Published online Jul 14, 2010. doi: 10.3748/wjg.v16.i26.3249
Revised: March 17, 2010
Accepted: March 24, 2010
Published online: July 14, 2010
AIM: To investigate the anti-diabetogenic mechanism of Nardostachys jatamansi extract (NJE).
METHODS: Mice were injected with streptozotocin via a tail vein to induce diabetes. Rat insulinoma RINm5F cells and isolated rat islets were treated with interleukin-1β and interferon-γ to induce cytotoxicity.
RESULTS: Treatment of mice with streptozotocin resulted in hyperglycemia and hypoinsulinemia, which was confirmed by immunohistochemical staining of the islets. The diabetogenic effects of streptozotocin were completely abolished when mice were pretreated with NJE. Inhibition of streptozotocin-induced hyperglycemia by NJE was mediated by suppression of nuclear factor (NF)-κB activation. In addition, NJE protected against cytokine-mediated cytotoxicity. Incubation of RINm5F cells and islets with NJE resulted in a significant reduction in cytokine-induced NF-κB activation and downstream events, inducible nitric oxide synthase expression and nitric oxide production. The protective effect of NJE was further demonstrated by the normal insulin secretion of cytokine-treated islets in response to glucose.
CONCLUSION: NJE provided resistance to pancreatic β-cell damage from cytokine or streptozotocin treatment. The β-cell protective effect of NJE is mediated by suppressing NF-κB activation.
-
Citation: Song MY, Bae UJ, Lee BH, Kwon KB, Seo EA, Park SJ, Kim MS, Song HJ, Kwon KS, Park JW, Ryu DG, Park BH.
Nardostachys jatamansi extract protects against cytokine-induced β-cell damage and streptozotocin-induced diabetes. World J Gastroenterol 2010; 16(26): 3249-3257 - URL: https://www.wjgnet.com/1007-9327/full/v16/i26/3249.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i26.3249
Type 1 diabetes mellitus is an autoimmune disease that causes selective destruction of insulin producing β-cells in the islets of Langerhans. In early-stage disease, infiltration of inflammatory cells into the pancreatic islets can be observed histologically[1]. The inflammatory cells produce and release cytokines, including interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ). IL-1β, alone or in combination with TNF-α or IFN-γ, upregulates inducible nitric oxide synthase (iNOS), and produces high levels of nitric oxide (NO) in pancreatic islets[2,3]. NO is produced by the oxidation of L-arginine to L-citruline by NOS, and generation of excess NO can inhibit mitochondrial metabolism, protein modification, and DNA cleavage; any one of which could lead to impaired insulin secretion and β-cell death[4,5]. Streptozotocin (STZ) is a diabetogenic agent that is toxic to pancreatic β-cells and is commonly used in diabetes research[6]. Streptozotocin contains a nitroso moiety and releases NO during its metabolism[7]. In rodents, STZ activates poly-ADP ribose polymerase, depletes cellular NAD and ATP, breaks DNA strands, and initiates β-cell necrosis[8].
NO production is regulated by transcription factors that bind to specific sites in the iNOS promoter. Nuclear factor (NF)-κB, which can be activated by cytokines and STZ, has been implicated as a key signaling mediator in iNOS induction[9,10]. When inactive, NF-κB is located in the cytosol complexed with NF-κB inhibitory factor (IκB). Various inducers cause complex dissociation, presumably via IκB phosphorylation. Released NF-κB translocates to the nucleus, where it interacts with recognition sites to mediate gene transcription[11]. We and others have shown that NF-κB-dependent NO production is involved in the dysfunction and destruction of β-cells, which suggests NO involvement in autoimmune diabetes pathogenesis[9,12-15].
Nardostachys jatamansi (N. jatamansi) is used in Ayurvedic medicine to treat mental disorders, hyperlipidemia, hypertension, and convulsions[16-18]. Various sesquiterpenes such as lignans and neolignans are present in root extracts of this plant[19]. N. jatamansi is suggested to protect cells and tissues through its antioxidative properties[20,21]. We found that N. jatamansi extract (NJE) protects against development of acute cerulean-induced pancreatitis[22]. However, as far as we are aware, no studies have reported on the antidiabetic effects of NJE. Therefore, in this study we examined the effect of NJE on cytokine- or STZ-stimulated pancreatic β-cell damage and the resultant development of type 1 diabetes.
Rat pancreatic β-cell line RINm5F was from the American Type Culture Collection and were grown at 37°C in a humidified 5% CO2 atmosphere in RPMI 1640 medium (Gibco BRL, Grand Island, NY, USA), supplemented with 10% fetal bovine serum and 2 mmol/L glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 2.5 μg/mL amphotericin B. IL-1β and IFN-γ were obtained from R&D Systems (Minneapolis, MN, USA). All reagents were from Sigma (St. Louis, MO, USA), unless otherwise noted.
N. jatamansi was from a standard commercial source (Omni Herb, Seoul, Korea), and its identity was confirmed at the Korean Drug Test Laboratory (Seoul, Korea). Voucher specimens (NO; Oh/wh/nj-43) were deposited at the School of Oriental Medicine Herbarium, Wonkwang University. NJE was prepared by decocting 200 g of dried herbs with 1800 mL boiling distilled water for approximately 2 h. The extract was filtered, freeze-dried and stored at 4°C.
