Published online Jul 14, 2010. doi: 10.3748/wjg.v16.i26.3299
Revised: December 11, 2009
Accepted: December 18, 2009
Published online: July 14, 2010
AIM: To investigate the prevalence of proximal small bowel (SB) lesions detected by wireless capsule endoscopy (WCE) in Crohn’s disease (CD).
METHODS: WCE was performed in 64 patients: 32 with CD of the distal ileum, and 32 controls with iron-deficiency anemia (IDA) or diarrhea. WCE was performed using the Given SB-WCE, followed by small intestine contrast ultrasonography (SICUS). Findings compatible with CD by using WCE included erosions, aphthoid or deep ulcers, and strictures/stenosis.
RESULTS: WCE detected proximal SB lesions in 16/32 (50%) patients (14 aphthoid ulcers, 2 deep ulcers, one stricture), which appeared not to be related to clinical parameters [epigastric pain, age, smoking, non-steroidal anti-inflammatory drugs (NSAIDs), IDA]. Among patients with proximal SB lesions, 6 (37%) were smokers, 3 (19%) NSAID users, 3 (19%) had epigastric pain and 4 (25%) had IDA. SICUS detected proximal SB lesions in 3/32 patients (19%) also showing lesions with WCE. No correlations were observed between proximal SB lesions assessed by WCE or by SICUS (χ2 = 1.5, P = 0.2).
CONCLUSION: The use of WCE allows the detection of previously unknown upper SB lesions in a high proportion of patients with a previous diagnosis of CD involving the distal ileum.
- Citation: Petruzziello C, Onali S, Calabrese E, Zorzi F, Ascolani M, Condino G, Lolli E, Naccarato P, Pallone F, Biancone L. Wireless capsule endoscopy and proximal small bowel lesions in Crohn’s disease. World J Gastroenterol 2010; 16(26): 3299-3304
- URL: https://www.wjgnet.com/1007-9327/full/v16/i26/3299.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i26.3299
Wireless capsule endoscopy (WCE) is a non-invasive technique for visualizing the mucosal surface of the small bowel (SB)[1-7]. However, a variable impact risk (from 0%-6.7%) has been reported[8]. WCE showed a high sensitivity and specificity for detecting lesions related to SB Crohn’s disease (CD)[1-7]. A meta-analysis showed an incremental diagnostic yield of WCE vs small bowel follow through (SBFT) (P < 0.001), ileocolonoscopy (P = 0.02), computed tomography enteroclysis (P = 0.001) and push enteroscopy (P < 0.001)[9]. WCE has in particular been shown to be able to detect minor lesions (erosions, aphthoid ulcer), not visualized by conventional radiologic techniques, which result in high radiation exposure. WCE has therefore been proposed as an alternative non-invasive technique for assessing CD lesions.
Ultrasonography also is a non-invasive technique proposed for detecting SB lesions in CD[10]. The use of an oral contrast [small intestine contrast ultrasonography (SICUS)] significantly increases, in experienced hands, the sensitivity of ultrasonography for assessing SB lesions in CD (> 95%)[11,12].
Although several studies concordantly showed that WCE is able to visualize superficial lesions in the SB, its role in defining the extent of the lesions in CD is undefined. In particular, the frequency and clinical relevance of superficial lesions in the upper SB as detected by WCE, but not by conventional techniques, in patients with an established diagnosis of CD involving the distal ileum, is currently unknown. Disease-specificity of the small lesions as detected by WCE is also under investigation.
On the basis of these observations we therefore aimed to assess, in a prospective longitudinal study in patients with a known diagnosis of CD of the distal ileum, the prevalence of lesions in the proximal SB (jejunum, proximal ileum) compatible with CD, as assessed by WCE. The secondary end point was to evaluate the possible concordance between WCE and SICUS, in detecting SB lesions compatible with CD. Additional end points included investigation of possible correlations between proximal SB lesions in WCE and specific signs and symptoms, including anemia and/or epigastric pain in patients with CD. A comparison between characteristics of SB lesions detected by WCE in patients with CD vs patients undergoing WCE for other indications was also performed. The safety of WCE in CD patients with no radiological or clinical evidence of sub/obstructive symptoms was further addressed.
In a prospective longitudinal study, WCE was performed in all consecutive CD patients referred to our Unit from May 2004 to May 2008, fulfilling the following inclusion criteria: (1) age 18-75 years; (2) regular follow-up; and (3) established diagnosis of CD involving the distal ileum, according to standard procedures.
