Published online Jul 21, 2010. doi: 10.3748/wjg.v16.i27.3437
Revised: March 10, 2010
Accepted: March 17, 2010
Published online: July 21, 2010
AIM: To evaluate the efficacy of transcatheter arterial chemoembolization (TACE) using a suspension of a fine-powder formulation of cisplatin (DDPH) for hepatocellular carcinoma (HCC).
METHODS: The study population was comprised of 164 patients who were treated by TACE alone. Of these patients, 76 underwent TACE using a suspension of DDPH in lipiodol (LPD) (DDPH group), and the remaining 88 underwent TACE with an emulsion of doxorubicin (ADM) with LPD (ADM group). We compared the DDPH group with the ADM group in terms of the objective early response rate, progression free survival (PFS) and overall survival (OS).
RESULTS: The objective early response rate in the DDPH group was significantly higher than that in the ADM group (54% vs 24%, P < 0.001). The PFS rate in the DDPH group was also significantly higher than that in the ADM group (P < 0.001). Moreover, the OS in the DDPH group was significantly longer than that in the ADM group (P = 0.002). Although the incidence rate of nausea or vomiting in the DDPH group was higher than that in the ADM group, the ADM group showed a higher incidence rate of the adverse events of hepatic arterial damage and leucopenia. No other serious complications were observed in either group.
CONCLUSION: We conclude that TACE using a suspension of DDPH in LPD could be a useful treatment for HCC.
- Citation: Kasai K, Ushio A, Sawara K, Miyamoto Y, Kasai Y, Oikawa K, Kuroda H, Takikawa Y, Suzuki K. Transcatheter arterial chemoembolization with a fine-powder formulation of cisplatin for hepatocellular carcinoma. World J Gastroenterol 2010; 16(27): 3437-3444
- URL: https://www.wjgnet.com/1007-9327/full/v16/i27/3437.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i27.3437
Hepatocellular carcinoma (HCC) is the cancer with the sixth highest incidence in the world[1]. The number of deaths from HCC is also increasing throughout the world[2-5]. Development of new treatments for HCC has helped improve the patient prognosis[6,7]. Local ablating therapies such as percutaneous ethanol injection (PEI) or radiofrequency ablation (RFA) have been effective in cases of limited tumor spread and are increasingly used[7,8]. However, the majority of patients are not eligible for these modalities because of large tumor size or diffuse tumor growth. In these patients regional transcatheter arterial chemoembolization (TACE) has been widely used as a palliative treatment[9,10]. Two randomized trials from Europe and Asia recently confirmed a survival benefit after TACE using gelfoam and iodized oil (lipiodol) compared to conservative treatment[11,12]. In recent years, TACE using an emulsion of doxorubicin (ADM) with lipiodol (LPD) (ADM-LPD emulsion) followed by embolization with a gelatin sponge has been employed commonly for HCC treatment[13,14]. However, the tumors have been demonstrated to show a high frequency of recurrence after TACE[10,15,16]. Cisplatin (CDDP), a platinum compound, is an effective anticancer agent used in the treatment of various malignancies[17]. Researchers have recently reported that TACE using a suspension of CDDP powder in LPD may be more effective against unresectable HCC as compared with TACE using ADM-LPD emulsion[18,19]. However, only limited institutions have used this for TACE because it is laborious to refine the CDDP powder. Since 2004, a fine-powder formulation of CDDP (DDPH, IA-call; Nippon Kayaku, Tokyo, Japan) has also been available as a therapeutic agent for intra-arterial infusion in Japan. As a result, TACE using DDPH has become widespread in Japanese institutions. Nevertheless, the efficacy of TACE using DDPH-LPD suspension has not yet been reported.
In this article, we compared the effectiveness with regard to the response rate (RR), progression free survival (PFS) and overall survival (OS) between TACE using a suspension of DDPH in LPD (DDPH-LPD suspension) and ADM-LPD emulsion. Moreover, we analyzed the prognostic factors for clinical outcome of patients treated with TACE.
Between January 2006 and July 2009, 164 HCC patients who showed no indication for surgical resection or local ablation therapy such as RFA and PEI therapy were enrolled in the study. HCC was diagnosed by the distinctive findings on ultrasonography (US), computed tomography (CT), magnetic resonance imaging (MRI) and angiography, and the serum levels of des-γ-carboxy prothrombin (DCP) and α-fetoprotein (AFP). Histologic examination was not always carried out. Liver function was evaluated according to the Child-Pugh classification[20]. Tumor stage was judged by the TNM classification established by the International Union Against Cancer[21]. The extent of portal vein invasion was classified as follows: Vp 0, no invasion of the portal vein; Vp 1, invasion of the third or more distal branch of the left or right portal vein; Vp 2, invasion of the second branch of the portal vein; Vp3, invasion of the first branch of the portal vein; and Vp4, invasion of the trunk of the portal vein. After being presented with the clinical results of previous studies of TACE using DDPH-LPD suspension or TACE using ADM-LPD emulsion, all 164 patients themselves selected the therapeutic option on the basis of informed consent. All of the enrolled patients met the eligibility criteria for inclusion in the analysis described in the next paragraph. The patients were divided into two groups: one group consisting of 76 patients who underwent TACE using DDPH-LPD suspension (DDPH group), and another group consisting of 88 patients who underwent TACE using ADM-LPD emulsion (ADM group). They were all treated by TACE alone.
