Published online Jul 21, 2010. doi: 10.3748/wjg.v16.i27.3406
Revised: March 30, 2010
Accepted: April 6, 2010
Published online: July 21, 2010
AIM: To evaluate fecal calprotectin concentrations (FCCs) in subjects with chronic gastritis and the correlation between FCCs and gastritis activity score.
METHODS: FCCs were measured in 61 patients with histological diagnosis of gastritis and in 74 healthy volunteers. Histological grading of gastritis was performed according to the updated Sydney gastritis classification. Patients were subdivided into 2 groups according to the presence/absence of an active gastritis. Patients with chronic active gastritis were divided into 3 subgroups on the basis of the activity score (mild, moderate, marked). FFCs in relation to Helicobacter pylori (H. pylori) infection and proton pump inhibitor (PPI) use were also evaluated.
RESULTS: FCCs in patients with chronic active gastritis were not significantly different to FCCs either in subjects with non active gastritis or in healthy controls. Among patients with chronic active gastritis (even marked), FCCs did not significantly differ according to activity score. No significant differences in FCCs were found when considering H. pylori, as well as when considering PPI chronic use.
CONCLUSION: FCCs were not significantly increased in subjects with chronic gastritis, even in those patients with a marked neutrophil infiltration.
- Citation: Montalto M, Gallo A, Ianiro G, Santoro L, D’Onofrio F, Ricci R, Cammarota G, Covino M, Vastola M, Gasbarrini A, Gasbarrini G. Can chronic gastritis cause an increase in fecal calprotectin concentrations? World J Gastroenterol 2010; 16(27): 3406-3410
- URL: https://www.wjgnet.com/1007-9327/full/v16/i27/3406.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i27.3406
Calprotectin is a calcium and zinc binding protein, mainly contained in neutrophils where it accounts for more than 60% of cytosolic proteins. It has well-known antimicrobial activity, both bacterial and fungicidal[1]. Elevated concentrations of calprotectin can be measured in plasma, synovial fluid, urine, liquor, saliva and feces when an inflammation process with recruitment of neutrophils is ongoing[2,3]. In particular, the presence of calprotectin in feces quantitatively relates to neutrophil migration towards the gastrointestinal tract[4]. Its levels are closely correlated with the fecal excretion of 111In-labelled leukocytes[5]. Therefore, it is considered a useful marker of intestinal inflammation[6]. Several recent studies reported a significant increase in fecal calprotectin concentrations (FCCs) in intestinal conditions characterized by a conspicuous neutrophil infiltrate, such as inflammatory bowel diseases (IBDs) and non-steroidal antiinflammatory drug (NSAID) enteropathy[7-9]. It may accurately distinguish IBD from non-IBD conditions (such as irritable bowel syndrome)[10,11]. It has also been proposed as a reliable marker able to predict clinical relapse in IBD patients[12,13]. Diagnostic accuracy of FCCs in colorectal neoplasia has not been univocally established yet[14].
Chronic gastritis represents a common and heterogeneous inflammatory process. It can be morphologically characterized by a variable inflammatory infiltrate in the lamina propria, within the epithelium and within the foveolar lumen[15]. According to the updated Sydney System, the presence of a neutrophil infiltrate characterizes the “activity” of gastritis[15].
The aim of our study was to evaluate FCCs in subjects with chronic gastritis and the possible correlation between FCCs and the activity score, according to the updated Sydney System gastritis classification.
