Published online Jul 21, 2010. doi: 10.3748/wjg.v16.i27.3427
Revised: April 27, 2010
Accepted: May 4, 2010
Published online: July 21, 2010
AIM: To investigate the prevalence of, and risk factors for, diabetes mellitus (DM) in Algerian patients with chronic hepatitis C virus (HCV) infection and in a control group.
METHODS: A cross-sectional study was undertaken. A total of 416 consecutive patients with viral chronic hepatitis attending the Internal Medicine Department of the University Hospital Center Touhami Benflis in Batna [290 HCV-infected and 126 hepatitis B virus (HBV)-infected patients] were prospectively recruited.
RESULTS: The prevalence of DM was higher in HCV-infected patients in comparison with HBV-infected patients (39.1% vs 5%, P < 0.0001). Among patients without cirrhosis, diabetes was more prevalent in HCV-infected patients than in HBV-infected patients (33.5% vs 4.3%, P < 0.0001). Among patients with cirrhosis, diabetes was more prevalent in HCV-infected patients, but the difference was not significant (67.4% vs 20%, P = 0.058). The logistic regression analysis showed that HCV infection [odds ratio (OR) 4.73, 95% CI: 1.7-13.2], metabolic syndrome (OR 12.35, 95% CI: 6.18-24.67), family history of diabetes (OR 3.2, 95% CI: 1.67-6.13) and increased hepatic enzymes (OR 2.22, 95% CI: 1.1-4.5) were independently related to DM in these patients.
CONCLUSION: The high prevalence of diabetes in HCV-infected patients, and its occurrence at early stages of hepatic disease, suggest that screening for glucose abnormalities should be indicated in these patients.
- Citation: Rouabhia S, Malek R, Bounecer H, Dekaken A, Amor FB, Sadelaoud M, Benouar A. Prevalence of type 2 diabetes in Algerian patients with hepatitis C virus infection. World J Gastroenterol 2010; 16(27): 3427-3431
- URL: https://www.wjgnet.com/1007-9327/full/v16/i27/3427.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i27.3427
Hepatitis C virus (HCV) infection is a common worldwide medical problem; it is one of the major causes of chronic liver disease. According to recent World Health Organization estimates the worldwide prevalence of HCV infection is 2.2%, affecting approximately 130 million people globally[1]. Patients with chronic HCV infection may develop various extrahepatic manifestations, including cryoglobulinemia, the presence of serum antibodies, glomerulonephritis, sialoadenitis and porphyria cutaneous tarda[2]. Several studies from different parts of the world have reported that HCV infection may also contribute to the development of diabetes mellitus (DM), and higher prevalence of type 2 DM has been observed in patients with HCV infection than in those with other forms of chronic hepatitis[3-5]. However, the prevalence of type 2 DM in patients with HCV infection has not been reported in Algeria, as far as we know.
Thus, in order to examine the prevalence of DM in patients with chronic HCV infection in Batna (Algeria), we conducted a cross-sectional study assessing the prevalence of this metabolic disorder in patients with HCV infection in comparison with the prevalence in those with hepatitis B virus (HBV) infection. In addition, risk factors associated with DM development such as age, body mass index (BMI), diabetic familial history and metabolic syndrome were also evaluated to clarify the possible role of chronic HCV infection in association with development of diabetes.
In this study, the prevalence of diabetes in patients with chronic HCV has not been compared to that in the general population, because patients with chronic liver disease, regardless of etiology, have a higher prevalence of diabetes mellitus[6]. We have chosen patients with chronic hepatitis B as a control group, because HBV infection is the second leading cause of chronic hepatitis after HCV in Algeria[7].
