients from two families with HHCS, confirmed by genetic analysis showing A to G mutation at position +40 in the L-ferritin gene, were recruited to undergo serum hepcidin and prohepcidin measurements using radioimmunoassay and enzyme linked immunoassay, respectively, and measurements were compared with levels in serum from 25 healthy volunteers (14 females), mean age 36 ± 11.9 years.
RESULTS: The serum hepcidin and prohepcidin levels in patients with HHCS were 19.1 ± 18.6 and 187 ± 120.9 ng/mL, respectively. Serum ferritin was 1716.3 ± 376 μg/L. Liver biopsy in one patient did not show any evidence of iron overload. Serum hepcidin and prohepcidin values in healthy controls (HCs) were 15.30 ± 15.71 and 236.88 ± 83.68 ng/mL, respectively, while serum ferritin was 110 ± 128.08 μg/L. There was no statistical difference in serum hepcidin level between the two cohorts (19.1 ± 18.6 ng/mL vs 15.30 ± 15.71 ng/mL, P = 0.612) using two-tailed t-test.
CONCLUSION: Serum hepcidin levels in HHCS patients is similar to that in HCs. Our study suggests that circulating ferritin is not a factor influencing hepcidin synthesis and does not have a role in the iron-sensing mechanism in hepatocytes.
- Citation: Arnold J, Sangwaiya A, Manglam V, Thursz M, Beaumont C, Kannengiesser C, Busbridge M. Hepcidin levels in hereditary hyperferritinemia: Insights into the iron-sensing mechanism in hepatocytes. World J Gastroenterol 2010; 16(28): 3541-3545
- URL: https://www.wjgnet.com/1007-9327/full/v16/i28/3541.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i28.3541
The iron-sensing mechanism has been widely studied but is not yet fully defined. In iron-replete states, circulating transferrin carrying iron to hepatocytes competes with hemochromatosis-Fe protein (HFE-protein) to bind to transferrin receptor-1 (TfR-1)[1]. Transferrin loaded with iron has greater affinity with TfR-1 than does HFE-protein. When transferrin binds to TfR-1, HFE-protein is freed up to bind to transferrin receptor-2 (TfR-2). This complex is thought to operate as an iron sensor mechanism and functions as an inducer of hepcidin production via SMAD (small mothers against decepentaplegic homologue) pathway[2]. Under normal circumstances, hepcidin expression and subsequent release into plasma prevents further absorption of iron from the duodenal enterocytes by preventing the efflux of iron by ferroportin channels and hence reduced amounts of iron delivery via transferrin to hepatocytes[3].
Ferritin is an iron storage protein and its synthesis is controlled at the level of transcription[4] and mRNA translation by an iron response mechanism. The control process depends on a highly conserved motif at the 5’ non-coding region of ferritin mRNA. Studies have shown that ferritin is synthesized in the liver and has two subunits, L (19 kDa, Light) and H (21 kDa, Heavy)[5]. Different proportions of component L and H subunits give rise to isoferritins with tissue specific distributions, with H-ferritin as the major iron storage protein. The L-ferritin and H-ferritin genes are based in chromosomes 19 and 11, respectively.
Regulation of ferritin synthesis involves an interaction between an iron binding protein, termed as the iron regulatory protein (IRP), and ferritin mRNA[6,7]. The translational regulation of ferritin mRNA involves two IRPs, IRP-1 and IRP-2. Only IRP-1, but not IRP-2, contains an iron-sulphur complex and is bifunctional, registering intracellular iron status mainly through an iron-sulphur switch mechanism and alternating between an active cytosolic aconitase form and an apoprotein that binds iron responsive elements (IREs). Although IRP-2 is homologous to IRP-1, IRP-2 activity is regulated primarily by iron-dependent degradation through the ubiquitin-proteasomal system in iron-replete cells. Targeted deletions of IRP-1 and IRP-2 in animals suggest that IRP-2 is the chief physiologic iron sensor[8]. A constant region of the ferritin mRNA molecule, termed the IRE, binds with IRP. The resultant IRE-IRP complex inhibits ribosomal binding to mRNA and prevents translation of the ferritin coding sequence[6]. A critical CAGUGU sequence within IRE is important for binding with IRP.