Specific pathogen-free male ICR mice, weighing 25-30 g, were purchased from Orientbio Inc. (Seoungnam, Korea) and housed at our animal facility for 1 wk. All mice were kept under specific pathogen-free conditions with free access to a standard commercial diet and were used at 5-6 wk of age. To induce diabetes, mice were injected via the tail vein with 80 mg/kg STZ dissolved in 0.1 mol/L sodium citrate buffer (pH 4.0), prepared within 5 min of administration. Mice were divided into the following groups: (1) non-treated controls; (2) STZ; (3) NJE; and (4) NJE + STZ (n = 5 for each group). Control animals received citrate buffer alone. Group 4 received intraperitoneal injections of 125 mg/kg NJE daily for 3 d before administration of STZ. The day on which STZ was first administered was defined as day 1. At day 5, mice were sacrificed by decapitation without anesthesia and trunk blood was collected in pre-chilled tubes that contained 1 mg/mL EDTA. Plasma glucose was assayed using the glucose oxidase-peroxidase method, and plasma insulin was measured using a radioimmunoassay kit (Linco Research, St. Charles, MO, USA). All experimental procedures were approved by the Institutional Animal Care and Use Committee at Chonbuk National University, Jeonbuk, Korea.
The viability of cultured cells was determined by the reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to formazan. RINm5F cells were seeded overnight in clear, flat-bottomed 96-well tissue culture plates at 105 cells/well in 100 μL medium. Cells were pretreated with NJE as indicated for 3 h, then IL-1β (1 U/mL) and IFN-γ (100 U/mL) were added for an additional 48 h. Cells were washed twice with PBS, and MTT was added (100 μg/100 μL PBS). After incubation at 37°C for 1 h, 100 μL DMSO was added to dissolve the formazan crystals, and absorbance was measured at 570 nm using a Spectra MAX PLUS spectrophotometer (Molecular Devices, Sunnyvale, CA, USA).
Biologically produced NO is rapidly oxidized to nitrite and nitrate in aqueous solutions. NO production was measured as nitrite concentration in cell-free culture supernatants using a colorimetric assay. Briefly, 5 × 105 RINm5F cells or 30 islet samples were pretreated with the indicated concentrations of NJE for 3 h prior to the addition of IL-1β (1 U/mL) and IFN-γ (100 U/mL). After 24 h, 100 μL aliquots of culture supernatant were incubated with 100 μL of a modified Griess reagent of a 1:1 mixture of 1% sulfanilamide in 30% acetic acid and 0.1% N-(1-naphthyl) ethylenediamine dihydrochloride in 60% acetic acid, at room temperature for 5 min, and the absorbance was measured at 540 nm using a spectrophotometer (Ultrospec 2100 pro; Amersham Biosciences). NO concentration was determined from a linear standard curve of serial dilutions of sodium nitrite in a working medium.
Cells, islets, or pancreatic tissues were washed with PBS and lysed in CytoBuster protein extraction buffer (Novagen, Madison, WI, USA). Lysate was centrifuged at 10 000 ×g for 5 min at 4°C, and the supernatant was used as whole cell protein extract. Cytosolic and nuclear extracts were prepared from cells using NE-PER Nuclear and Cytoplasmic Extraction Reagent (Pierce Biotechnology, Rockford, IL, USA).
RINm5F cells (5 × 106) or 30 islet samples were homogenized in 100 μL ice-cold lysis buffer (20 mmol/L HEPES, pH 7.2, 1% Triton X-100, 10% glycerol, 1 mmol/L PMSF, 10 μg/mL leupeptin, 10 μg/mL aprotinin) and 20 μg protein separated by SDS-PAGE and transferred to nitrocellulose membranes. Blots were probed with 1 μg/mL primary antibody against p50, p65, iNOS, actin, or PCNA (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and detected with horseradish peroxidase-conjugated IgG (Zymed, South San Francisco, CA, USA).
NF-κB activation was assayed using a gel mobility shift assay with nuclear extracts from control and treated cells. An oligonucleotide that contained the κ-chain binding site (κB, 5′-CCGGTTAACAGAGGGGGCTTTCCGAG-3′) was used as a probe. The two complementary strands were annealed and labeled with [α-32P]dCTP. Binding reactions that contained labeled oligonucleotide (10 000 cpm), 10 μg nuclear extract protein, and binding buffer (10 mmol/L Tris-HCl, pH 7.6, 500 mmol/L KCl, 10 mmol/L EDTA, 50% glycerol, 100 ng poly (dI·dC), 1 mmol/L dithiothreitol) in a final volume of 20 μL were incubated for 30 min at room temperature. Reactions were separated on 4% polyacrylamide gels in 0.5 × Tris-borate buffer, and the gels were dried and visualized by autoradiography. Specificity of the DNA/NF-κB interaction was demonstrated by competitive assays with 50-fold excess unlabeled oligonucleotide.