As a control group (C), WCE was performed in all consecutive patients referred to our Unit from May 2004 to May 2008, with the following inclusion criteria: (1) age 18-75 years; and (2) clinical indication for WCE such as iron-deficiency anemia (IDA) or chronic diarrhea of unknown origin with no diagnosis by conventional procedures. No patients showed evidence of stenoses/strictures. Written informed content was provided by all patients.
CD group: Before WCE, recorded parameters included: findings at physical examination, activity (CD activity index, CDAI)[13], epigastric pain (yes/no), IDA, laboratory tests (complete blood count, hemoglobin, serum iron, ferritin, creatine phosphate, erythrocyte sedimentation rate), non-steroidal antiinflammatory drug (NSAID) use, and smoking habit. Before WCE, all 32 patients were studied by both SICUS and ileocolonoscopy (median time interval 1 mo, range 0-7 mo, and 1 mo, range 0-14 mo, respectively). After WCE, patients were clinically followed up at least 12 mo (median 24 mo, range 12-36 mo).
C group: Before WCE, recorded parameters included: findings at physical examination, gastrointestinal symptoms, laboratory tests (as above), NSAID use, and smoking habit.
SICUS was performed after 375 mL polyethylene glycol (PEG) ingestion[11] using 3.5 and 5 MHz convex and linear-array transducers, by the same expert gastroenterologist (> 2000 examinations). Findings compatible with CD included: increased bowel wall thickness (BWT) (≥ 3 mm), SB dilation (diameter > 2.5 cm), bowel stricture (diameter < 1 cm, at the level of the maximally distended loop)[11,12]. Fistulas or abscesses were considered.
All endoscopies were performed by the same gastroenterologist, according to standard procedures.
WCE was performed with the Given Pillcam SB capsule system (Given Imaging Limited, Yoqneam, Israel)[1] after 3 d of a fiber-free diet and bowel preparation [2 L PEG, (Promefarm, Milano, Italy)]. Images were reviewed by a single gastroenterologist unaware of the SICUS findings.
CD group: The following WCE findings were considered compatible with CD: aphthoid ulcers (> 3), deep ulcers, strictures or stenoses. Erosions, villous dropouts and mucosal breaks were reported, although considered not related to CD. As no standard criteria for defining upper SB lesions using WCE were available, distal SB lesions were considered lesions proximal to the ileo-cecal valve or to the ileo-colonic anastomosis. Upper SB lesions were considered the SB lesions proximal to these areas (jejunum, proximal ileum).
In a subgroup of 10 CD patients with ileo-colonic resection, WCE findings were blindly scored by 2 independent gastroenterologists. For this purpose, lesions in the peri-anastomotic area and in the upper SB were graded as follows[5]: absent (G0), erythema/loss of villi (G1), erosions/aphthoid ulcers (G2), deep ulcers (G3).
C group: Any lesion detected by WCE was reported. A comparison with the CD group considered only those lesions in the upper or distal SB compatible with CD, including: aphthoid or deep ulcers, strictures, stenoses. Planar X-ray of the abdomen was performed in all patients with no WCE excretion after 48-72 h. Retention was defined as WCE persistence after 14 d. Incomplete studies were defined when WCE did not reach the cecum.
Comparison between WCE and SICUS in terms of findings compatible with SB lesions related to CD was made using the following parameters: presence (yes/no), site (upper vs distal SB) and severity of the lesions (deep vs aphthoid ulcers) by using WCE and presence (yes/no) and site (upper vs distal SB) of increased BWT (≥ 3 mm) using SICUS. Correlations between WCE findings compatible with upper SB lesions and clinical parameters (age, smoking habits, epigastric pain, IDA) were determined.
Results were expressed as median and range both in the text and in the tables. Differences between groups were assessed by the Student’s t-test. The interobserver variation in terms of presence and severity of upper SB lesions detected by WCE was assessed.