Informed consent was obtained from all of the patients. The study protocol was approved by the Ethics Committee of Iwate Medical University and the study was conducted in accordance with the Declaration of Helsinki 1975.
The eligibility criteria of the patients for this study were as follows: (1) No indication for surgical resection or local ablation therapy such as RFA and PEI therapy; (2) No evidence of extra-hepatic metastasis; (3) No tumor thrombus in the main trunk of portal vein; (4) No evidence of active heart or renal diseases meeting the contraindications for ADM and CDDP therapy, respectively; (5) Eastern Cooperative Oncology Group (ECOG) performance status (PS)[22] level 0-2; (6) Hypervascular tumors showing enhancement during angiography; (7) Bidimensionally measurable hepatic lesions; (8) No uncontrolled ascites or pleural effusion; and (9) Total serum bilirubin (T-Bil) less than 3 mg/dL.
The presence of underlying liver diseases such as hepatitis or cirrhosis was confirmed by laboratory, radiological examinations and pathological examinations. We classified the chronic hepatitis patients into Child-Pugh class A, because chronic hepatitis is a known pre-cirrhotic condition.
We used DDPH or ADM (Adriacin; Kyowa Hakko Kogyo, Tokyo, Japan) mixed with LPD (iodized oil; Andre Guerget, Aulnay-sous-Bois, France).
The DDPH-LPD suspension was prepared by mixing 50 mg of DDPH into 3-10 mL of LPD.
The ADM-LPD emulsion was prepared by the following procedure: 10-30 mg of ADM was dissolved in 1-2 mL of a contrast medium (Iomeron; Eisai Co., Ltd., Tokyo, Japan) and then mixed with 3-10 mL LPD.
The dosage of LPD and the anticancer drugs was adjusted depending on the tumor size, number of tumors, degree of liver impairment and renal function, however, the maximum dose of LPD was not allowed to exceed 10 mL.
Hepatic arteriography, superior mesenteric arterial porto-venography, CT during arteriography and CT during arterio-portography were performed to define the size and locations of tumor nodules and to exclude tumor thrombus in the main trunk of the portal vein. Following hepatic angiography, a catheter was selectively inserted into the hepatic artery supplying the target tumor and the DDPH-LPD suspension or the ADM-LPD emulsion was injected. In patients with several tumors in the liver, superselective catheterization was performed for each lesion. If superselective catheterization was not possible, the DDPH-LPD suspension or the ADM-LPD emulsion was injected into the right and left main hepatic artery distal to the origin of the cystic artery. After the injection, arterioembolization was performed used gelatin sponge particles (Gelpart; Nippon Kayaku, Tokyo, Japan) mixed with contrast medium.
All the patients were followed up with US, CT and/or MRI after 1 mo and every 3 mo thereafter. TACE was undertaken again when relapse of the treated lesions and/or new hepatic lesions were detected. These patients received additional TACE using the same agent during the follow-up period. The TACE was repeated until complete regression of the tumor was obtained, or until the patient could no longer be treated.
Early tumor response was assessed by US, CT and/or MRI, conducted 1 mo after the initial treatment. We regarded LPD accumulation in the tumor as representing a necrotic area, based on previous reports of such LPD retention areas corresponding to the necrotic areas on CT[23-26]. By measurement of the two largest perpendicular diameters of the tumor, we classified the tumor response into four categories using the following criteria: complete response (CR), complete disappearance or 100% necrosis of all tumors; partial response (PR), reduction and/or necrosis, with at least 50% decrease of all the measurable lesions; progressive disease (PD), an increase of the tumor size exceeding 25% of all the measurable lesions or appearance of a new lesion; stable disease (SD), disease not qualifying for classification as CR, PR, PD.
Toxicity was evaluated by the National Cancer Institute-Common Terminology Criteria for Adverse Events, version 3.0 (CTCAE v3.0).
The differences in the background clinical characteristics of the patients between the DDPH group and ADM group were assessed by Mann-Whitney’s U test, logistic regression test, or the χ2 test, as appropriate.