RNA was isolated from RINm5F cells or islets using Trizol reagent (Invitrogen, Carlsbad, CA, USA). RNA was precipitated with isopropanol and dissolved in DEPC-treated distilled water. Total RNA (2 μg) was treated with RNase-free DNase (Invitrogen), and first-strand cDNA was generated using random hexamer primer in a first-strand cDNA synthesis kit (Applied Biosystems, Foster City, CA, USA). Specific primers for iNOS were designed using primer express software (Applied Biosystems): iNOS (accession No. NM_012611), 5′-TGTGCTAATGCGGAAGGTCAT-3′ (forward), and 5′-CGACTTTCCTGTCTCAGTAGCAAA-3′ (reverse). Control 18S rRNA was purchased from Applied Biosystems and was used as the invariant control. Real-time RT-PCR mixtures consisted of 10 ng reverse transcribed total RNA, 167 nmol/L forward and reverse primers, and 2 × PCR Master Mix in a final volume of 10 μL. Reactions were carried out in 384-well plates using the ABI Prism 7900HT Sequence Detection System (Applied Biosystems). All experiments were performed in triplicate.
Pancreatic islets were isolated from 250-300 g male Sprague-Dawley rats, using the collagenase digestion method[23]. Islets were cultured for 24 h with IL-1β and IFN-γ in the presence or absence of NJE, then washed three times in Krebs-Ringer bicarbonate buffer (25 mmol/L HEPES, 115 mmol/L NaCl, 24 mmol/L NaHCO3, 5 mmol/L KCl, 1 mmol/L MgCl2, 2.5 mmol/L CaCl2, 0.1% bovine serum albumin, pH 7.4), which contained 3 mmol/L D-glucose. Insulin secretion assays were performed with either 5.5 or 20 mmol/L D-glucose. All experiments were performed in triplicate.
Immunohistochemical staining was performed with the DAKO Envision system (DAKO, Carpinteria, CA, USA), which used dextran polymers conjugated with horseradish peroxidase to avoid contamination with endogenous biotin. Pancreases were removed and immediately placed in fixative (10% formalin solution in 0.1 mol/L PBS). Histological sections of 4 μm were cut from formalin-fixed, paraffin-embedded tissue blocks. After deparaffinization, tissue sections were treated using a microwave antigen retrieval procedure in 0.01 mol/L sodium citrate buffer. After blocking endogenous peroxidase, the sections were incubated with Protein Block Serum-Free (DAKO) to block nonspecific staining, then with anti-insulin antibody (Santa Cruz Biotechnology). Peroxidase activity was detected with 3-amino-9-ethylcarbazole.