In iron overload, as in hereditary hemochromatosis, the IRE-IRP inhibitory system is suppressed and ferritin synthesis is increased. When this form of iron storage is saturated, iron is deposited as hemosiderin in tissue[9]. Thus ferritin is a sequel of intracellular iron metabolism and not a part of the iron sensor mechanism itself.
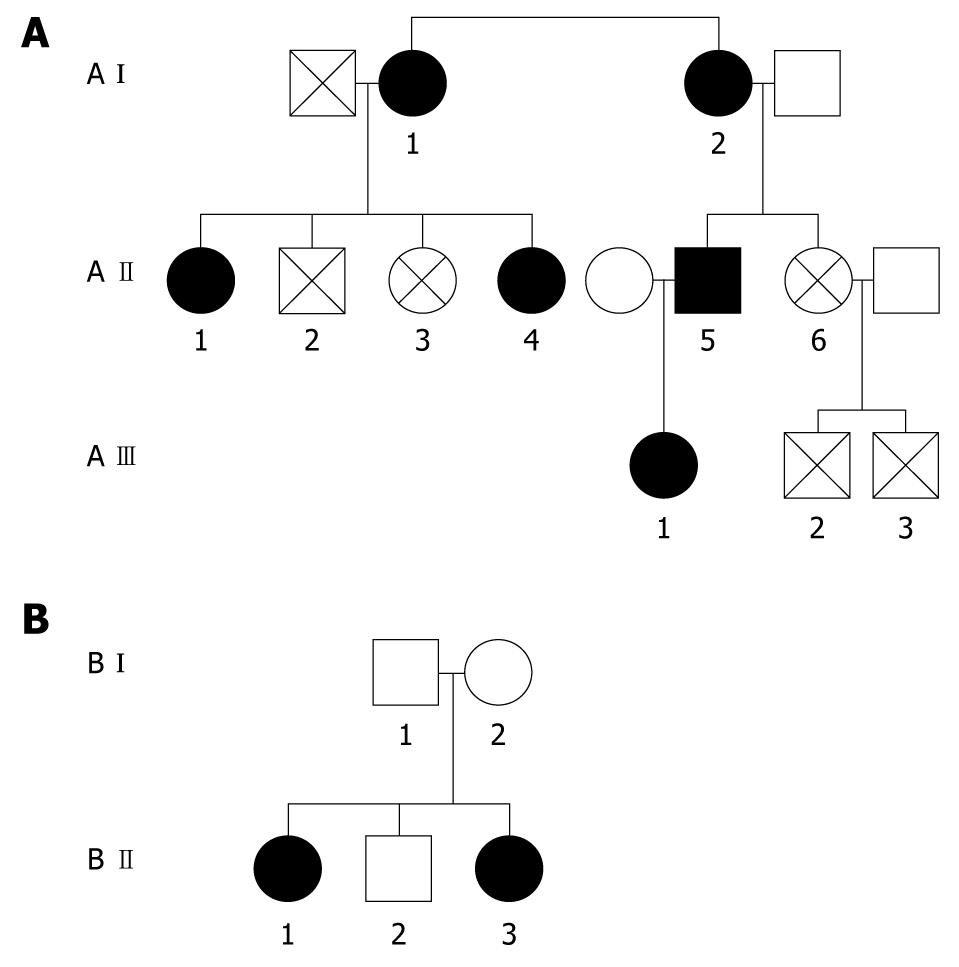
Hereditary hyperferritinemia cataract syndrome (HHCS) is an autosomal dominant disorder characterized by premature cataract formation and raised serum L-ferritin in the absence of iron overload[10,11]. We have earlier described this syndrome in a family with 11 members, six members from three generations having genetic mutation at +40 (A to G mutation) corresponding to the critically conserved nucleotide motif in L-ferritin mRNA IRE on chromosome 19[12]. We have recently discovered another family with five members from two generations; two members confirmed as having the genetic mutation as described above. In this manuscript, we describe serum levels of hepcidin and prohepcidin in HHCS and discuss the iron-sensing mechanism in hepatocytes.