From September 2004 to September 2007, we conducted a cross-sectional study by enrolling patients with chronic viral hepatitis admitted to the University Hospital Center Touhami Benflis in Batna, Algeria. The diagnosis of HCV infection was made if patients were positive for anti-HCV antibody and HCV RNA. The presence of anti-HCV antibody was assessed using the third generation microparticle enzyme immunoassay test. The presence of HCV RNA was confirmed by Cobas Ampliprep/Roche Taq Man (Pasteur Institute, Algiers and Sadelaoud Laboratory, Batna, Algeria). HBV infection was diagnosed if patients had evidence of hepatitis B surface antigen. Patients with concomitant HCV and HBV infection were excluded. There was no serologic evidence of co-infection with other hepatotropic viruses or with human immunodeficiency virus. Patients having other causes of liver disease, in particular those known to be involved in the pathogenesis of diabetes, such as hemochromatosis or alcoholic liver disease, were excluded. None of the study patients had received corticosteroids during the previous 6 mo before the study. Patients with a history of, or evidence of, pancreatitis, pancreatic tumor, hepatic tumor or cirrhosis with Child-Pugh category C were excluded from the study. None of the study patients had previously received anti-viral treatment. No woman was pregnant in this study. Patients who were infected with HCV or HBV after being diagnosed with diabetes were also excluded from the analysis.
According to the American Diabetes Association criteria[8], patients were assigned a diagnosis of DM if they were using oral hypoglycemic medication or insulin, or if they showed fasting glucose greater than 126 mg/dL on two occasions, or glucose greater than 200 mg/dL, 2 h after an oral glucose tolerance test, performed in patients with impaired fasting glucose (fasting glucose concentration ≥ 110 mg/dL and < 126 mg/dL).
A diagnosis of cirrhosis was established either by histology or by presumptive diagnosis made when patients had ascites, hematologic evidence of hypersplenism, esophageal varices or relevant ultrasonographic findings. Liver biopsy was performed in patients with increased alanine aminotransferase (ALT) and who gave their informed consent beforehand. Liver biopsy specimens were analyzed by a single experienced pathologist, who was informed of clinical and biologic data. Fibrosis was assessed using the METAVIR score[9]. Fibrosis stage (F) was scored as F0 (absent), F1 (portal fibrosis), F2 (portal fibrosis with few septa), F3 (septal fibrosis), and F4 (cirrhosis).
The BMI and family history of DM were recorded for each patient during enrollment. The BMI was expressed as the body weight divided by the square of the body length (kg/m2). Overweight was defined as a BMI 25-29.9 kg/m2 and obesity as a BMI ≥ 30 kg/m2. The family history of diabetes was obtained from the patients themselves and was recorded as positive if their first-degree relatives had DM. The metabolic syndrome was diagnosed according to the National Cholesterol Education Program’s Adult Treatment Panel III definition[10].
All data values were expressed as mean ± standard deviation. Results were compared between HCV and HBV patients using the χ2 test for categorical variables and Student t-test for continuous variables. Stepwise multivariate logistic regression was performed to evaluate the predictive variables associated with the presence of diabetes in the study patients. All statistical analyses were performed using Epi info 2000 (Statistics Program for Public Health. CDC, Atlanta, USA), and a P value < 0.05 was considered significant.
In total, 416 patients (290 HCV-infected patients and 126 HBV-infected patients) were enrolled in this study. Considerable differences could be noted when the demographic characteristics of the two groups were compared (Table 1). Patients with hepatitis C were older than those with hepatitis B (55 ± 9 years vs 40 ± 13 years, P < 0.0001). Percentage of men was lower in the HCV-infected patients than in those with HBV (26.9% vs 62.7%, P < 0.0001). In addition, there were significantly more patients with metabolic syndrome among the hepatitis C patients, and more cirrhosis in the hepatitis C group.
| Clinical features | Virological diagnosis | P | |
| HBV (n = 126) | HCV (n = 290) | ||
| Age (yr) | |||
| < 40 | 57 (45.2) | 13 (4.5) | < 0.001 |
| 40-59 | 60 (47.6) | 193 (66.6) | < 0.001 |
| ≥ 60 | 9 (7.1) | 84 (29) | < 0.001 |
| Male sex | 79 (62.7) | 78 (26.9) | < 0.001 |
| Family history of diabetes | 38 (30.2) | 103 (35.5) | 0.28 |
| BMI (kg/m2), mean ± SD | 25.22 ± 4.48 | 26.27 ± 4.51 | 0.05 |
| BMI ≥ 25 kg/m2 | 68 (54) | 161 (55.5) | 0.42 |
| Metabolic syndrome | 20 (15.9) | 122 (42.1) | < 0.0001 |
| Increased ALT | 15 (11.9) | 177 (61) | < 0.0001 |
| Cirrhosis | 5 (4) | 51 (17.6) | 0.00018 |
DM was observed more often in HCV-infected patients than in HBV-infected patients (39.1% vs 5%, P < 0.0001). However, this difference is statistically significant only in patients aged between 40 and 60 years.