CD group: Thirty two consecutive patients (16 male, median age 32 years, range 19-65 years) with an established diagnosis of CD of the distal ileum fulfilled the inclusion criteria. Clinical characteristics of the enrolled patients are summarized in Table 1.
| Parameter | n (%) |
| Gender | |
| Male | 16 (50) |
| Female | 16 (50) |
| Clinical activity | |
| Active (CDAI > 150) | 5 (16) |
| Inactive (CDAI < 150) | 27 (84) |
| Lesions extent before WCE | |
| Distal ileum only | 27 (84) |
| Distal ileum and colon | 2 (6) |
| Distal ileum and esophagus/stomach | 3 (10) |
| Previous intestinal resections | |
| Yes | 25 (78) |
| Smoking habits | |
| Smokers | 14 (44) |
| Ex-smokers | 4 (12) |
| Non-smokers | 14 (44) |
| NSAID use | |
| Yes | 3 (9) |
Treatment included budesonide (9 mg/d) in 6 (19%) and mesalazine 2.4 g/d in 26 (81%) patients. Among the 32 patients, 4 (12%) had IDA and 7 (22%) epigastric pain.
C group: Thirty two consecutive C patients (16 male, median age 42 years, range 18-72 years) undergoing WCE fulfilled the inclusion criteria. Clinical indications for WCE included IDA in 17 (53%) and chronic diarrhea in 15 (47%) patients.
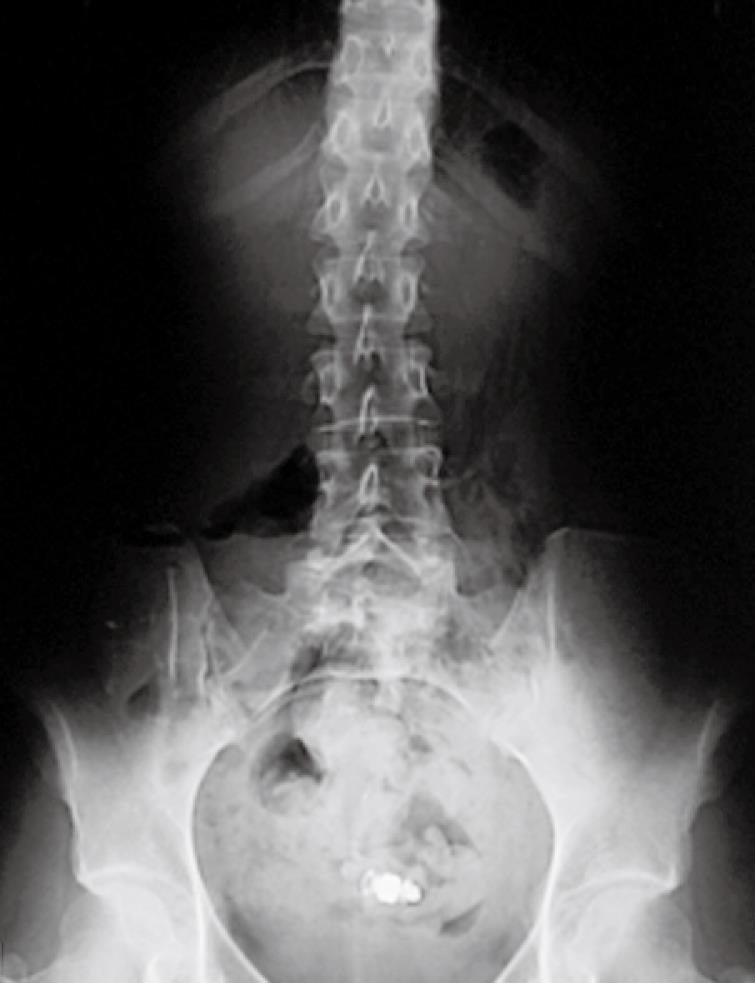
CD group: Retention was observed in one patient (3%) with 2 anastomoses (ileo-ileal and ileo-colonic), showing no symptoms despite capsule retention for > 12 wk (Figure 1). Surgical removal of WCE was required after 2 unsuccessful therapeutic endoscopies. During surgery, WCE was detected within the “cul de sac” of the side-to-side ileo-ileal anastomosis not reachable by the endoscope.
C group: No adverse events were reported.