PFS and OS were calculated from the date of start of the therapy to the date on which tumor progression was documented and the date of death of the patient, respectively. Both were assessed by the Kaplan-Meier life-table method, and the differences between the two treatment groups were evaluated by the log rank test. Univariate analysis to identify the predictors of survival in the patients was conducted by the Kaplan-Meier life-table method, and the differences between the two groups were evaluated by the log rank test. Multivariate analysis to identify the predictors of survival was conducted using the Cox proportional hazards model. Statistical significance was defined as a P value of less than 0.05. All of the above analyses were performed using the SPSS software (version 11, SPSS, Chicago, IL, USA).
The characteristics of the 164 patients of both groups are summarized in Table 1. There were 109 male and 55 female patients, ranging in age from 21 to 90 years old (mean, 68 years old).
| Characteristics | DDPH group | ADM group | P value |
| No. of patients | 76 | 88 | |
| Age (yr) [median, (range)] | 67 (32-87) | 69 (21-90) | 0.093 |
| Gender (male/female) | 57/19 | 52/36 | 0.031 |
| Etiology (HBV/HCV/NBNC) | 11/50/15 | 8/64/16 | 0.508 |
| Child-Pugh classification (A/B/C) | 47/26/3 | 45/36/7 | 0.303 |
| TNM classification (I-II/III-IV) | 10/66 | 24/64 | 0.026 |
| Tumor size ( ≤ 3.0/> 3.0 cm) | 21/55 | 30/58 | 0.373 |
| Number of tumors (1-3/≥ 4) | 35/41 | 46/42 | 0.427 |
| PVTT (Vp0-2 /Vp3) | 62/14 | 80/8 | 0.080 |
| Total bilirubin ( ≤ 1.5/> 1.5 mg/dL) | 66/10 | 75/13 | 0.906 |
| Albumin ( ≤ 3.5/> 3.5 g/dL) | 38/38 | 45/42 | 0.822 |
| AFP ( ≤ 1000/> 1000 ng/mL) | 68/8 | 79/8 | 0.776 |
| DCP ( ≤ 1000/> 1000 mAU/mL) | 59/14 | 73/14 | 0.609 |
Regarding the assessment of differences in the characteristics of the patients, there were significant differences in the gender distribution and in the TNM classification between the two groups, i.e. there was a higher proportion of males (P = 0.031) and more subjects with advanced TNM classification (P = 0.026) in the DPHH group. There were no significant differences in any of the other characteristics between the two groups.
The median follow-up period was 13.1 mo (range: 1-40 mo). We performed 392 TACE procedures (157 sessions in the DDPH group, 235 sessions in the ADM group) in 164 patients. The median number of TACE procedures was 2 sessions (range: 1-5 sessions) in the DDPH group and 3 sessions (range: 1-6 sessions) in the ADM group. The median interval to the re-treatment with TACE was 9.4 mo in the DDPH group and 3.8 mo in the ADM group. One hundred and ten sessions (70.1%) in the DDPH group and 170 sessions (72.3%) in the ADM group were treated by superselectivity of TACE. There was no significant difference in the incidence of superselectivity of TACE between the two groups.
In the DDPH group, 2 (3%), 39 (51%), 23 (30%) and 12 (16%) patients showed CR, PR, SD and PD, respectively. In the ADM group, 5 (6%), 16 (18%), 5 (6%) and 62 (70%) patients showed CR, PR, SD and PD, respectively. Therefore, the objective early response rate of the DDPH group (54%) was significantly higher than that in the ADM group (24%). The difference in the rate between the two groups was statistically significant (P < 0.001) (Table 2).
| DDPH (n = 76) | ADM (n = 88) | P value | |
| CR | 2 (3) | 5 (6) | |
| PR | 39 (51) | 16 (18) | |
| SD | 23 (30) | 5 (6) | |
| PD | 12 (16) | 62 (70) | |
| CR + PR | 41 (54) | 21 (24) | < 0.001 |
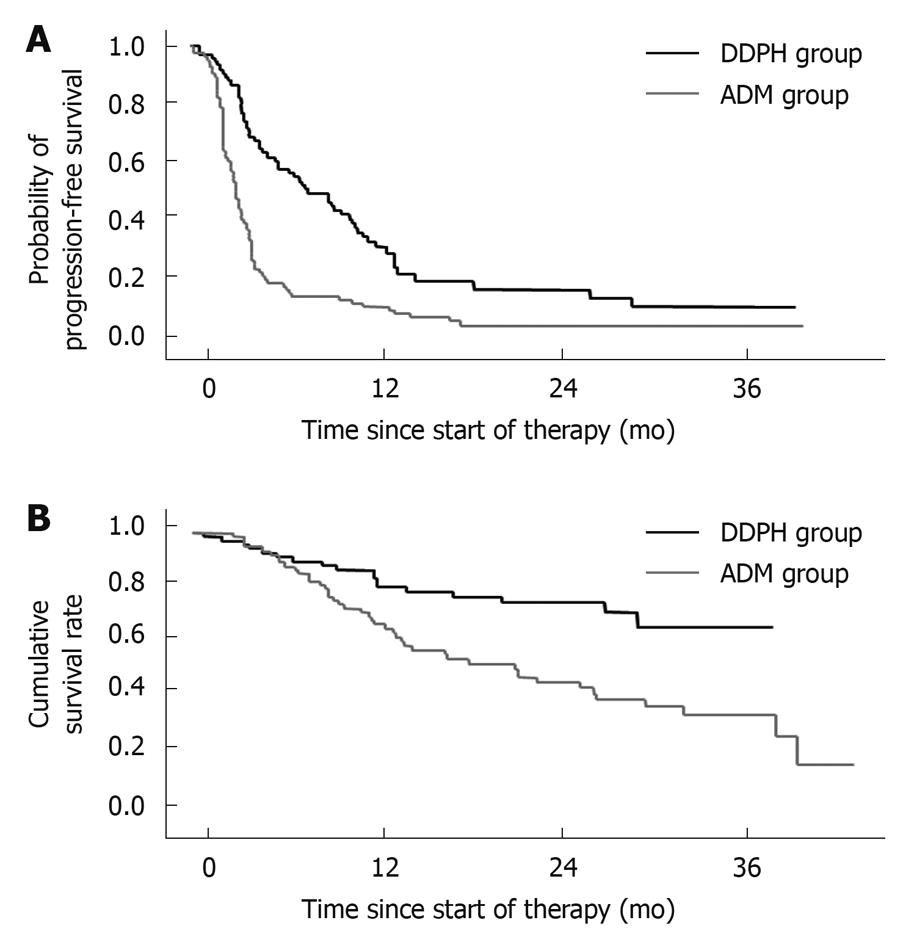