We compared variables associated with diabetes in patients with patent diabetes (6 HBV-infected patients and 102 infected HCV patients) and in those who were non-diabetics (115 HBV-infected patients and 159 HCV-infected patients). Patients with impaired fasting glucose (5 HBV-infected patients and 29 HCV-infected patients) were excluded from this comparison.
A family history of diabetes appeared to be matched in the present study; of the subjects infected with HCV and HBV, 35.5% and 30.2%, respectively, had a familial history of DM. Patients with a family history of diabetes were more likely to have DM compared with those without (41.08% vs 21.73%, P < 0.0001). For patients with a family history of DM, the prevalence of diabetes was significantly higher in subjects with HCV infection compared with those with HBV infection (56.5% vs 2.7%, P < 0.000001) (Table 2).
| Variables | Virological diagnosis | P | |
| HBV (n = 121) | HCV (n = 261) | ||
| Diabetic | 6 (5) | 102 (39.1) | < 0.0001 |
| Age (yr) | |||
| < 40 | 0/56 (0) | 1/12 (8.3) | |
| 40-59 | 4/58 (6.9) | 61/174 (35.1) | < 0.0001 |
| ≥ 60 | 2/7 (28.6) | 40/75 (52.6) | 0.56 |
| Family history of diabetes | 1/37 (2.7) | 52/92 (56.5) | < 0.0001 |
| BMI ≥ 25 kg/m2 | 5/66 (7.6) | 70/147 (47.6) | < 0.0001 |
| Metabolic syndrome | 4/18 (22.2) | 75/108 (69.4) | < 0.001 |
| Increased ALT | 2/14 (14.3) | 79/155 (51) | 0.01 |
| Cirrhosis | 1/5 (20) | 29/43 (67.4) | 0.058 |
| No cirrhosis | 5/116 (4.3) | 73/218 (33.5) | < 0.0001 |
Metabolic syndrome was more frequent in HCV-infected patients (42.1% vs 15.9%, P < 0.0001). Patients with metabolic syndrome were more likely to have DM compared with those without (62.69% vs 11.32%, P < 0.000001). For patients with metabolic syndrome, DM was significantly more frequent in HCV-infected patients than in those with HBV infection (69.4% vs 22.2%, P < 0.001). In obese or overweight subjects (BMI ≥ 25 kg/m2), DM was more frequent in HCV-infected patients (47.6% vs 7.6%, P < 0.0001).
Liver disease appeared more severe in the HCV group. Cirrhosis was more frequent in HCV-infected patients than in those with HBV infection (17.6% vs 4%, P < 0.001). In patients without cirrhosis, DM was more frequent in patients with HCV infection than in those with HBV infection (33.5% vs 4.3%, P < 0.00001). However, in patients with cirrhosis, diabetes was more prevalent in HCV-infected patients, but the difference was not significant (67.4% vs 20%, P = 0.058).