Statistical analyses were performed using ANOVA and Duncan’s tests. Differences with P < 0.05 were considered statistically significant.
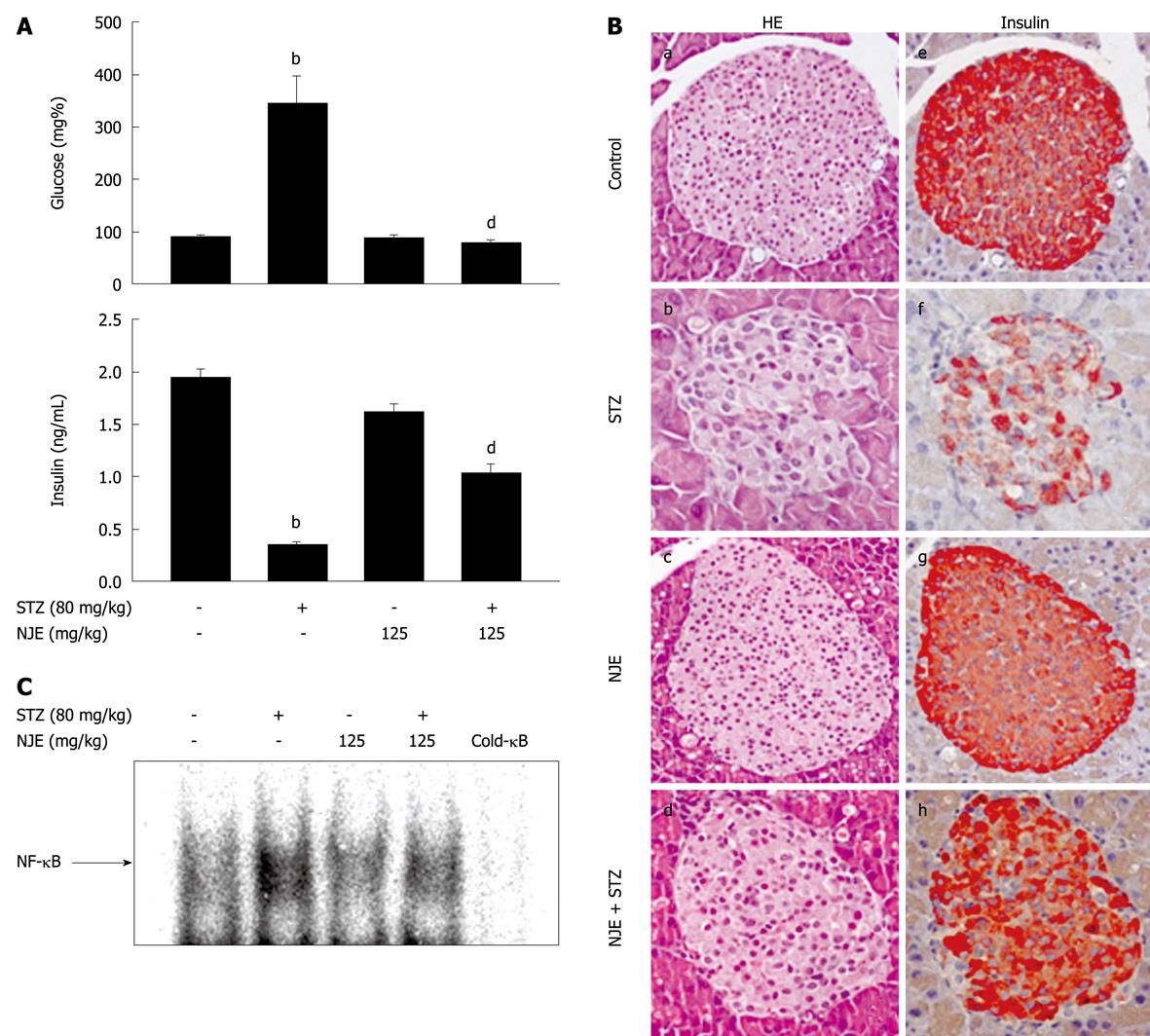
Mice injected with STZ gradually became hyperglycemic, with an increased incidence of diabetes observed starting at day 3. At day 5, the average blood glucose level of mice injected with STZ was 344 ± 52.8 mg/dL. Mice that were pretreated with NJE were fully resistant to diabetes development (Figure 1A), and treatment with NJE alone did not affect blood glucose concentration. In addition, the mean plasma insulin level at day 5 in the STZ group decreased by 84.2% compared with the control (from 1.9 ± 0.1 to 0.3 ± 0.1 ng/mL), while the severity of hypoinsulinemia was attenuated in mice pretreated with NJE (Figure 1A). These results indicate that NJE is protective against STZ-induced diabetes.
The preventative effect of NJE on STZ-induced diabetes was histologically examined. Pancreatic tissues at 5 d after STZ administration, with or without NJE pretreatment, were subjected to hematoxylin and eosin (HE) staining and immunohistochemistry. STZ-treated mice showed degenerative and necrotic changes and islet shrinkage (Figure 1B, b), as well as weak insulin-reactivity in a few β-cells (Figure 1B, f). However, tissues from STZ-treated mice pretreated with NJE showed round, nearly normal, and clearly defined islets that were strongly positive for insulin (Figure 1B, d and h).
To elucidate the antidiabetogenic mechanism of NJE, we examined its effect on STZ-induced NF-κB activation. Figure 1C is a representative electrophoretic mobility shift assay (EMSA) that shows the 32P-DNA/NF-κB complex formed with nuclear extracts from the pancreas 30 min after STZ administration. The findings were similar to those of our previous study, with STZ treatment resulting in increased NF-κB binding to DNA[10]. This complex was not detected in pancreatic nuclear extracts from NJE-pretreated mice. Taken together, these results show that NJE inhibits NF-κB activation and prevents type 1 diabetes development in mice.