The median PFS was 8.6 mo in the DDPH group and 3.0 mo in the ADM group. The PFS rates at 6, 12, 24 and 36 mo were 58%, 32%, 18% and 11%, respectively, in the DDPH group. In contrast, the corresponding values were 18%, 10%, 5% and 5%, respectively, in the ADM group. The PFS rates in the DDPH group were significantly higher than those in the ADM group (P < 0.001) (Figure 1A).
The median survival time (MST) in the DDPH and ADM groups was “not reached” and 20.8 mo, respectively. The OS values at 6, 12, 24, and 36 mo were 92%, 81%, 76% and 67%, respectively, in the DDPH group. The corresponding values in the ADM group were 87%, 68%, 46% and 37%, respectively. The OS in the DDPH group was significantly longer than that in the ADM group (P = 0.002) (Figure 1B).
Univariate analysis to identify the predictors of survival indicated five possible factors affecting the survival: TNM classification; tumor size; number of tumors; portal vein tumor thrombosis (PVTT) and serum DCP level. The treatment regimen was close to being statistically significant (P = 0.065) for survival (Table 3). Multivariate analysis performed using factors that were considered significant (P < 0.1) on univariate analysis identified the treatment regimen, gender, number of tumors, PVTT, and serum albumin as independent factors affecting the survival (Table 4).
| Variable | Hazard ratio | 95% CI | P value |
| Treatment regimen (ADM vs DDPH) | 0.580 | 0.325-1.035 | 0.065 |
| Age ( ≤ 65 yr vs > 65 yr) | 1.286 | 0.741-2.231 | 0.372 |
| Gender (female vs male) | 1.651 | 0.944-2.888 | 0.079 |
| Etiology (NBNC vs HBV/HCV) | 0.734 | 0.432-1.246 | 0.252 |
| Child Pugh classification (A vs B/C) | 1.142 | 0.689-1.891 | 0.607 |
| TNM classification (I-II vs III-IV) | 2.765 | 1.252-6.106 | 0.012 |
| Tumor size ( ≤ 3.0 cm vs > 3.0 cm) | 2.094 | 1.161-3.776 | 0.014 |
| Number of tumors (1-3 vs≥ 4) | 2.612 | 1.535-4.444 | 0.001 |
| PVTT (Vp0-2 vs Vp3) | 4.714 | 2.520-8.819 | < 0.001 |
| Total bilirubin ( ≤ 1.5 mg/dL vs > 1.5 mg/dL) | 1.730 | 0.874-3.422 | 0.116 |
| Albumin ( ≤ 3.5 g/dL vs > 3.5 g/dL) | 0.996 | 0.603-1.647 | 0.989 |
| AFP ( ≤ 1000 ng/mL vs > 1000 ng/mL) | 1.323 | 0.528-3.315 | 0.551 |
| DCP ( ≤ 1000 mAU/mL vs > 1000 mAU/mL) | 2.396 | 1.288-4.459 | 0.005 |
| Variable | Hazard ratio | 95% CI | P value |
| Treatment regimen (ADM vs DDPH) | 0.329 | 0.149-0.726 | 0.006 |
| Gender (female vs male) | 2.291 | 1.174-4.470 | 0.015 |
| Number of tumors (1-3 vs≥ 4) | 6.541 | 3.201-13.363 | < 0.001 |
| PVTT (Vp0-2 vs Vp3) | 6.704 | 2.581-17.418 | < 0.001 |
| Albumin ( ≤ 3.5 g/dL vs > 3.5 g/dL) | 0.311 | 0.157-0.612 | 0.001 |
Table 5 shows a summary of the adverse effects in the two groups. The incidence rate of nausea/vomiting in the DDPH group was significantly higher than that in the ADM group (P < 0.001). In addition, the incidence rates of hepatic arterial damage (HAD) after TACE and leucopenia in the ADM group were significantly higher than those in the DDPH group (P < 0.001 and P = 0.002, respectively). We observed HAD in 17 patients. Although one patient in the DDPH group was observed to have slight wall irregularity of the hepatic artery (HA), HAD associated with TACE did not interfere with catheterization at the next TACE session. On the other hand, in the ADM group, we observed slight wall irregularity of HA in six patients, overt stenosis of HA in four patients and occlusion of HA in six patients. In six patients who were observed as having occlusion of HA, it became impossible to treat with repeated TACE.