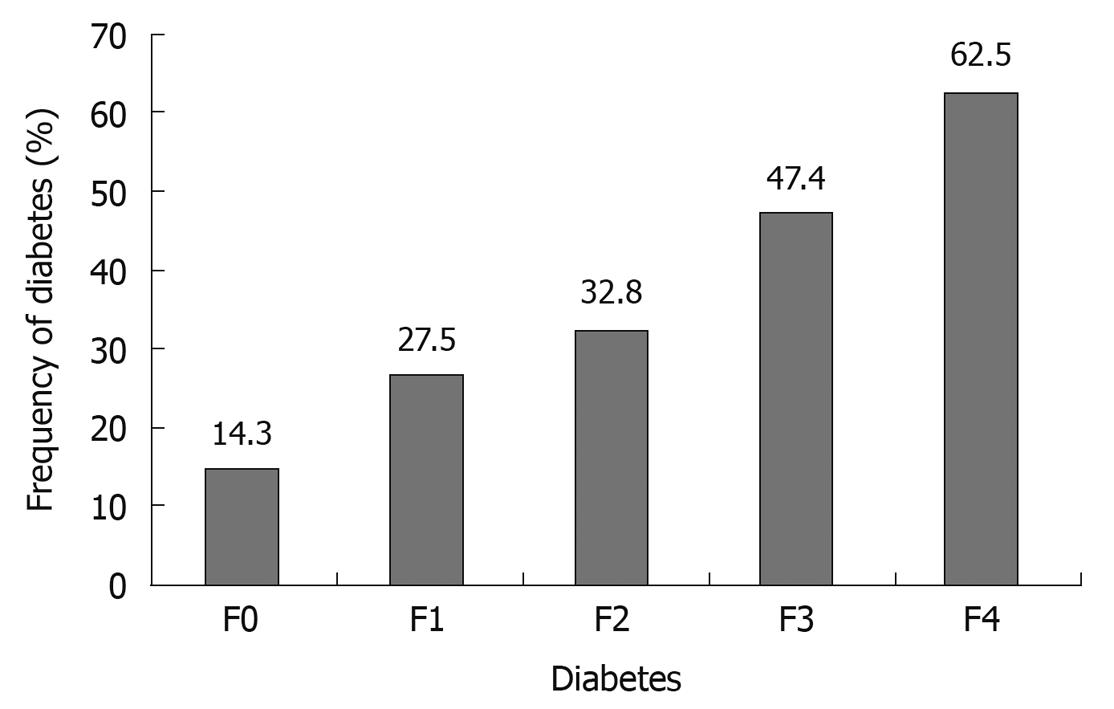
In HCV-infected patients in whom liver biopsy was performed, DM prevalence increased progressively and significantly with the fibrosis stage (Figure 1). DM was more frequent in patients with increased ALT plasma concentration (> 40 IU/L) compared with those with normal ALT plasma concentration (47.92% vs 12.67%, P < 0.00001). In patients with increased ALT plasma concentration, DM was significantly more frequent in HCV-infected patients than in those with HBV infection (51% vs 14.3%, P = 0.01).
The multiple regression analysis revealed that the major independent variables associated with type 2 diabetes were metabolic syndrome [odds ratio (OR) 12.35, P = 0.0001, 95% CI: 6.18-24.67], HCV infection (OR 4.73, P = 0.0029, 95% CI: 1.69-13.20), family history of diabetes (OR 3.2, P = 0.0004, 95% CI: 1.67-6.13) and increased ALT (OR 2.22, P = 0.027, 95% CI: 1.09-4.52) (Table 3).
| Variables | Odds ratio | 95% CI | P value |
| Metabolic syndrome | 12.35 | 6.18-24.67 | 0.00001 |
| Hepatitis C | 4.73 | 1.69-13.20 | 0.0029 |
| Family history of diabetes | 3.2 | 1.67-6.13 | 0.0004 |
| Increased ALT | 2.22 | 1.09-4.52 | 0.027 |
Our epidemiological and virological data suggest that HCV infection is more closely related to diabetes than HBV infection. Diabetes was observed in 39.1% of patients with HCV infection, as compared with 5% of HBV-infected subjects in our population. However, our study has some limitations, related to the small size of the control group. Indeed, chronic hepatitis B is much less common than chronic hepatitis C in our area[7]. Our findings are in concordance with similar epidemiological studies from different part of the world.
Allison et al[11] published, in 1994, the first article about a link between viral hepatitis C and diabetes. In their retrospective study of 100 cirrhotic patients listed for transplantation, these authors reported that the prevalence of type 2 DM was higher in patients with HCV-associated cirrhosis than in cirrhotics with other underlying liver diseases. In a cross-sectional survey including 9841 persons, Mehta et al[12] found that HCV-positive persons who were older than 40 years had an increased risk for type 2 diabetes mellitus, more than 3-fold when compared to persons without HCV infection. However, no difference was seen between HBV-infected subjects and the general population[12].
In a retrospective analysis of 1117 patients with chronic viral hepatitis, diabetes was present in significantly more patients with HCV compared to those with HBV infection (21% vs 12%)[13]. In a separate case-control trial included in the same report, the prevalence of HCV infection was significantly higher among patients with diabetes than among controls (4.2% vs 1.6%).