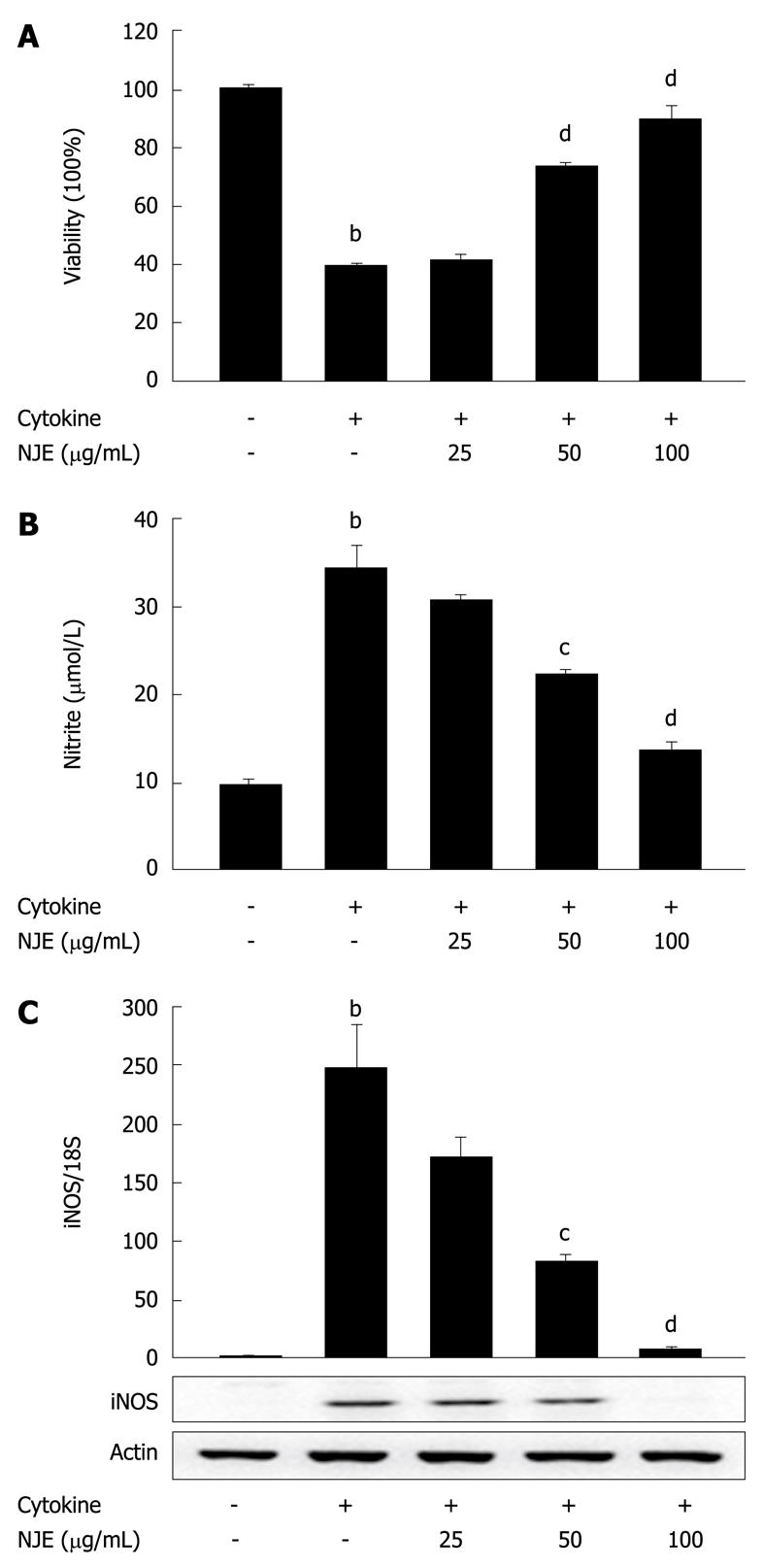
We investigated the antidiabetogenic effect of NJE at the cellular level. Untreated RINm5F cells or cells pretreated with NJE for 3 h were exposed to cytokine for 48 h, and viability was assessed using an MTT assay. Treatment with cytokine significantly reduced cell viability to 39.8% ± 0.6% of the controls (Figure 2A). Conversely, NJE increased the viability of cytokine-treated RINm5F cells in a concentration-dependent manner. Treatment with NJE alone did not affect cell viability at the concentrations used in this study (data not shown).
NO production was also evaluated. In 24 h, control RINm5F cells generated 9.8 ± 0.5 μmol/L nitrite, while cytokine-treated cells generated 34.2 ± 2.7 μmol/L (Figure 2B). A concentration-dependent reduction in cytokine-mediated nitrite production was observed in RINm5F cells treated with cytokine plus NJE. Near complete inhibition of nitrite production was observed in cells that were pretreated with 100 μg/mL NJE.
To investigate the regulatory effects of NJE on NO production, we examined the effects of NJE on cytokine-induced iNOS mRNA and protein expression, using real-time RT-PCR and Western blotting. Cytokine increased iNOS mRNA and protein levels (Figure 2C). However, when cells were treated with NJE prior to cytokine treatment, mRNA and protein levels decreased in a concentration-dependent manner. Treatment with 100 μg/mL NJE completely blocked iNOS expression.
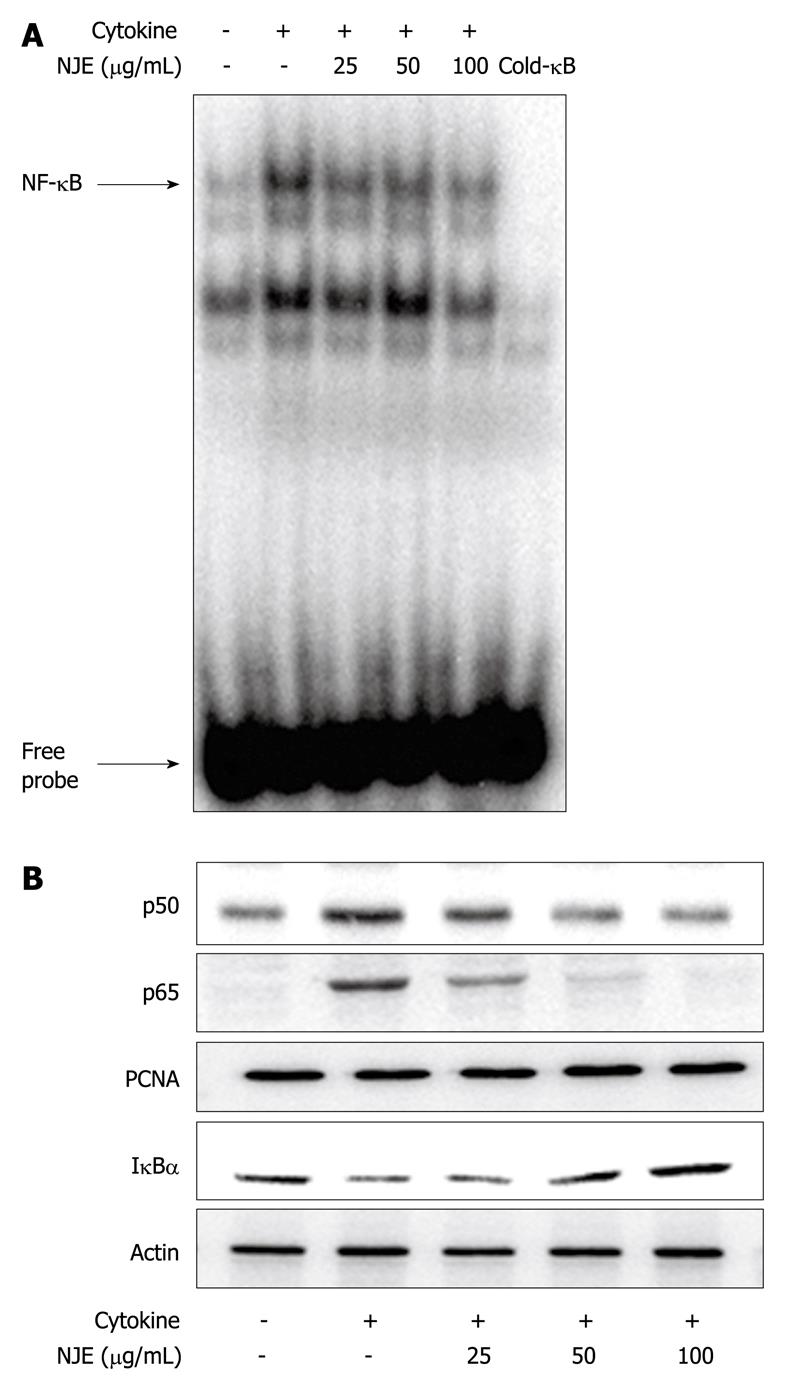
NF-κB was implicated in STZ toxicity (Figure 1C). Therefore, the effect of NJE on the cytokine-stimulated translocation of NF-κB from the cytosol to the nucleus in RINm5F cells was examined. Nuclear extracts from cytokine-stimulated RINm5F cells showed increased NF-κB binding activity (Figure 3A, lane 2), as well as increased nuclear levels of p65 and p50 subunits (Figure 3B), compared to those of unstimulated cells. In contrast, cytokine-induced