A prospective study was performed with approval of our Regional Ethics Committee and written consent was obtained from all patients and healthy volunteers in accordance with the Declaration of Helsinki. Patients and healthy volunteers were recruited from a single hospital with mixed ethnicity mainly comprising Caucasians and South Asians living in West London.
The serum study comprised of 25 healthy controls (HCs); 14 were females, mean age was 36 ± 11.9 years (age range 21-62 years), who were hospital colleagues recruited to measure serum hepcidin-25, prohepcidin and ferritin as well as routine biochemical profile. HCs on supplemental vitamins and oral iron were excluded. None of the participants had received any blood products in the past. A morning fasting venous blood sample was collected and serum stored at -20°C for analysis.
Six patients from two families with HHCS were recruited for serum hepcidin, prohepcidin and ferritin analysis. All six patients had cataracts with limited reduction in visual acuity and all had hyperferritinemia but normal transferrin saturation. Venous blood samples were obtained and serum separated and stored at -20°C until analysis. None of the recruited patients had chronic inflammatory disease and none were on oral iron supplementation.
Liver biopsy was performed on one of the affected individuals (A II-1) to determine tissue iron status using Perl’s Prussian blue staining. Blood samples from family members were analyzed for genetic mutation within the L-ferritin IRE.
Polymerase chain reaction amplification and DNA sequencing were carried out as described previously[12,13]. Direct cycle sequencing of the 5’ untranslated region of the L-ferritin gene from three affected members from Family A (A I-2, A II-1 and A II-5) revealed that all three patients were heterozygous for A to G point mutation at position +40. Family B (B II-1 and B II-3) underwent genetic analysis and revealed the same mutation as mentioned above. This corresponds to position 2 of the CAGUGU motif within the L-ferritin IRE mRNA.
We have earlier described development of a radioimmunoassay for measurement of serum hepcidin-25[14]. Serum prohepcidin was measured by enzyme linked immunoassay (DRG Diagnostics, UK). Serum ferritin was measured using a standard solid phase, two site chemiluminescent immunometric assay (Immulite).
Quantitative variables were compared using unpaired t-test. A value of P < 0.05 was considered significant. All statistical analyses were carried out using the statistical package GraphPad Prism, version 5.00 for Windows, (GraphPad Software, San Diego California USA, http://www.graphpad.com).
The serum hepcidin and transferrin saturation values for all six patients with HHCS were similar to HCs. Figure 1 describes the relationship between the various family members and Table 1 describes the relation of serum ferritin to cataract. The mean ± SD serum hepcidin in patients with HHCS was 19.1 ± 18.6 ng/mL as compared to an HC mean value of 15.68 ± 15.7 ng/mL, and there was no statistically significant difference between cohorts (P = 0.612). However, there was a highly significant difference (P < 0.0001) in serum ferritin level between the two cohorts; mean serum ferritin for HHCS patients was 1716 ± 376 μg/L, as compared to mean value of 110 ± 128 μg/L for HCs (Table 2). Liver biopsy performed on one patient did not reveal iron overload.