Diabetes mellitus has been more often seen in cirrhotic patients[14]. However, in a cohort of 45 non-cirrhotic patients with chronic hepatitis C the prevalence of type 2 DM was 33%, higher than in the matched control group and in a group of patients with chronic hepatitis B[15]. Furthermore, in a large retrospective study DM was present in 23.6% of patients with hepatitis C, and in 9.4% of those with hepatitis B[16].
Recently, in a Spanish study which included 525 chronic hepatitis C patients treated with peginterferon plus ribavirin, patients were followed up after treatment. The incidence of altered baseline glucose and the appearance of type 2 DM was greater in non-responders than in sustained responders, even after multivariate analysis including such confounding variables as previous type 2 DM in relatives, age older than 40 years and male sex. Thus, hepatitis C virus clearance induced a decrease in insulin resistance index during short time follow-up and decreased the incidence of type 2 DM in long-term follow-up[17].
Shintani et al[18], in an experimental model, observed that the HCV core antigen transgenic mouse had higher basal insulin levels than non-transgenic mice, and readily developed diabetes when fed a high-fat diet, in addition to exhibiting marked insulin resistance as demonstrated by the insulin tolerance test.
In the present study, logistic regression analysis confirmed that family history of diabetes, metabolic syndrome and increased transaminases were the major independent variables associated with DM. This finding is consistent with reports in the literature[19-21].
The mechanisms by which hepatitis C induces increased insulin resistance and the risk for development of diabetes has not been completely understood. Liver fibrosis progression has long been considered responsible for the appearance of insulin resistance and type 2 DM in patients with chronic liver diseases[22]. However, in our study, diabetes occurs in the early stages of liver disease. The mechanism through which HCV is associated with insulin resistance involves direct viral effects, proinflammatory cytokines and suppressors of cytokine signaling[23-25].
In conclusion, this study shows a higher prevalence of DM in patients with HCV infection than in those with HBV infection, and that DM occurs at an early stage of hepatic disease. However, other factors such as metabolic syndrome, family history of diabetes and increased transaminases seem also to be important risk factors for the development of diabetes in Algeria.
A higher prevalence of diabetes mellitus (DM) has been observed in patients with hepatitis C virus (HCV) infection than in those with other forms of chronic hepatitis and several mechanisms have been implicated in the pathogenesis of DM. However, there is no information from Algeria regarding this issue, and few reports from Africa.
Recent data link HCV infection with diabetes. However, diabetes is a multifactorial disease; other factors such as age, weight, family history of diabetes and cirrhosis contribute to the development of diabetes.
This is a cross-sectional study assessing the prevalence of diabetes in patients with HCV infection in comparison with the prevalence in those with hepatitis B virus (HBV) infection. It is the first study of its kind performed in Algeria. In addition, risk factors associated with DM development were also evaluated.
Diabetes plays a role in the initiation and progression of liver injury. The high prevalence of diabetes in HCV-infected patients, and its occurrence at early stages of hepatic disease, suggest that screening for glucose abnormalities should be indicated in these patients.
The authors used 290 HCV-infected and 126 HBV-infected patients. It would be better if they had used equal numbers for both the groups or slightly fewer.