NF-κB activation was markedly suppressed by NJE pretreatment, which suggested that NJE inhibited iNOS expression by suppressing NF-κB activation. We previously have reported that IκBα, but not IκBβ, is the major participant in cytokine-induced NF-κB activation[23]. Therefore, we investigated IκBα levels in the cytosol following cytokine treatment (Figure 3B). Cytokine-treated RINm5F cells showed a decreased level of IκBα protein in the cytosol compared to a similar fraction from unstimulated cells; however, increased IκBα degradation as a result of cytokine treatment was markedly suppressed by pretreatment with NJE.
NJE suppressed the cytokine-induced NF-κB pathway and preserved glucose-stimulated insulin secretion in rat islets
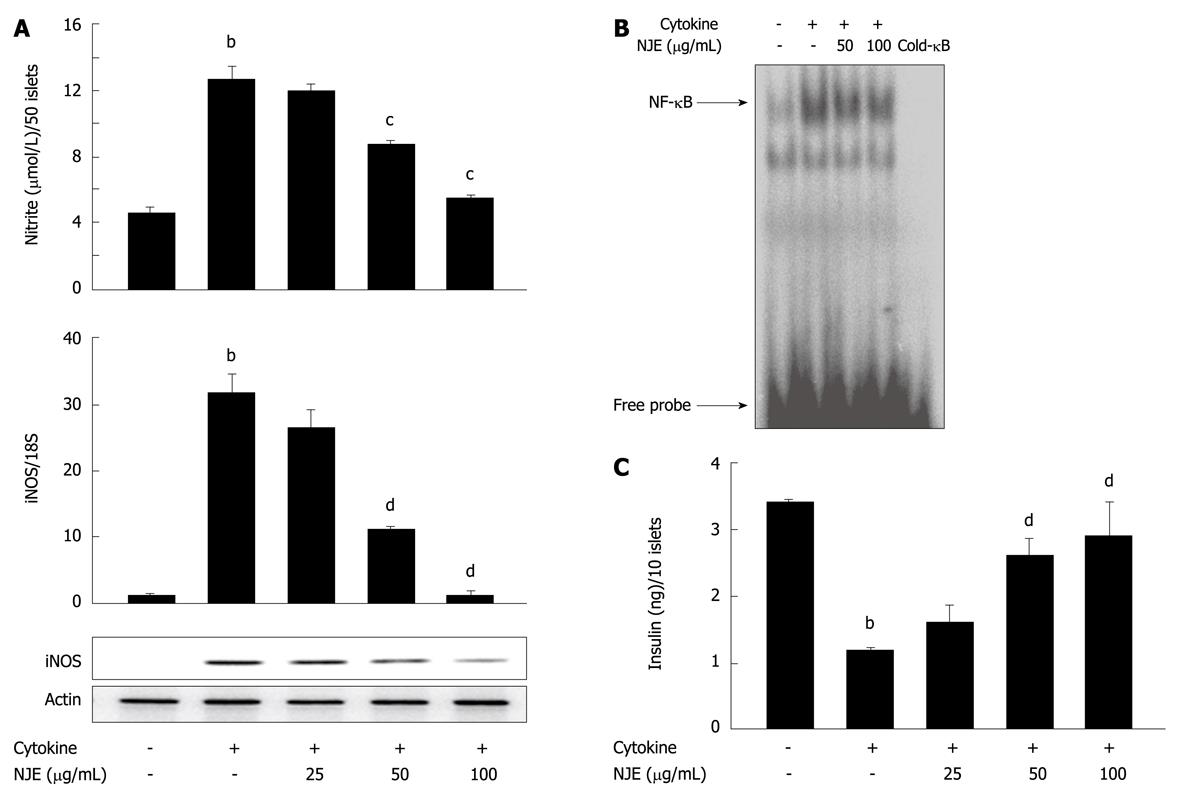
We further assayed the preventive effects of NJE using rat pancreatic islets isolated from male Sprague-Dawley rats. Incubation of rat islets with cytokine for 24 h resulted in a 2.8-fold increase in NO production (Figure 4A). Real time RT-PCR and Western blotting revealed that iNOS mRNA and protein levels were markedly increased by cytokines (Figure 4A). Consistent with results from RINm5F cells, pretreatment of islets with NJE abolished the cytokine effects and reduced NO production and iNOS expression to the level of those of control islets. Additionally, treatment with cytokine increased NF-κB DNA binding activity in islets (Figure 4B), and pretreatment of islets with NJE completely abolished these effects. To add functional data, NJE protection against cytokine-induced impairment of glucose-stimulated insulin secretion (GSIS) was evaluated. After 24 h of cytokine exposure, insulin secretion was assayed in response to 20 mmol/L glucose. Control islets secreted 3.4 ± 0.4 ng/mL insulin, while cytokine-treated islets secreted significantly less, at 1.2 ± 0.2 ng/mL (P < 0.01) (Figure 4C). However, pretreatment with NJE blocked the cytokine effect and maintained islet cell insulin secretion to levels similar to those of the controls. In addition, treatment with NJE alone did not affect insulin secretion in response to glucose (data not shown).