| Family member | Serum ferritin (μg/L) | Transferrin saturation (%) | Cataract |
| A I 1 | 1542 | 18 | Yes |
| A I 2 | 1581 | 17 | Yes |
| A II 1 | 2049 | 22 | Yes |
| A II 4 | 988 | 17 | Yes |
| A II 5 | 1855 | 26 | Yes |
| A III 1 | 902 | 37 | Yes |
| A II 2 | 37 | 21 | No |
| A II 3 | 49 | NA | No |
| A III 2 | 15 | 16 | No |
| A III 3 | 19 | NA | No |
| B I 1 | NA | NA | Yes |
| B I 2 | NA | NA | No |
| B II 1 | 2143 | 9 | Yes |
| B II 2 | NA | NA | Yes |
| B II 3 | 1128 | 37 | Yes |
| Healthy controls | HHCS cohort | Reference values | |
| No. (M:F) | 25 (11:14) | 6 (1:5) | |
| Age (range), yr | 36 (21-62) | 44.65 (21-75) | |
| Hb (g/dL) | 13.8 ± 1.3 | 12.8 ± 2.3 | 13-17 |
| MCV (fL) | 87.8 ± 5.8 | 90.4 ± 3.6 | 80-100 |
| Iron (μmol/L) | 16.4 ± 4.0 | 15.6 ± 6.7 | 10.6-28.3 |
| Ferritin (μg/L) | 110 ± 128 | 1716.3 ± 376 | 30-400 |
| Prohepcidin (ng/mL) | 236.88 ± 83.68 | 187 ± 120.9 | NA |
| Hepcidin (ng/mL) | 15.68 ± 15.7 | 19.1 ± 18.6 | NA |
| TIBC (μmol/L) | 59.2 ± 9.6 | 70.67 ± 19.9 | 41-77 |
| Tsat % | 28.8 ± 9.6 | 21.5 ± 9.5 | NA |
| Vitamin B12 (pg/mL) | 280.4 ± 153 | 324.2 ± 127.2 | 180-914 |
| Serum folate (ng/mL) | 8.2 ± 4.0 | 5.5 ± 1.9 | 3.1-17.5 |
| Bilirubin (μmol/L) | 10.44 ± 5.5 | 5.3 ± 3 | 0-20 |
| ALP (IU/L) | 73.3 ± 26.6 | 77.5 ± 11 | 40-129 |
| ALT (IU/L) | 30.3 ± 22.4 | 17.7 ± 6.9 | 10-50 |
| Albumin (g/L) | 46.2 ± 2.4 | 42.7 ± 4.2 | 34-50 |
HHCS is now a well recognized entity[15,16]. The only clinical manifestation in these patients appears to be early onset cataracts. Further studies on other family members who have undergone cataract extraction have confirmed that the cataracts represent deposits of L-ferritin subunits[17]. To our knowledge, this is the first ever study of hepcidin and prohepcidin levels in patients with HHCS. Our patients had normal transferrin saturation with liver biopsy on one patient ruling out tissue iron overload. Serum hepcidin levels in HHCS patients were similar to those in HCs.
The iron-sensing mechanism in hepatocytes is poorly understood. Unlike the ferritin mRNA, there is no iron regulatory element sequence in the hepcidin gene that is directly influenced by cellular iron[18]. Kemna et al[19] have put forward the hypothesis that several pathways are involved in hepcidin regulation. Three of these are active regulation pathways (erythropoietic activity derived regulation, iron store based regulation, and inflammation induced regulation) and one is an independent mandatory signaling pathway.
Hemojuvelin (HJV)-controlled transcription factors, such as the bone morphogenic protein (BMP)/SMAD signaling pathway, appears to be a mandatory signaling process for the influence of iron stores and erythropoiesis derived hepcidin regulation[19,20]. Any direct influence of intracellular iron on hepcidin regulation is not well elucidated. It is known that iron activates BMP-6, an upstream regulator of hepcidin. Moreover, it has also been suggested that hepcidin responds to increases in transferrin saturation. Recent reports suggest that a transmembrane protease (matriptase-2) inhibits hepcidin activation by cleaving membrane HJV[21,22].
The inflammation regulatory pathway for hepcidin regulation is mainly induced by interleukin-6 (IL-6) with a cascade involving IL-6 receptor Janus kinase, signal transducer and activator of transcription (STAT 3)[23].
A syndrome of non-hereditary liver iron overload in patients with modestly raised serum ferritin but normal transferrin saturation has also been reported. This disorder is distinct from both hereditary hemochromatosis and HHCS and seems to be associated with hyperlipidemia and impaired glucose tolerance[24]. The metabolic syndrome candidate genes, upstream stimulating factor (USF) 1 and 2 in chromosome 19, are in direct control of the hepcidin anti-microbial peptide gene (HAMP gene, chromosome 19 q13), suggesting a link between lipid, glucose, and iron metabolism.