Peer reviewer: Dr. Shivananda Nayak, PhD, Department of Preclinical Sciences, Biochemistry Unit, Faculty of Medical Sciences, The University of The West Indies, Building 36, EWMSC, Mount Hope, Trinidad and Tobago
S- Editor Wang YR L- Editor Logan S E- Editor Lin YP
| 1. | Alter MJ. Epidemiology of hepatitis C virus infection. World J Gastroenterol. 2007;13:2436-2441. |
| 2. | Zignego AL, Ferri C, Pileri SA, Caini P, Bianchi FB. Extrahepatic manifestations of Hepatitis C Virus infection: a general overview and guidelines for a clinical approach. Dig Liver Dis. 2007;39:2-17. |
| 3. | Bernsmeier C, Heim MH. Insulin resistance in chronic hepatitis C: mechanisms and clinical relevance. Swiss Med Wkly. 2009;139:678-684. |
| 4. | Lonardo A, Adinolfi LE, Petta S, Craxì A, Loria P. Hepatitis C and diabetes: the inevitable coincidence? Expert Rev Anti Infect Ther. 2009;7:293-308. |
| 5. | Negro F, Alaei M. Hepatitis C virus and type 2 diabetes. World J Gastroenterol. 2009;15:1537-1547. |
| 6. | Pazhanivel M, Jayanthi V. Diabetes mellitus and cirrhosis liver. Minerva Gastroenterol Dietol. 2010;56:7-11. |
| 7. | Khelifa F, Thibault V. [Characteristics of hepatitis B viral strains in chronic carrier patients from North-East Algeria]. Pathol Biol (Paris). 2009;57:107-113. |
| 8. | Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539-553. |
| 9. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. |
| 10. | Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143-3421. |
| 11. | Allison ME, Wreghitt T, Palmer CR, Alexander GJ. Evidence for a link between hepatitis C virus infection and diabetes mellitus in a cirrhotic population. J Hepatol. 1994;21:1135-1139. |
| 12. | Mehta SH, Brancati FL, Sulkowski MS, Strathdee SA, Szklo M, Thomas DL. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Intern Med. 2000;133:592-599. |
| 13. | Mason AL, Lau JY, Hoang N, Qian K, Alexander GJ, Xu L, Guo L, Jacob S, Regenstein FG, Zimmerman R. Association of diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;29:328-333. |
| 14. | Thuluvath PJ, John PR. Association between hepatitis C, diabetes mellitus, and race. a case-control study. Am J Gastroenterol. 2003;98:438-441. |
| 15. | Knobler H, Schihmanter R, Zifroni A, Fenakel G, Schattner A. Increased risk of type 2 diabetes in noncirrhotic patients with chronic hepatitis C virus infection. Mayo Clin Proc. 2000;75:355-359. |
| 16. | Caronia S, Taylor K, Pagliaro L, Carr C, Palazzo U, Petrik J, O'Rahilly S, Shore S, Tom BD, Alexander GJ. Further evidence for an association between non-insulin-dependent diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;30:1059-1063. |
| 17. | Romero Gómez M, Fernandez-Rodriguez C, Alonso S, Pons JA, López-Serrano P, Gutiérrez ML, Pérez C, Temiño R, García P, Grande L. Sustained response in chronic hepatitis C reduces the risk to develop impaired fasting glucose and/or diabetes type 2. J Hepatol. 2006;44:548, page S204. |
| 18. | Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Tsukamoto K, Kimura S, Moriya K, Koike K. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004;126:840-848. |
| 19. | Zein CO, Levy C, Basu A, Zein NN. Chronic hepatitis C and type II diabetes mellitus: a prospective cross-sectional study. Am J Gastroenterol. 2005;100:48-55. |
| 20. | Narita R, Abe S, Kihara Y, Akiyama T, Tabaru A, Otsuki M. Insulin resistance and insulin secretion in chronic hepatitis C virus infection. J Hepatol. 2004;41:132-138. |
| 21. | Taura N, Ichikawa T, Hamasaki K, Nakao K, Nishimura D, Goto T, Fukuta M, Kawashimo H, Fujimoto M, Kusumoto K. Association between liver fibrosis and insulin sensitivity in chronic hepatitis C patients. Am J Gastroenterol. 2006;101:2752-2759. |
| 22. | Romero-Gómez M. Insulin resistance and hepatitis C. World J Gastroenterol. 2006;12:7075-7080. |
| 23. | Aytug S, Reich D, Sapiro LE, Bernstein D, Begum N. Impaired IRS-1/PI3-kinase signaling in patients with HCV: a mechanism for increased prevalence of type 2 diabetes. Hepatology. 2003;38:1384-1392. |
| 24. | Kawaguchi T, Ide T, Taniguchi E, Hirano E, Itou M, Sumie S, Nagao Y, Yanagimoto C, Hanada S, Koga H. Clearance of HCV improves insulin resistance, beta-cell function, and hepatic expression of insulin receptor substrate 1 and 2. Am J Gastroenterol. 2007;102:570-576. |
| 25. | Douglas MW, George J. Molecular mechanisms of insulin resistance in chronic hepatitis C. World J Gastroenterol. 2009;15:4356-4364. |