In this study, we present a mode of action for NJE protection against development of type 1 diabetes. Intraperitoneal administration of NJE prevented diabetes development after STZ and preserved β-cell mass. In addition, NJE protected β-cells from cytokine toxic challenge in RINm5F cells and islets.
We demonstrated that NJE prevented STZ-induced diabetes in mice. STZ destroys islet cells through several mechanisms, including the production of reactive oxygen species (ROS)[24], activation of pancreatic NF-κB[25], and induction of pronounced immune and inflammatory responses[26]. STZ-treated mice showed marked islet destruction and relatively small numbers of insulin-positive β-cells, while NJE pre-treated mice showed well-defined islets and strong insulin staining. An EMSA revealed increased NF-κB binding activity in pancreatic nuclear extracts derived from STZ-treated hyperglycemic diabetic mice. However, pretreatment with NJE prevented NF-κB activation, which resulted in the maintenance of plasma glucose and insulin levels in the normal range. NF-κB participates in the transcriptional regulation of pro-inflammatory genes, and their activation results in the production of pro-inflammatory mediators[27,28]. Therefore, NF-κB might be a key regulator in local cytokine response pathways in STZ-mediated β-cell destruction. Alternatively, the NJE anti-diabetic effect could be related to its antioxidative properties. Several studies have confirmed that NJE is an ROS scavenger[20,21], and that ROS induces NF-κB activation[29,30], which suggests that ROS scavenging by NJE suppresses STZ-induced NF-κB activation. Taken together, these results suggest that manipulation of NF-κB activity by NJE in pancreatic β-cells allows these cells to withstand and survive STZ-mediated immune attack.
NJE not only protected against STZ-induced diabetes, but also protected RINm5F cells and rat islets against cytokine toxicity. IL-1β has been implicated in early events in β-cell destruction. Suppression of IL-1β production or inhibition of its interaction with corresponding cellular receptors significantly inhibits IL-1β-mediated deleterious effects on β-cells[31,32]. IL-1β exerts its main effects through the NF-κB pathway[33-35]. IFN-γ alone does not stimulate iNOS expression in rodent or human islets, but it reduces the concentration of IL-1β required to induce iNOS expression in rat islets, and a combination of IL-1β and IFN-γ is required to induce iNOS expression and β-cell dysfunction in mouse and human islets[36]. In addition to cytokines, different intracellular pathways that lead to β-cell death (e.g. oxidative stress, chemical generation of NO, mitogen-activated protein kinase activation, JAK-STAT activation, and endoplasmic reticulum stress) partially converge at NF-κB[12,14,37,38]. Hypothetically, this gives us, employing only one approach, the ability to block NF-κB signaling, to save β-cell mass and thereby prevent diabetes development. In this model, NJE completely inhibits NO production in IL-1β- and IFN-γ-stimulated RINm5F cells and islets, through suppression of NF-κB-dependent iNOS expression, thereby protecting RINm5F cells and islets from IL-1β and IFN-γ cytotoxicity. In addition to increased cell viability, we observed the preservation of insulin secretion in NJE pretreated rat islets. The molecular mechanism by which NJE inhibits NF-κB activation by IL-1β and IFN-γ appears to involve both inhibition of IκBα degradation and translocation of p65 and p50 into the nucleus.
Flavonoids comprise the most common group of plant polyphenols and provide much of the flavor and color to fruits and vegetables. Several flavonoids have been shown to inhibit the expression of NF-κB-dependent cytokines, iNOS, and cyclooxygenase-2 genes[39]. Therefore, it would be interesting to analyze flavonoid composition in NJE extracts.
In summary, this study is believed to be the first to demonstrate that NJE has a β-cell protective effect. Specifically, NJE protected β-cells from cytokine-induced injury in vitro and counteracted the development of type 1 diabetes in response to STZ in vivo. This β-cell protective effect might be mediated, at least in part, by suppressing NF-κB activation. NJE did not cause serious side effects in mice, therefore, it could be a therapeutic alternative for rescuing β-cells in cases of ongoing β-cell destruction.