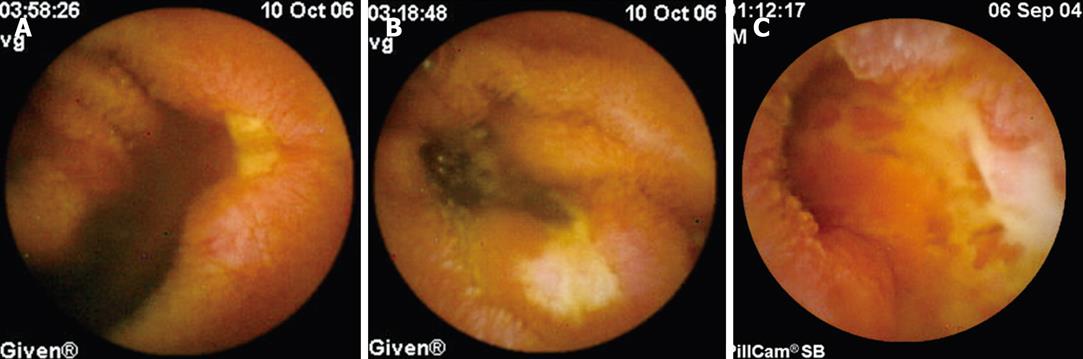
CD group: WCE detected previously unknown proximal SB lesions compatible with CD in 16/32 (50%) patients. Among these 16 patients, lesions included > 3 aphthoid ulcers in 14 (87.5%), deep ulcers in 2 (12.5%) and one ulcerated stricture identified by WCE in one patient (Figure 2A-C). All lesions compatible with CD appeared discontinuous and surrounded by macroscopically uninvolved mucosa. In all 16 patients showing lesions in the upper SB, WCE also detected lesions in the distal ileum compatible with CD.
The median age was comparable in patients showing or not (n = 16 for both) proximal SB lesions at WCE (44 years, range 20-65 years vs 32 years, range 19-48 years, P = NS).
Table 2 summarizes the clinical characteristics of the 32 patients grouped according to the presence or not of upper SB lesions in WCE. No statistically significant concordance was observed between upper SB lesions in WCE for both clinical parameters and risk factors considered (CD site χ2 = 3.3, P = 0.18, epigastric pain χ2 = 0.0, P = 1.0, smoking habits χ2 = 1.3, P = 0.5, NSAIDs use χ2 = 1.5, P = 0.2, IDA χ2 = 0.0, P = 1.0).
| Small bowel lesions at WCE (%) | ||
| Yes (n = 16) | No (n = 16) | |
| Known CD extent before WCE | ||
| Distal ileum | 12 (75) | 15 (94) |
| Distal ileum and colon | 1 (6) | 1 (6) |
| Distal ileum and esophagus stomach | 3 (19) | 0 |
| Epigastric pain | ||
| Yes | 3 (19) | 4 (25) |
| No | 13 (81) | 12 (75) |
| Smoking habits | ||
| Smokers | 6 (37) | 8 (50) |
| Ex-smokers | 3 (19) | 1 (6) |
| Non-smokers | 7 (44) | 7 (44) |
| NSAID use | ||
| Yes | 3 (19) | 0 |
| No | 13 (81) | 16 (100) |
| IDA | ||
| Yes | 4 (25) | 5 (31) |
| No | 12 (75) | 11 (69) |
The interobserver agreement for SB lesions visualized by WCE (score 0-4)[5] was very high when considering proximal SB lesions (κ = 0.86) and high when considering distal SB lesions (κ = 0.61). At 12 mo, none of the 32 patients showing lesions in the upper SB developed related symptoms (anemia, epigastric pain) or symptomatic SB stenosis.
C group: WCE detected proximal SB lesions not compatible with CD in 5/32 (16%) patients, represented by erosions in 3 (9%) and angiodysplasia in 2 (6%). In contrast to CD patients, none of the 32 C patients showed aphthoid or deep ulcers in the SB with WCE.
SICUS detected proximal SB lesions in 3/32 (9%) CD patients, represented by increased jejunal BWT with bowel dilation, associated with stenosis in one patient.
When considering the 16 CD patients showing upper SB lesions with WCE, only 3 (19%) also showed SICUS findings compatible with CD lesions in the same area (BWT > 3 mm). Therefore, all 3 (9%) patients showing SICUS results compatible with CD lesions in the proximal SB had the findings confirmed by WCE. Upper SB lesions in WCE in these patients included deep ulcers in 2 and aphthoid ulcers in one patient. The only stricture identified by WCE was also visualized by SICUS. However, these 2 techniques showed no significant concordance in detecting proximal SB lesions (χ2 = 1.5, P = 0.2).