Our study confirms that L-ferritin in patients with HHCS is not a factor in the regulation of hepcidin synthesis. It is therefore most likely that iron saturation of transferrin plays a key role in the iron-sensing mechanism in hepatocytes. The iron overload reported in patients with congenital atransferrinemia also supports this hypothesis[25].
Hereditary hyperferritinemia cataract syndrome (HHCS) is an autosomal dominant disorder characterized by L-ferritin hyperferritinemia and premature cataract formation. The defect is due to a point mutation or deletion in the L-ferritin iron responsive element (IRE) and the resultant changes in the structure of the L-ferritin IRE preventing its interaction with the iron regulatory protein. Hepcidin is an iron regulatory peptide predominantly formed in the liver. Hepcidin has been shown to be low in the various types of hereditary hemochromatosis such as hemochromatosis-Fe-related C282Y/C282Y, hemojuvelin- and transferrin receptor-2-related hemochromatosis. No data has been available on the levels of hepcidin in HHCS to date. This research study examined serum levels of hepcidin in two families with HHCS and set out to explain the iron-sensing mechanism in the liver.
Hepcidin and its role in iron metabolism has been a topic of intense research recently. The initial hurdle to measure hepcidin in serum has partly been resolved with the development of assays to measure the peptide in serum, e.g. radioimmunoassay and enzyme-linked immunosorbent assay. HHCS is a benign disorder of iron metabolism and we report for the first time serum levels of hepcidin in this disorder. This should help in further understanding the regulation of hepcidin in various disorders.
The authors report for the first time the level of serum hepcidin in patients with HHCS. Results prove that circulating ferritin has no role in the iron-sensing mechanism.
The authors used radioimmunoassay to measure serum levels of hepcidin in patients and healthy controls. Use of this assay would help explore the role of hepcidin in various iron disorders.
In this work Arnold et al measure the iron regulatory hormone hepcidin (and its precursor pro-hepcidin) in sera from patients with HHCS. They report that levels of hepcidin are within the normal range in these patients and conclude that serum ferritin is not involved in body iron sensing. These findings are not surprising, but are nevertheless interesting and worthy of publication.
Peer reviewers: Kostas Pantopoulos, Associate Professor, Department of Medicine, McGill University, Lady Davis Institute for Medical Research, 3755 Cote Ste-Catherine Road, Montreal, Quebec, H3T 1E2, Canada; Sebastian Mueller, MD, PhD, Professor of Medicine, Department of Internal Medicine, Salem Medical Center, and Center for Alcohol Research, University of Heidelberg, Zeppelinstraße 11 - 33, Heidelberg 69121, Germany
S- Editor Wang JL L- Editor Logan S E- Editor Lin YP
| 1. | Giannetti AM, Björkman PJ. HFE and transferrin directly compete for transferrin receptor in solution and at the cell surface. J Biol Chem. 2004;279:25866-25875. |
| 2. | Gao J, Chen J, Kramer M, Tsukamoto H, Zhang AS, Enns CA. Interaction of the hereditary hemochromatosis protein HFE with transferrin receptor 2 is required for transferrin-induced hepcidin expression. Cell Metab. 2009;9:217-227. |
| 3. | Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090-2093. |
| 4. | Koorts AM, Viljoen M. Ferritin and ferritin isoforms I: Structure-function relationships, synthesis, degradation and secretion. Arch Physiol Biochem. 2007;113:30-54. |
| 5. | Crichton RR. Ferritin: structure, synthesis and function. N Engl J Med. 1971;284:1413-1422. |