Between May 2008 and December 2008, subjects who were referred to the Endoscopy Center of “Gemelli Hospital” for upper gastrointestinal endoscopy, were invited to enter the study. In those subjects who agreed to participate in the study, the extraction of at least 5 biopsy samples (2 from the antrum, 2 from the corpus and one from the incisura angularis) had been undertaken to correctly characterize an eventual gastritis process, in accordance with Sydney’s recommendations[15]. However, when esophageal lesions, gastric ulcers, gastric polyps or duodenal lesions were found during the endoscopy, the necessary biopsy specimens were taken, and these subjects were not included in the study. In addition, subjects with IBDs or family history of IBDs, colorectal cancer, chronic use of NSAIDs, history of gastric resection, coexisting and severe cardiopulmonary, hepatic, renal, neurologic, psychiatric, endocrine and rheumatologic diseases, malignancy, pregnancy, alcohol abuse, other intestinal disorders characterized by increased mucosal permeability and inflammatory changes, were not considered for the study.
All the eligible subjects were asked to provide a stool sample for measurement of calprotectin levels, within 2 d of endoscopic examination, before starting specific therapy. Stools were also examined to exclude infectious intestinal diseases. All subjects were asked if they were taking proton pump inhibitor (PPI) therapy for at least since 1 mo before the endoscopy.
According to the updated Sydney System, depending on the presence/absence of a neutrophil infiltrate, patients with chronic gastritis were divided into 2 groups: group A which consisted of patients with active gastritis and group B which consisted of patients with non active gastritis. Furthermore, adult healthy volunteers participated as a further control group, providing their own stool sample.
Procedures were in accordance with the ethical standards of the Helsinki Declaration of 1975, as revised in 1983. Each subject gave written informed consent for the study. The study was approved by the Institutional Board of Department of Internal Medicine, Catholic University of Rome.
The biopsy samples were fixed in 4% buffered formalin, processed in the usual manner, and paraffin embedded. The sections were stained with hematoxylin and eosin for histological evaluation; Giemsa stain was also used to evaluate the presence of Helicobacter pylori (H. pylori). The sections were evaluated by 2 separate expert gastrointestinal pathologists working blind. The degree of activity of inflammation was assessed using a semiquantitative 3-tiered scale (mild, moderate, marked) according to the updated Sydney System[15]. The infiltration of neutrophil granulocytes was defined as “mild” when isolated cells of this type were identified in the lamina propria only with difficulty, after a thorough search; it was defined as “moderate” if neutrophils were either easily detectable in the lamina propria or were found within the epithelium, provided they were not crowded; finally, the infiltration was defined as “marked” when a dense neutrophil infiltrate, usually involving both lamina propria and epithelium, was strikingly evident at low power magnification. When activity differed among antrum, corpus and incisura angularis, the activity grade in the most severely affected compartment was considered; when activity grade changed among different biopsies of the same gastric compartment, the predominant grade was considered, according to the updated Sydney classification[16]. H. pylori status was evaluated as present/absent in all the examined biopsy samples. H. pylori density score was graded as mild, moderate and marked, according to the updated Sydney classification[16].
Each subject was instructed to collect and return a single stool sample within 48 h of defecation. Upon receipt, the stools were frozen and stored at -20°C for subsequent biomarker determination.
The stool samples were prepared and analyzed according to the manufacturer’s instructions (Calprest; Eurospital SpA, Trieste, Italy). A portion of each sample (40-120 mg) was measured and an extraction buffer containing citrate and urea was added in a weight per volume ratio of 1:50. The samples were mixed for 30 s by a vortex method and homogenized for 25 min. One milliliter of the homogenate was transferred to a tube and centrifuged for 20 min. Finally, the supernatant was collected and frozen at -20°C. In most cases, time from sampling to preparation and freezing was estimated to be 1-3 d, except for a few samples that took 4-6 d before handling. The supernatants were thawed and analyzed later with Calprest, a quantitative calprotectin ELISA, for determination of calprotectin in stools. The within-assay coefficient of variation was 1.5%. Calprotectin was expressed as μg/g of feces.
Statistical comparison of age and sex among patients with chronic active gastritis, non active gastritis and healthy controls was performed by the t-test for unpaired data and χ2 test. FCCs among subjects with active gastritis, non active gastritis and healthy controls groups were compared by the t-test for unpaired data.