CD group: Findings compatible with CD lesions in the distal SB were detected by WCE in 30/32 (93%) patients. In the remaining 2 patients, WCE did not visualize the colon, thus not allowing the evaluation of the distal SB. Of the 30 patients with available distal SB images with WCE, lesions included erosions in 2 (7%), aphthoid ulcers in 13 (43%), deep ulcers in 11 (37%), both aphthoid and deep ulcers in 3 (10%) and one single ulcerated substenosis in one (3%) patient. In all patients showing lesions in the upper SB, WCE also showed lesions in the distal SB.
One patient (PL) showed WCE impaction at the level of the anastomosis as detected by a plain film of the abdomen showing capsule retention for 12 wk, with no associated symptoms (Figure 1). The patient had 2 anastomoses (ileo-ileal and ileo-colonic) for CD-related surgery. Surgical removal of the WCE was required, showing the capsule retained within the “cul de sac” of the side-to-side ileo-ileal anastomosis not reachable by the endoscope. In a second patient (CE) with IDA, WCE images stopped at the level of a bleeding ulcerated substenosis in the proximal SB, with no retention (Figure 2C). In this patient indication for surgery was also determined after WCE examination, followed by resection of the ulcerated substenosis not detected by conventional techniques, with histological findings compatible with CD.
C group: WCE detected distal SB lesions not compatible with CD in 2/32 (6%) patients, including erosions in one patient and one single angiodysplasia in the other patient. No patients showed aphthoid or deep ulcers compatible with CD.
CD group: SICUS detected distal SB lesions in 30/32 (93%) patients, including all 3 patients showing upper SB lesions by SICUS. In both patients with normal SICUS, conventional techniques detected lesions in the distal ileum.
Findings compatible with CD in the distal SB were detected by both WCE and SICUS in 30/32 (93%) patients, as SICUS detected no lesions in 2 patients. These 2 techniques showed no significant concordance in detecting proximal SB lesions (χ2 = 0.5, P = 0.4).
CD group: WCE detected gastric and/or duodenal lesions in 7/32 (22%) patients. Gastric lesions were detected in 5/32 (15%) patients, including aphthoid ulcers in one and erosions in 4 patients. Duodenal lesions (erosions) were detected by WCE in 5/32 (15%) patients, and were also visualized in the stomach in 4 patients. Of the 7 patients showing gastric/duodenal lesions at WCE, 5 (71%) also showed upper SB lesions with WCE.
C group: Additional findings were detected in 6/32 (19%) C patients, including gastric/duodenal erosions in 5 and colonic angiodysplasia in 2 (with gastric erosions in one).
Proximal SB lesions are detected in a low proportion of CD patients (about 5%)[14-16]. However, these rates have been reported using radiologic techniques which show a low sensitivity for visualizing superficial lesions. Recently, WCE has been shown to visualize the inner SB surface, providing a high sensitivity in detecting minor lesions (i.e. erosions, aphthoid ulcers)[2-4]. Two independent studies reported that WCE visualizes lesions related to early CD recurrence in the SB[17,18]. As the frequency, natural history and clinical relevance of proximal SB lesions in CD is undefined, we investigated this issue in a prospective longitudinal study. A high frequency of WCE findings compatible with proximal SB lesions related to CD was observed. As WCE does not allow an histological characterization of the lesions, WCE findings in CD patients were compared with those observed in control patients requiring WCE for IDA or chronic diarrhea. No control patients showed aphthoid or deep ulcers, thus supporting the specificity of the upper SB lesions detected by WCE in our CD population. No correlations were observed between risk factors and proximal SB lesions, supporting the disease-specificity of our findings.
The frequency of WCE impaction was within the expected range (3%)[8]. The finding of WCE impaction within the “cul the sac” of a side-to-side ileo-ileal anastomosis may indicate a higher impaction risk in these patients, even in the absence of overt stenosis. In addition, a WCE examination allowed the detection of a previously unknown ulcerated substenosis in one additional patient with IDA associated with CD of the distal ileum. This observation further supports the role of WCE in identifying upper SB lesions not detected by conventional radiology.
No concordance was observed between proximal SB lesions at WCE and related signs/symptoms, even when patients were followed up for at least 12 mo. This observation provides additional evidence for a diffuse involvement of the SB in patients with CD of the distal ileum, even if not associated with overt symptoms. Present findings also suggest that no treatment changes may be required in CD patients showing upper SB lesions at WCE. The observed frequency of upper SB lesions is in agreement with previous findings in the early postoperative period (56%)[17].