| 6. | Jaffrey SR, Haile DJ, Klausner RD, Harford JB. The interaction between the iron-responsive element binding protein and its cognate RNA is highly dependent upon both RNA sequence and structure. Nucleic Acids Res. 1993;21:4627-4631. |
| 7. | Theil EC. Iron regulatory elements (IREs): a family of mRNA non-coding sequences. Biochem J. 1994;304:1-11. |
| 8. | Rouault TA. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat Chem Biol. 2006;2:406-414. |
| 9. | Niederau C, Fischer R, Sonnenberg A, Stremmel W, Trampisch HJ, Strohmeyer G. Survival and causes of death in cirrhotic and in noncirrhotic patients with primary hemochromatosis. N Engl J Med. 1985;313:1256-1262. |
| 10. | Girelli D, Corrocher R, Bisceglia L, Olivieri O, De Franceschi L, Zelante L, Gasparini P. Molecular basis for the recently described hereditary hyperferritinemia-cataract syndrome: a mutation in the iron-responsive element of ferritin L-subunit gene (the "Verona mutation"). Blood. 1995;86:4050-4053. |
| 11. | Allerson CR, Cazzola M, Rouault TA. Clinical severity and thermodynamic effects of iron-responsive element mutations in hereditary hyperferritinemia-cataract syndrome. J Biol Chem. 1999;274:26439-26447. |
| 12. | Arnold JD, Mumford AD, Lindsay JO, Hegde U, Hagan M, Hawkins JR. Hyperferritinaemia in the absence of iron overload. Gut. 1997;41:408-410. |
| 13. | Ferrari F, Foglieni B, Arosio P, Camaschella C, Daraio F, Levi S, García Erce JA, Beaumont C, Cazzola M, Ferrari M. Microelectronic DNA chip for hereditary hyperferritinemia cataract syndrome, a model for large-scale analysis of disorders of iron metabolism. Hum Mutat. 2006;27:201-208. |
| 14. | Busbridge M, Griffiths C, Ashby D, Gale D, Jayantha A, Sanwaiya A, Chapman RS. Development of a novel immunoassay for the iron regulatory peptide hepcidin. Br J Biomed Sci. 2009;66:150-157. |
| 15. | Beaumont C, Leneuve P, Devaux I, Scoazec JY, Berthier M, Loiseau MN, Grandchamp B, Bonneau D. Mutation in the iron responsive element of the L ferritin mRNA in a family with dominant hyperferritinaemia and cataract. Nat Genet. 1995;11:444-446. |
| 16. | Bonneau D, Winter-Fuseau I, Loiseau MN, Amati P, Berthier M, Oriot D, Beaumont C. Bilateral cataract and high serum ferritin: a new dominant genetic disorder? J Med Genet. 1995;32:778-779. |
| 17. | Mumford AD, Cree IA, Arnold JD, Hagan MC, Rixon KC, Harding JJ. The lens in hereditary hyperferritinaemia cataract syndrome contains crystalline deposits of L-ferritin. Br J Ophthalmol. 2000;84:697-700. |
| 18. | Bayele HK, McArdle H, Srai SK. Cis and trans regulation of hepcidin expression by upstream stimulatory factor. Blood. 2006;108:4237-4245. |
| 19. | Kemna EH, Tjalsma H, Willems HL, Swinkels DW. Hepcidin: from discovery to differential diagnosis. Haematologica. 2008;93:90-97. |
| 20. | Malyszko J. Hemojuvelin: the hepcidin story continues. Kidney Blood Press Res. 2009;32:71-76. |
| 21. | Silvestri L, Pagani A, Nai A, De Domenico I, Kaplan J, Camaschella C. The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metab. 2008;8:502-511. |
| 22. | Finberg KE, Heeney MM, Campagna DR, Aydinok Y, Pearson HA, Hartman KR, Mayo MM, Samuel SM, Strouse JJ, Markianos K. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA). Nat Genet. 2008;40:569-571. |
| 23. | Verga Falzacappa MV, Vujic Spasic M, Kessler R, Stolte J, Hentze MW, Muckenthaler MU. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood. 2007;109:353-358. |
| 24. | Moirand R, Mortaji AM, Loréal O, Paillard F, Brissot P, Deugnier Y. A new syndrome of liver iron overload with normal transferrin saturation. Lancet. 1997;349:95-97. |
| 25. | Beutler E, Felitti V, Gelbart T, Ho N. Genetics of iron storage and hemochromatosis. Drug Metab Dispos. 2001;29:495-499. |