In subjects with chronic active gastritis, FCC differences among the subgroups identified by density of neutrophil infiltration (activity score) were analyzed by means of one-way analysis of variance (ANOVA). The post hoc effect was assessed by Bonferroni t-test. Comparison between FCCs and H. pylori status, FCCs and PPI use, was performed by means of the t-test for unpaired data. FCC differences among the subgroups identified by density of H. pylori infection were analyzed by ANOVA. The post hoc effect was assessed by the Bonferroni t-test. The statistical analysis of categorical parameters was performed by the χ2 test. All values were assessed as mean ± SD. A P-value of 0.05 or less was regarded as significant.
During the study period, 929 subjects had an upper intestinal endoscopy; 696 were ruled out on the basis of the above-mentioned exclusion criteria. Of the 247 eligible patients, 61 Caucasians (28 male, 33 female, mean age 49.64 ± 13.80 years) gave their consent to participate in the study. Seventy four adult healthy volunteers (32 male, 42 female, mean age 45.93 ± 12.42 years) entered the study as controls. The demographic data of the different study groups are summarized in Table 1. There were no significant differences between the groups regarding age and sex.
| Groups | n | Sex (M/F) | Age (yr) | FCCs (μg/g) |
| Patients | 61 | 28/33 | 49.64 ± 13.80 | 28.25 ± 23.43 |
| Active gastritis | 35 | 15/20 | 49.66 ± 14.15 | 29.70 ± 21.26 |
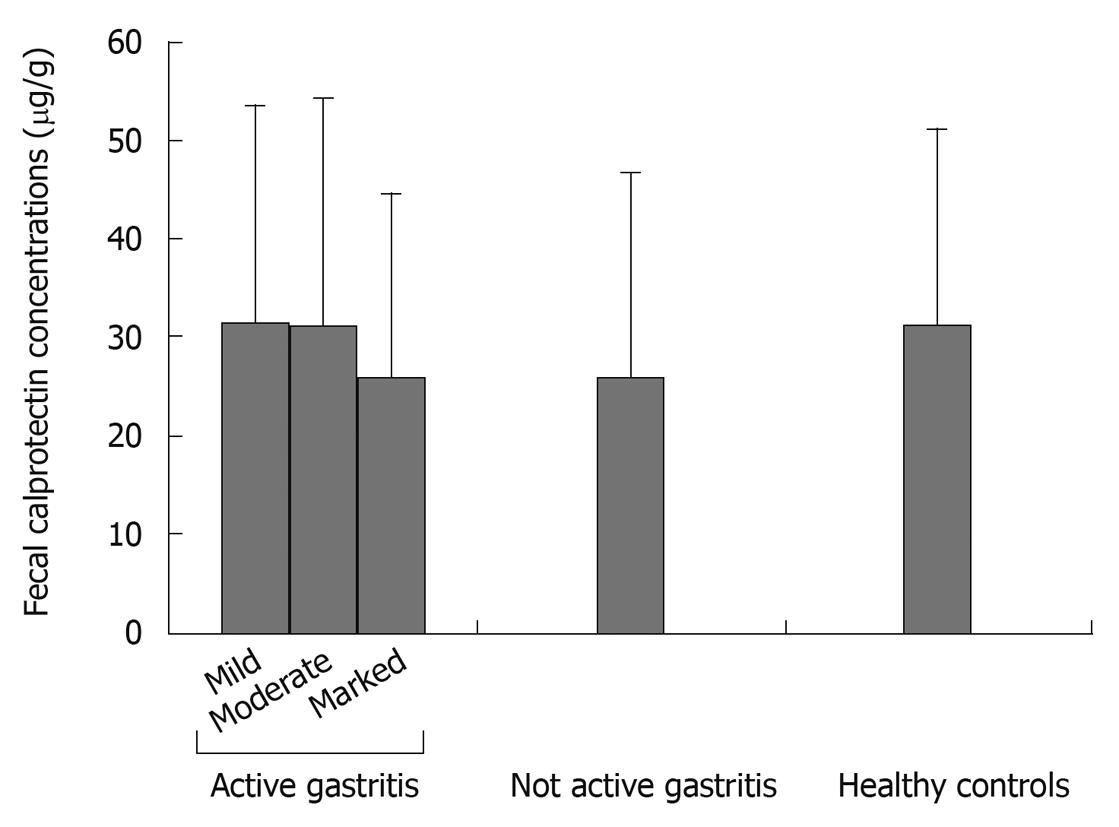
| Mild | 15 | 6/9 | 48.07 ± 14.56 | 31.44 ± 22.55 |
| Moderate | 10 | 7/3 | 47.60 ± 13.72 | 31.08 ± 23.68 |
| Marked | 10 | 2/8 | 53.30 ± 14.64 | 26.57 ± 17.66 |
| Non active gastritis | 26 | 13/13 | 49.90 ± 13.61 | 25.97 ± 22.55 |
| Healthy controls | 74 | 32/42 | 45.93 ± 12.42 | 31.20 ± 19.18 |
According to the updated Sydney System classification, 35 patients showed chronic active gastritis (group A); in particular, 15 showed mild activity, 10 moderate activity and 10 marked activity. When separately analyzed by antrum gastritis activity (AGA) and corpus gastritis activity (CGA), 21 patients showed predominant antral activity (4 mild AGA without CGA; 7 moderate AGA, 6 with mild CGA and 1 without CGA; 10 marked AGA, 7 with mild CGA and 3 with moderate CGA), 2 showed predominant corpus activity (both with mild CGA without AGA), and the other 12 patients showed a concordant activity between antrum and corpus (9 mild and 3 moderate in both AGA and CGA).