When WCE and SICUS findings were compared, a small proportion of patients (3 of 16) showing upper SB lesions with WCE, also showed the same finding with SICUS. This discrepancy is in agreement with our previous studies[18,19] and may be related to the observation that WCE and SICUS provide a different view of the SB (i.e. intraluminal vs extraluminal). In addition, WCE allows the visualization of superficial lesions not detected by SICUS[18]. In the 3 patients showing upper SB lesions by both WCE and SICUS, lesions were represented by deep ulcers at WCE in 2, suggesting that discrepancies are mainly observed for superficial lesions. In contrast to the upper SB lesions, WCE and SICUS findings appeared comparable in the distal SB. This observation indicates that the characteristics of the SB lesions, including not only the severity (deep vs aphthoid ulcers) and number, but also the site (upper vs distal SB) may influence the sensitivity of ultrasonography. A good interobserver agreement was observed, supporting previous findings[17]. Although some patients were studied by SBFT, this technique was not included for ethical and economic reasons, in relation to both the high radiation exposure and to the known low sensitivity of SBFT in detecting superficial SB lesions. In addition, there was no clinical indication for SBFT in our population, including only 5 active patients with an established diagnosis of CD.
To our knowledge, no studies have investigated the role of WCE in comparison with SICUS in detecting the presence and clinical relevance of upper SB lesions in patients with CD involving the distal ileum, diagnosed by conventional radiological techniques. The present findings supports that WCE is a non-invasive technique which allows the visualization of superficial proximal SB lesions in a high proportion of patients with an established diagnosis of CD of the distal ileum. Despite no significant clinical relevance appearing to be associated with these findings even in the long term, the use of WCE in CD involving the distal SB may add clues in defining the extent of the lesions and its relation with clinical manifestations of the disease.
Wireless capsule endoscopy (WCE) is a non-invasive technique visualizing the mucosal surface of the small bowel (SB). WCE showed a high sensitivity and specificity for detecting lesions related to SB Crohn’s disease (CD). WCE has in particular been shown to be able to detect minor lesions, not visualized by conventional radiologic techniques, providing high radiation exposure. WCE has therefore been proposed as an alternative non-invasive technique for assessing CD lesions. Ultrasonography also is a non-invasive technique proposed for detecting SB lesions in CD. The use of an oral contrast (SICUS) significantly increases, in experienced hands, the sensitivity of ultrasonography for assessing SB lesions in CD (> 95%). Although several studies concordantly showed that WCE is able to visualize superficial lesions in the SB, its role in defining the extent of the lesions in CD is undefined.
Proximal small bowel lesions are detected in a low proportion of CD patients (about 5%). However, these frequencies have been reported using radiologic techniques which have a low sensitivity for visualizing superficial lesions. WCE has been shown to visualize the inner SB surface, providing a high sensitivity in detecting minor lesions (i.e. erosions, aphthoid ulcers). Two independent studies reported that WCE visualizes lesions related to early CD recurrence in the SB. However, the frequency, natural history and clinical relevance of proximal SB lesions in CD is currently undefined.
The present study showed that WCE is a non-invasive technique allowing the visualization of superficial proximal small bowel lesions in a high proportion of patients with an established diagnosis of CD of the distal ileum.
Despite no significant clinical manifestations appearing to be associated with these findings even in the long term, the use of WCE in CD involving the distal SB may add clues in defining the extent of the lesions and the relationship with clinical symptoms of the disease.
WCE is a non-invasive technique able to visualize the inner surface of the small intestine. SICUS is also a non invasive technique showing, in experienced hands, a high sensitivity and specificity in terms of assessment of small bowel lesions, including increased bowel wall thickness in ileal CD.
This is a small (32 patients) prospective study, with a control population, that evaluates the diagnostic accuracy of WCE in CD, and compares it with different diagnostic tools. It is well presented and performed in an ethical manner.