| Adverse effect | Treatment group (%) | P value | |
| DDPH group (n = 76) | ADM group (n = 88) | ||
| Nausea/vomiting | 64 (84) | 48 (55) | < 0.001 |
| Fever | 61 (80) | 73 (83) | 0.571 |
| Abdominal pain | 53 (69) | 63 (72) | 0.958 |
| Elevation of transaminase levels | 55 (72) | 62 (71) | 0.993 |
| Liver abscess | 1 (1) | 2 (2) | 0.765 |
| Hepatic arterial damage | 1 (1) | 16 (18) | < 0.001 |
| Renal or liver failure | 0 (0) | 2 (2) | 0.229 |
| Leucopenia | 3 (4) | 12 (14) | 0.002 |
| Thrombocytopenia | 4 (5) | 6 (7) | 0.650 |
| Fatigue | 21(28) | 27 (31) | 0.839 |
No other serious complications or treatment-related deaths were observed in either group.
TACE has been widely used for the treatment of unresectable HCC[9,10]. The most commonly used agent used in TACE for HCC treatment is ADM-LPD emulsion, followed by embolization with a gelatin sponge[13,14]; however, the tumors frequently recur[10,15,16] or residual tumors are observed at a high incidence. CDDP is an effective anticancer agent used in the treatment of various malignancies[17]. It has been reported to exert its actions by binding to the DNA in cancer cells, inhibiting DNA synthesis and subsequent cellular division. The antitumor activity of CDDP is closely associated with the serum concentration of the drug[27]. Therefore, the antitumor activity can be enhanced by increase of the dose. LPD acts as a selective carrier of anticancer agents and as an embolic material[23]; the anticancer agent is gradually released from the iodized oil. Although the mechanism of topical accumulation of LPD in the tumor is not yet precisely understood, it is used nonetheless to achieve a targeting drug delivery system with long-lasting accumulation in the tumor and gradual drug release. Consequently, augmented antitumor efficacy and milder side-effects have come to be expected with the use of this substance for TACE. In fact, Morimoto et al[28] investigated the pharmacological advantages of TACE using DDPH for hypervascular hepatic tumors in animal experiments. They reported that the tumor concentration of the platinum agent in the DDPH-LPD-TACE group was about 14 times higher than that in the DDPH-hepatic arterial infusion (HAI) group. In addition, they reported that the plasma concentrations of the platinum agent at 5 and 10 min from start of the infusion were lower in the DDPH-LPD-TACE group than those in the DDPH-HAI group. Recently, Ono et al[18] reported that TACE using a suspension of CDDP powder in LPD was more effective than that using ADM-LPD emulsion against unresectable HCC. Other investigators have also frequently reported favorable results obtained with TACE using a suspension of CDDP powder in LPD in HCC patients[19,29]. However, the CDDP powder for this therapy is difficult to produce because of the characteristics of the drug formulation. Therefore, CDDP powder had to be a custom-made formulation in individual institutions[30]. Consequently, when an institution was able to dispense CDDP powder in its own pharmacy department, TACE using a suspension of CDDP powder in LPD was undertaken.
A fine-powder formulation of CDDP, namely “DDPH”, for intra-arterial infusion has been available for HCC treatment since 2004 in Japan. Dispensing of CDDP powder improved with the development of DDPH, and DDPH has now come to replace CDDP powder. Using DDPH-LPD suspension for TACE in HCC patients was expected to yield better therapeutic outcomes; therefore, TACE using DDPH became widespread in Japanese institutions. Nevertheless, the efficacy of TACE using DDPH-LPD suspension has not yet been reported. Therefore, we compared the outcomes of TACE using DDPH-LPD suspension and ADM-LPD emulsion.