Of the 26 patients with non active chronic gastritis (group B), 15 showed a predominant chronic mononuclear infiltrate, 4 intestinal metaplasia and 7 glandular atrophy.
Mean FCCs were not significantly different between group A and group B, and they both did not differ significantly from FCCs in healthy volunteers (P = NS for all comparisons).
When considering only patients with chronic active gastritis, mean FCCs were not significantly different among the 3 subgroups identified by the different degree of neutrophil infiltrate (Table 1 and Figure 1). Also, when separately considering antrum and corpus gastritis, mean FCCs did not correlate with the degree of activity in either subgroup.
When considering the presence of H. pylori infection in the whole study group, 24 patients were H. pylori positive (7 with mild infection, 8 moderate and 9 marked), while 37 patients were H. pylori negative; mean FCCs neither significantly differed between the 2 subgroups (27.35 ± 22.64 vs 28.84 ± 24.21, P = NS), nor correlated with degree of H. pylori infection (P = NS for all comparisons). On the other hand, both the presence and density of H. pylori significantly correlated with neutrophilic infiltration. In particular, in subjects with chronic active gastritis, 5/15 (33%) with a mild active gastritis, 8/10 (80%) with a moderate active gastritis, and 10/10 (100%) with a severe active gastritis, were H. pylori positive, whereas in the group with non active gastritis, only 1/26 (3.8%) was H. pylori positive (P < 0.001). In addition, when considering H. pylori density, of the 7 patients with a mild H. pylori density score, 4 showed mild active gastritis and 3 moderate active gastritis; of the 8 patients with moderate H. pylori density, 3 showed moderate active gastritis and 4 showed marked active gastritis, while one had non active gastritis; of the 9 patients with marked H. pylori density, one showed mild active gastritis, 2 moderate active gastritis and 6 marked active gastritis (P < 0.05).
Finally, when considering PPI use, 22 patients were on PPI therapy and 39 patients were not; mean FCCs were not significantly different between the 2 groups (32.88 ± 25.90 vs 25.64 ± 25.83, P = NS).