Peer reviewer: Javier San Martín, Chief, Gastroenterology and Endoscopy, Sanatorio Cantegril, Av. Roosevelt y P 13, Punta del Este 20100, Uruguay
S- Editor Wang JL L- Editor Cant MR E- Editor Ma WH
| 2. | Sidhu R, Sanders DS, Morris AJ, McAlindon ME. Guidelines on small bowel enteroscopy and capsule endoscopy in adults. Gut. 2008;57:125-136. |
| 3. | Fireman Z, Mahajna E, Broide E, Shapiro M, Fich L, Sternberg A, Kopelman Y, Scapa E. Diagnosing small bowel Crohn's disease with wireless capsule endoscopy. Gut. 2003;52:390-392. |
| 4. | Eliakim R, Suissa A, Yassin K, Katz D, Fischer D. Wireless capsule video endoscopy compared to barium follow-through and computerised tomography in patients with suspected Crohn's disease--final report. Dig Liver Dis. 2004;36:519-522. |
| 5. | Buchman AL, Miller FH, Wallin A, Chowdhry AA, Ahn C. Videocapsule endoscopy versus barium contrast studies for the diagnosis of Crohn's disease recurrence involving the small intestine. Am J Gastroenterol. 2004;99:2171-2177. |
| 6. | Fireman Z, Mahajna E, Broide E, Shapiro M, Fich L, Sternberg A, Kopelman Y, Scapa E. Diagnosing small bowel Crohn's disease with wireless capsule endoscopy. Gut. 2003;52:390-392. |
| 7. | Costamagna G, Shah SK, Riccioni ME, Foschia F, Mutignani M, Perri V, Vecchioli A, Brizi MG, Picciocchi A, Marano P. A prospective trial comparing small bowel radiographs and video capsule endoscopy for suspected small bowel disease. Gastroenterology. 2002;123:999-1005. |
| 8. | Cheifetz AS, Kornbluth AA, Legnani P, Schmelkin I, Brown A, Lichtiger S, Lewis BS. The risk of retention of the capsule endoscope in patients with known or suspected Crohn's disease. Am J Gastroenterol. 2006;101:2218-2222. |
| 9. | Triester SL, Leighton JA, Leontiadis GI, Gurudu SR, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with non-stricturing small bowel Crohn's disease. Am J Gastroenterol. 2006;101:954-964. |
| 10. | Sonnenberg A, Erckenbrecht J, Peter P, Niederau C. Detection of Crohn's disease by ultrasound. Gastroenterology. 1982;83:430-434. |
| 11. | Calabrese E, La Seta F, Buccellato A, Virdone R, Pallotta N, Corazziari E, Cottone M. Crohn's disease: a comparative prospective study of transabdominal ultrasonography, small intestine contrast ultrasonography, and small bowel enema. Inflamm Bowel Dis. 2005;11:139-145. |
| 12. | Parente F, Greco S, Molteni M, Anderloni A, Sampietro GM, Danelli PG, Bianco R, Gallus S, Bianchi Porro G. Oral contrast enhanced bowel ultrasonography in the assessment of small intestine Crohn's disease. A prospective comparison with conventional ultrasound, x ray studies, and ileocolonoscopy. Gut. 2004;53:1652-1657. |
| 13. | Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976;70:439-444. |
| 14. | Wagtmans MJ, Verspaget HW, Lamers CB, van Hogezand RA. Clinical aspects of Crohn's disease of the upper gastrointestinal tract: a comparison with distal Crohn's disease. Am J Gastroenterol. 1997;92:1467-1471. |
| 15. | D'Haens G, Rutgeerts P, Geboes K, Vantrappen G. The natural history of esophageal Crohn's disease: three patterns of evolution. Gastrointest Endosc. 1994;40:296-300. |
| 16. | Carter MJ, Lobo AJ, Travis SP. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004;53 Suppl 5:V1-V16. |
| 17. | Bourreille A, Jarry M, D'Halluin PN, Ben-Soussan E, Maunoury V, Bulois P, Sacher-Huvelin S, Vahedy K, Lerebours E, Heresbach D. Wireless capsule endoscopy versus ileocolonoscopy for the diagnosis of postoperative recurrence of Crohn's disease: a prospective study. Gut. 2006;55:978-983. |
| 18. | Biancone L, Calabrese E, Petruzziello C, Onali S, Caruso A, Palmieri G, Sica GS, Pallone F. Wireless capsule endoscopy and small intestine contrast ultrasonography in recurrence of Crohn's disease. Inflamm Bowel Dis. 2007;13:1256-1265. |
| 19. | Calabrese E, Petruzziello C, Onali S, Condino G, Zorzi F, Pallone F, Biancone L. Severity of postoperative recurrence in Crohn's disease: correlation between endoscopic and sonographic findings. Inflamm Bowel Dis. 2009;15:1635-1642. |