Analysis of the results in our study revealed that the objective response rate in the DDPH group was significantly higher than that in the ADM group. Moreover, the OS of the patients in the DDPH group was significantly longer than that of the patients in the ADM group. This could be explained as being due to the fact that TACE with ADM cannot be repeated as required because of the high frequency of adverse effects of ADM such as leucopenia, severe vascular changes and occlusion of the hepatic artery[18,31,32]. In fact, the incidences of leucopenia and HAD in the ADM group in our study were significantly higher than those in the DDPH group. Considering the fact that TACE is often repeated in most patients, longer patency of the hepatic artery is preferable for properly deploying the lipiodol mixture and embolic agents into the tumor. In addition, we conclude that anthracyclines such as ADM may be relatively less effective against HCC; this is because of the high expression level of P-glycoprotein, which transports antitumor agents such as anthracyclines or vinca alkaloids from cells with a high active efflux mechanism, in HCC tumors[33].
On the other hand, Pelletier et al[34] reported that TACE with CDDP sometimes caused severe complications, such as acute hepatic failure. The treatment also did not produce any significant improvement of the survival rate in this study. Severe complications could be expected with the high doses (2 mg/kg) of CDDP used in their study. Therefore, we performed TACE using DDPH-LPD suspension in our study with half of the dose (50 mg = 1 mg/kg) that they had used. Modification of the CDDP dose used for the treatment to DDPH 50 mg in our study resulted in a lower severity of complications.
Takayasu et al[35] reported a nationwide prospective cohort study which was performed in 8510 patients with unresectable HCC who underwent TACE using an emulsion of lipiodol and anticancer agents followed by gelatin sponge particles as an initial treatment. In their report, multivariate analysis for the factors affecting survival showed significant differences in degree of liver damage, AFP, maximum tumor size, number of lesions, and PVTT. In contrast to their report, we could not observe AFP value as a prognostic factor in our multivariate analysis. This may be due to fewer in the study population and a shorter observation period in our study compared with their study. In addition, a cut-off value for AFP of 1000 ng/mL in our study was much higher than that (400 ng/mL) in their study because we aimed to analyze the difference in the effect of TACE with the extent that HCC had progressed. Therefore, we could not observe AFP value as a prognostic factor in our multivariate analysis.
This study was not a well-controlled prospective study. Nevertheless, the patients in the two groups had fairly similar characteristics with regard to age, etiology, Child-Pugh classification, tumor size, number of tumors, PVTT, total bilirubin, albumin, AFP, and DCP. In relation to the differences in the characteristics of the patients, the DDPH group had a significantly higher proportion of males and a more advanced stage in TNM classification than the ADM group. Several investigators[36,37] have shown that TNM classification and tumor stage are independent prognostic factors for survival of patients who are treated by TACE. Therefore, we forecast that the prognosis of the CDDP group was worse than that of the ADM group, because the DDPH group had more advanced stage in TNM classification than the ADM group. However, the OS in the DDPH group was significantly longer than that in the ADM group. Moreover, to avoid the confounding effects of any deviations in the patient characteristics causing an impact on the results, we used the multivariate analysis for comparison of the efficacy between the regimens. The analysis identified the treatment regimen employed for the TACE as one of the most important prognostic factors. Compared to a previous report[18] describing TACE using a suspension of CDDP powder in LPD, the objective response rate and OS in the DDPH group in our study were significantly higher.
Considering these facts, we conclude that TACE using DDPH-LPD suspension could be a useful treatment strategy for HCC patients. To confirm these results, randomized controlled trials comparing TACE using DDPH-LPD suspension with TACE using ADM-LPD suspension for patients with HCC are mandatory.
In recent years, transcatheter arterial chemoembolization (TACE) using an emulsion of doxorubicin (ADM) with lipiodol (LPD) (ADM-LPD emulsion) followed by embolization with a gelatin sponge has been employed commonly for hepatocellular carcinoma (HCC) treatment. However, the tumors have been demonstrated to show a high frequency of recurrence after TACE.
Cisplatin, a platinum compound, is an effective anticancer agent used in the treatment of various malignancies. Since 2004, a fine-powder formulation of cisplatin (DDPH, IA-call; Nippon Kayaku, Tokyo, Japan) has also been available as a therapeutic agent for intra-arterial infusion in Japan. Researchers have recently reported that TACE using a suspension of cisplatin powder in LPD may be more effective against unresectable HCC as compared with TACE using ADM-LPD emulsion. Therefore, TACE using DDPH has become widespread in Japanese institutions. However, the efficacy of TACE using DDPH-LPD suspension has not yet been reported.
In this article, the authors reported the effectiveness of TACE using DDPH-LPD suspension compared with that using ADM-LPD emulsion.