Our study showed no significant differences in FCCs between patients with chronic active gastritis and non active chronic gastritis controls, regardless of the degree of neutrophil infiltration. In addition, FCCs in both groups did not significantly differ with regard to that in healthy controls.
Fecal calprotectin has recently emerged as a reliable marker of intestinal inflammation[14]. Different studies regarding fecal calprotectin have been carried out in bowel diseases, mainly IBDs[8-11]. Up to now, no specific studies have been designed to evaluate FCCs in upper gastrointestinal tract diseases. The few available data on this topic can only be gathered from studies evaluating FCCs in different conditions throughout the gastrointestinal tract. In this regard, only Summerton et al[17], in 2002, performed a study evaluating FCCs in different gastrointestinal inflammatory and cancer conditions. In particular, 26 patients showed upper gastrointestinal inflammation because of gastritis and duodenitis. FCCs were in the normal range in all these subjects. Nevertheless, a correlation between FCCs and histological severity of inflammation was not performed in this study.
Chronic gastritis is a very common clinical condition. The updated Sydney System provided the term “activity” as an expression of the presence of neutrophils on a background of chronic inflammation[15].
As expected, we found that patients with non active chronic gastritis did not show increased FCCs, since a neutrophil infiltrate is lacking in these conditions. Nevertheless, we also found that FCCs were not significantly increased in active chronic gastritis, even in subjects with a marked activity score (and so a high grade of neutrophil infiltration). This result could be explained by the consideration that the inflammatory process, and in particular the neutrophil recruitment occurring in gastritis, is far less severe than in other intestinal conditions, mainly IBDs. Furthermore, our findings can be also explained by the smaller extent of inflamed tissue found in gastritis with respect to that in IBDs. In this regard, Sipponenn et al[18] reported that subjects with ileal Crohn’s disease showed lower fecal markers (calprotectin and lactoferrin) compared to subjects with colonic involvement. They supposed that this finding might be explained by the limited extent of ileal disease, even in the presence of endoscopic and histological inflammation. In addition, in 39 children with IBDs, it has been shown that FCCs were closely related not only to disease severity, but also to disease extent[19].
In our study, we did not consider all those subjects with endoscopic findings involving the esophagus, duodenum or with gastric polyps and ulcers, because our aim was to evaluate FCCs only in chronic gastritis, relating these levels to the neutrophil infiltrate classified according to a validated histological score. Further studies, specifically aimed at this purpose, should clarify if FCCs might be increased in other upper gastrointestinal diseases different from chronic gastritis.
It has been reported that a neutrophil infiltrate is almost always present in H. pylori gastritis and usually disappears within a few days of antibiotic therapy[20]. In agreement with data in the literature, we found that the gastritis activity score was closely correlated with the degree of H. pylori infection. In particular, only one patient with an absent neutrophil infiltrate was H. pylori positive, while all 10 patients with marked active gastritis showed H. pylori infection. However, no significant differences were found when FCCs was compared between H. pylori-positive and H. pylori-negative subjects, regardless of H. pylori density score.
Concerning the relationship between PPI therapy and FCCs, Poullis et al[21], in a letter, reported that patients using PPIs had significantly higher FFCs compared to those not on PPIs. Nevertheless, data on their population were lacking; they did not undergo endoscopy, and other causes of increased FCCs, such as IBDs and NSAID use were not excluded. On the other hand, it has been reported that PPIs may also inhibit proton pumps present on membranes of phagolysosomes of neutrophils, interfering with neutrophil release of reactive oxygen species, commonly mediated by lysosomal acidification[22,23]. We found that FCCs were not significantly different between subjects taking PPIs and subjects who did not, thus suggesting that gastric pH is unlikely to be responsible for the low levels of FCC we found. On the other hand, it is not possible to exclude that gastric acidity could interfere with FCCs. Until now, no data have been available on this topic and further studies should be encouraged.
In conclusion, we showed that in subjects with chronic active gastritis, even marked, FCCs were not significantly increased when compared with FCCs either in subjects with non active gastritis or in healthy controls. Thus we recommend that in subjects with high FCCs, causes of gut inflammation other than chronic gastritis should be checked.
Fecal calprotectin is a valid marker of intestinal inflammation, being quantitatively related to neutrophil migration towards the gastrointestinal tract. Fecal calprotectin concentrations (FCCs) are significantly increased in intestinal diseases characterized by a conspicuous neutrophil infiltration, mainly inflammatory bowel diseases. Chronic gastritis morphologically shows a variable neutrophil infiltrate, which characterizes the gastritis activity, according to the updated Sydney System classification.
No systematic study has ever been performed to evaluate FCCs in subjects with chronic gastritis and the possible correlation between FCCs and gastritis activity score.
The authors found no significant difference between FCCs in patients with chronic active gastritis and FCCs either in subjects with non active gastritis or in healthy controls. Among patients with chronic active gastritis (even marked), FCCs did not correlate with the activity score.
The authors recommend that in subject with high FCCs, causes of gut inflammation other than chronic gastritis should be checked.
Calprotectin: A calcium and zinc binding protein, mainly contained in neutrophils where it accounts for more than 60% of cytosolic proteins. It has well-known antimicrobial activity, both bacterial and fungicidal. Elevated concentrations of calprotectin can be measured in plasma, synovial fluid, urine, liquor, saliva and feces when an inflammation process with recruitment of neutrophils is ongoing. The presence of calprotectin in feces quantitatively relates to neutrophil migration towards the gastrointestinal tract. Active gastritis: according to the updated Sydney System, the presence of a neutrophil infiltrate characterizes the “activity” of gastritis.
The study is set up correctly. The material studied is big enough to allow conclusions to be drawn. The paper is written sufficiently well, the Introduction gives a good overview of the study background and the authors clearly raised the hypothesis of the study. The description of the method and material studied is accurate. The aim of the study is fulfilled. The Results are presented clearly and have been discussed sufficiently well.
Peer reviewer: Tamara Vorobjova, Senior Researcher in Immunology, Department of Immunology, Institute of General and Molecular Pathology, University of Tartu, Ravila, 19, Tartu, 51014, Estonia
S- Editor Tian L L- Editor Cant MR E- Editor Ma WH
| 1. | Costa F, Mumolo MG, Bellini M, Romano MR, Ceccarelli L, Arpe P, Sterpi C, Marchi S, Maltinti G. Role of faecal calprotectin as non-invasive marker of intestinal inflammation. Dig Liver Dis. 2003;35:642-647. |
| 2. | Johne B, Fagerhol MK, Lyberg T, Prydz H, Brandtzaeg P, Naess-Andresen CF, Dale I. Functional and clinical aspects of the myelomonocyte protein calprotectin. Mol Pathol. 1997;50:113-123. |
| 3. | Bjerke K, Halstensen TS, Jahnsen F, Pulford K, Brandtzaeg P. Distribution of macrophages and granulocytes expressing L1 protein (calprotectin) in human Peyer’s patches compared with normal ileal lamina propria and mesenteric lymph nodes. Gut. 1993;34:1357-1363. |
| 4. | Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut. 2006;55:426-431. |