Although randomized controlled trials comparing TACE using DDPH-LPD suspension with TACE using ADM-LPD suspension for patients with HCC are needed, this study shows that TACE using DDPH-LPD suspension can be a useful treatment strategy for HCC patients.
TACE: Transarterial chemoembolization, a procedure in which the blood supply to a tumor is blocked (embolized) and chemotherapy is administered directly into the tumor.
Kasai et al evaluated the efficacy of TACE using a suspension of DDPH for HCC. The authors indicated that early response rate, progression free survival and overall survival in the DDPH group was significantly higher than that in the ADM group.
Peer reviewers: Satoshi Mamori, MD, PhD, Department of Gastroenterology and Hepatology, Shinko Hospital, 1-4-47 Wakihama-cho, Chuo-ku, Kobe, Hyogo 651-0072, Japan; Susumu Ohwada, Associate Professor, Department of Surgery, Gunma University Graduate School of Medicine, 3-39-15 Shoma-Machi, Maebashi 371-8511, Japan
S- Editor Tian L L- Editor Logan S E- Editor Ma WH
| 1. | Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137-2150. |
| 2. | El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745-750. |
| 3. | Fattovich G, Giustina G, Degos F, Tremolada F, Diodati G, Almasio P, Nevens F, Solinas A, Mura D, Brouwer JT. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463-472. |
| 4. | La Vecchia C, Lucchini F, Franceschi S, Negri E, Levi F. Trends in mortality from primary liver cancer in Europe. Eur J Cancer. 2000;36:909-915. |
| 5. | Okuda K, Fujimoto I, Hanai A, Urano Y. Changing incidence of hepatocellular carcinoma in Japan. Cancer Res. 1987;47:4967-4972. |
| 6. | Ohtomo K, Furui S, Kokubo T, Yamauchi T, Itai Y, Yashiro N, Iio M. Transcatheter arterial embolization (TAE) in treatment for hepatoma--analysis of three-year survivors. Radiat Med. 1985;3:176-180. |
| 7. | Shiina S, Teratani T, Obi S, Hamamura K, Koike Y, Omata M. Nonsurgical treatment of hepatocellular carcinoma: from percutaneous ethanol injection therapy and percutaneous microwave coagulation therapy to radiofrequency ablation. Oncology. 2002;62 Suppl 1:64-68. |
| 8. | Shiina S, Tagawa K, Unuma T, Terano A. Percutaneous ethanol injection therapy for the treatment of hepatocellular carcinoma. AJR Am J Roentgenol. 1990;154:947-951. |
| 9. | Kanematsu T, Furuta T, Takenaka K, Matsumata T, Yoshida Y, Nishizaki T, Hasuo K, Sugimachi K. A 5-year experience of lipiodolization: selective regional chemotherapy for 200 patients with hepatocellular carcinoma. Hepatology. 1989;10:98-102. |
| 10. | Yamada R, Sato M, Kawabata M, Nakatsuka H, Nakamura K, Takashima S. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology. 1983;148:397-401. |
| 11. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. |
| 12. | Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171. |
| 13. | Shimamura Y, Gunvèn P, Takenaka Y, Shimizu H, Shima Y, Akimoto H, Arima K, Takahashi A, Kitaya T, Matsuyama T. Combined peripheral and central chemoembolization of liver tumors. Experience with lipiodol-doxorubicin and gelatin sponge (L-TAE). Cancer. 1988;61:238-242. |
| 14. | Takayasu K, Suzuki M, Uesaka K, Muramatsu Y, Moriyama N, Yoshida T, Yoshino M, Okazaki N, Hasegawa H. Hepatic artery embolization for inoperable hepatocellular carcinoma; prognosis and risk factors. Cancer Chemother Pharmacol. 1989;23 Suppl:S123-S125. |
| 15. | Takayasu K, Shima Y, Muramatsu Y, Moriyama N, Yamada T, Makuuchi M, Hasegawa H, Hirohashi S. Hepatocellular carcinoma: treatment with intraarterial iodized oil with and without chemotherapeutic agents. Radiology. 1987;163:345-351. |
| 16. | Kawai S, Okamura J, Ogawa M, Ohashi Y, Tani M, Inoue J, Kawarada Y, Kusano M, Kubo Y, Kuroda C. Prospective and randomized clinical trial for the treatment of hepatocellular carcinoma--a comparison of lipiodol-transcatheter arterial embolization with and without adriamycin (first cooperative study). The Cooperative Study Group for Liver Cancer Treatment of Japan. Cancer Chemother Pharmacol. 1992;31 Suppl:S1-S6. |
| 17. | Uchiyama N, Kobayashi H, Nakajo M, Shinohara S. Treatment of lung cancer with bronchial artery infusion of cisplatin and intravenous sodium thiosulfate rescue. Acta Oncol. 1988;27:57-61. |
| 18. | Ono Y, Yoshimasu T, Ashikaga R, Inoue M, Shindou H, Fuji K, Araki Y, Nishimura Y. Long-term results of lipiodol-transcatheter arterial embolization with cisplatin or doxorubicin for unresectable hepatocellular carcinoma. Am J Clin Oncol. 2000;23:564-568. |
| 19. | Kamada K, Nakanishi T, Kitamoto M, Aikata H, Kawakami Y, Ito K, Asahara T, Kajiyama G. Long-term prognosis of patients undergoing transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: comparison of cisplatin lipiodol suspension and doxorubicin hydrochloride emulsion. J Vasc Interv Radiol. 2001;12:847-854. |
| 20. | Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1-85. |
| 21. | Hermanek P, Scheibe O, Spiessl B, Wagner G. [TNM classification of malignant tumors: the new 1987 edition]. Rontgenblatter. 1987;40:200. |
| 22. | Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649-655. |
| 23. | Nakakuma K, Tashiro S, Hiraoka T, Uemura K, Konno T, Miyauchi Y, Yokoyama I. Studies on anticancer treatment with an oily anticancer drug injected into the ligated feeding hepatic artery for liver cancer. Cancer. 1983;52:2193-2200. |
| 24. | Jinno K, Moriwaki S, Tanada M, Wada T, Mandai K, Okada Y. Clinicopathological study on combination therapy consisting of arterial infusion of lipiodol-dissolved SMANCS and transcatheter arterial embolization for hepatocellular carcinoma. Cancer Chemother Pharmacol. 1992;31 Suppl:S7-S12. |
| 25. | Imaeda T, Yamawaki Y, Seki M, Goto H, Iinuma G, Kanematsu M, Mochizuki R, Doi H, Saji S, Shimokawa K. Lipiodol retention and massive necrosis after lipiodol-chemoembolization of hepatocellular carcinoma: correlation between computed tomography and histopathology. Cardiovasc Intervent Radiol. 1993;16:209-213. |
| 26. | Okusaka T, Okada S, Ueno H, Ikeda M, Yoshimori M, Shimada K, Yamamoto J, Kosuge T, Yamasaki S, Iwata R. Evaluation of the therapeutic effect of transcatheter arterial embolization for hepatocellular carcinoma. Oncology. 2000;58:293-299. |
| 27. | Takahashi K, Ebihara K, Honda Y, Nishikawa K, Kita M, Oomura M, Shibasaki C. [Antitumor activity of cis-dichlorodiammineplatinum(II) and its effect on cell cycle progression]. Gan To Kagaku Ryoho. 1982;9:624-631. |
| 28. | Morimoto K, Sakaguchi H, Tanaka T, Yamamoto K, Anai H, Hayashi T, Satake M, Kichikawa K. Transarterial chemoembolization using cisplatin powder in a rabbit model of liver cancer. Cardiovasc Intervent Radiol. 2008;31:981-985. |
| 29. | Kasugai H, Kojima J, Tatsuta M, Okuda S, Sasaki Y, Imaoka S, Fujita M, Ishiguro S. Treatment of hepatocellular carcinoma by transcatheter arterial embolization combined with intraarterial infusion of a mixture of cisplatin and ethiodized oil. Gastroenterology. 1989;97:965-971. |
| 30. | Yamamoto K, Shimizu T, Narabayashi I. Intraarterial infusion chemotherapy with lipiodol-CDDP suspension for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2000;23:26-39. |
| 31. | Doroshow JH, Locker GY, Myers CE. Experimental animal models of adriamycin cardiotoxicity. Cancer Treat Rep. 1979;63:855-860. |
| 32. | Olson HM, Capen CC. Subacute cardiotoxicity of adriamycin in the rat: biochemical and ultrastructural investigations. Lab Invest. 1977;37:386-394. |
| 33. | Itsubo M, Ishikawa T, Toda G, Tanaka M. Immunohistochemical study of expression and cellular localization of the multidrug resistance gene product P-glycoprotein in primary liver carcinoma. Cancer. 1994;73:298-303. |
| 34. | Pelletier G, Ducreux M, Gay F, Luboinski M, Hagège H, Dao T, Van Steenbergen W, Buffet C, Rougier P, Adler M. Treatment of unresectable hepatocellular carcinoma with lipiodol chemoembolization: a multicenter randomized trial. Groupe CHC. J Hepatol. 1998;29:129-134. |
| 35. | Takayasu K, Arii S, Ikai I, Omata M, Okita K, Ichida T, Matsuyama Y, Nakanuma Y, Kojiro M, Makuuchi M. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131:461-469. |
| 36. | Ji SK, Cho YK, Ahn YS, Kim MY, Park YO, Kim JK, Kim WT. Multivariate analysis of the predictors of survival for patients with hepatocellular carcinoma undergoing transarterial chemoembolization: focusing on superselective chemoembolization. Korean J Radiol. 2008;9:534-540. |