| 5. | Tibble J, Teahon K, Thjodleifsson B, Roseth A, Sigthorsson G, Bridger S, Foster R, Sherwood R, Fagerhol M, Bjarnason I. A simple method for assessing intestinal inflammation in Crohn’s disease. Gut. 2000;47:506-513. |
| 6. | Røseth AG, Fagerhol MK, Aadland E, Schjønsby H. Assessment of the neutrophil dominating protein calprotectin in feces. A methodologic study. Scand J Gastroenterol. 1992;27:793-798. |
| 7. | Poullis A, Foster R, Mendall MA, Fagerhol MK. Emerging role of calprotectin in gastroenterology. J Gastroenterol Hepatol. 2003;18:756-762. |
| 8. | Montalto M, Curigliano V, Santoro L, Armuzzi A, Cammarota G, Covino M, Mentella MC, Ancarani F, Manna R, Gasbarrini A. Fecal calprotectin in first-degree relatives of patients with ulcerative colitis. Am J Gastroenterol. 2007;102:132-136. |
| 9. | Gisbert JP, McNicholl AG. Questions and answers on the role of faecal calprotectin as a biological marker in inflammatory bowel disease. Dig Liver Dis. 2009;41:56-66. |
| 10. | Shitrit AB, Braverman D, Stankiewics H, Shitrit D, Peled N, Paz K. Fecal calprotectin as a predictor of abnormal colonic histology. Dis Colon Rectum. 2007;50:2188-2193. |
| 11. | Schoepfer AM, Trummler M, Seeholzer P, Seibold-Schmid B, Seibold F. Discriminating IBD from IBS: comparison of the test performance of fecal markers, blood leukocytes, CRP, and IBD antibodies. Inflamm Bowel Dis. 2008;14:32-39. |
| 12. | Tibble JA, Sigthorsson G, Bridger S, Fagerhol MK, Bjarnason I. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology. 2000;119:15-22. |
| 13. | Gisbert JP, Bermejo F, Pérez-Calle JL, Taxonera C, Vera I, McNicholl AG, Algaba A, López P, López-Palacios N, Calvo M. Fecal calprotectin and lactoferrin for the prediction of inflammatory bowel disease relapse. Inflamm Bowel Dis. 2009;15:1190-1198. |
| 14. | von Roon AC, Karamountzos L, Purkayastha S, Reese GE, Darzi AW, Teare JP, Paraskeva P, Tekkis PP. Diagnostic precision of fecal calprotectin for inflammatory bowel disease and colorectal malignancy. Am J Gastroenterol. 2007;102:803-813. |
| 15. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. |
| 16. | Price AB. The Sydney System: histological division. J Gastroenterol Hepatol. 1991;6:209-222. |
| 17. | Summerton CB, Longlands MG, Wiener K, Shreeve DR. Faecal calprotectin: a marker of inflammation throughout the intestinal tract. Eur J Gastroenterol Hepatol. 2002;14:841-845. |
| 18. | Sipponen T, Kärkkäinen P, Savilahti E, Kolho KL, Nuutinen H, Turunen U, Färkkilä M. Correlation of faecal calprotectin and lactoferrin with an endoscopic score for Crohn’s disease and histological findings. Aliment Pharmacol Ther. 2008;28:1221-1229. |
| 19. | Fagerberg UL, Lööf L, Lindholm J, Hansson LO, Finkel Y. Fecal calprotectin: a quantitative marker of colonic inflammation in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2007;45:414-420. |
| 20. | Stolte M, Eidt S. Chronic erosions of the antral mucosa: a sequela of Helicobacter pylori-induced gastritis. Z Gastroenterol. 1992;30:846-850. |
| 21. | Poullis A, Foster R, Mendall MA, Shreeve D, Wiener K. Proton pump inhibitors are associated with elevation of faecal calprotectin and may affect specificity. Eur J Gastroenterol Hepatol. 2003;15:573-574; author reply 574. |
| 22. | Luciani F, Spada M, De Milito A, Molinari A, Rivoltini L, Montinaro A, Marra M, Lugini L, Logozzi M, Lozupone F. Effect of proton pump inhibitor pretreatment on resistance of solid tumors to cytotoxic drugs. J Natl Cancer Inst. 2004;96:1702-1713. |
| 23. | Kedika RR, Souza RF, Spechler SJ. Potential anti-inflammatory effects of proton pump inhibitors: a review and discussion of the clinical implications. Dig Dis Sci. 2009;54:2312-2317. |