Type 1 diabetes mellitus is an autoimmune disease that causes selective destruction of insulin-producing β-cells in the islets of Langerhans. Once those cells are destroyed, they do not ever produce insulin again. Type 1 diabetes affects younger individuals and requires lifelong insulin treatment. Without treatment, the blood glucose rises to levels which can cause hyperglycemia. Type 1 diabetes cannot be prevented. There is no practical way to predict who will develop the disease because most people who develop it are otherwise healthy. Therefore, the best way to control type 1 diabetes is understanding the disease better and finding a therapeutic regimen to preserve functional β-cell damage.
Cytokines such as interleukin (IL)-1β and interferon (IFN)-γ, which are released during islet inflammation, are believed to participate in β-cell damage during the development of autoimmune type 1 diabetes. Evidence has suggested that activation of nuclear factor (NF)-κB in response to cytokines is an important component of the signal that triggers β-cell death. For this reason, NF-κB has been targeted for preventing type 1 diabetes development.
Nardostachys jatamansi is used in Ayurvedic medicine to treat mental disorders, hyperlipidemia, hypertension, and convulsions. N. jatamansi has been suggested to protect cells and tissues through its antioxidative properties. However, no studies to date have reported the antidiabetic effects of N. jatamansi. In this study, the authors observed that N. jatamansi extract (NJE) had antidiabetic effects in in vitro and in vivo models of diabetes.
NJE did not cause serious side effects in vivo, therefore, it might be a therapeutic alternative for rescuing β-cells in cases of ongoing β-cell destruction. NJE-treated islets can also be used to increase islet survival in an allograft transplantation model.
IL-1β and IFN-γ are cytokines that are secreted from infiltrated inflammatory cells into the pancreatic islets. Streptozotocin is a diabetogenic drug and particularly toxic to pancreatic β-cells.
The article is written well, however, there are many major queries which have to be answered by the authors. Authors have to report on the phytochemical constituents of the extract (at least qualitative analysis).
Peer reviewer: Dr. Shivananda Nayak, PhD, Department of Preclinical Sciences, Biochemistry Unit, Faculty of Medical Sciences, The University of The West Indies, Building 36, EWMSC, Mount Hope, Trinidad and Tobago
S- Editor Wang YR L- Editor Kerr C E- Editor Ma WH
| 1. | Papaccio G. Insulitis and islet microvasculature in type 1 diabetes. Histol Histopathol. 1993;8:751-759. |
| 2. | Cnop M, Welsh N, Jonas JC, Jörns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54 Suppl 2:S97-S107. |
| 3. | Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat Rev Endocrinol. 2009;5:219-226. |
| 4. | Corbett JA, McDaniel ML. Does nitric oxide mediate autoimmune destruction of beta-cells? Possible therapeutic interventions in IDDM. Diabetes. 1992;41:897-903. |
| 5. | Stadler J, Billiar TR, Curran RD, Stuehr DJ, Ochoa JB, Simmons RL. Effect of exogenous and endogenous nitric oxide on mitochondrial respiration of rat hepatocytes. Am J Physiol. 1991;260:C910-C916. |
| 6. | Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50:537-546. |
| 7. | Kwon NS, Lee SH, Choi CS, Kho T, Lee HS. Nitric oxide generation from streptozotocin. FASEB J. 1994;8:529-533. |
| 8. | Yamamoto H, Uchigata Y, Okamoto H. Streptozotocin and alloxan induce DNA strand breaks and poly(ADP-ribose) synthetase in pancreatic islets. Nature. 1981;294:284-286. |
| 9. | Heimberg H, Heremans Y, Jobin C, Leemans R, Cardozo AK, Darville M, Eizirik DL. Inhibition of cytokine-induced NF-kappaB activation by adenovirus-mediated expression of a NF-kappaB super-repressor prevents beta-cell apoptosis. Diabetes. 2001;50:2219-2224. |
| 10. | Kwon KB, Kim EK, Jeong ES, Lee YH, Lee YR, Park JW, Ryu DG, Park BH. Cortex cinnamomi extract prevents streptozotocin- and cytokine-induced beta-cell damage by inhibiting NF-kappaB. World J Gastroenterol. 2006;12:4331-4337. |
| 11. | Melloul D. Role of NF-kappaB in beta-cell death. Biochem Soc Trans. 2008;36:334-339. |
| 12. | Kim EK, Kwon KB, Song MY, Seo SW, Park SJ, Ka SO, Na L, Kim KA, Ryu DG, So HS. Genistein protects pancreatic beta cells against cytokine-mediated toxicity. Mol Cell Endocrinol. 2007;278:18-28. |
| 13. | Lv N, Song MY, Kim EK, Park JW, Kwon KB, Park BH. Guggulsterone, a plant sterol, inhibits NF-kappaB activation and protects pancreatic beta cells from cytokine toxicity. Mol Cell Endocrinol. 2008;289:49-59. |
| 14. | Song MY, Kim EK, Moon WS, Park JW, Kim HJ, So HS, Park R, Kwon KB, Park BH. Sulforaphane protects against cytokine- and streptozotocin-induced beta-cell damage by suppressing the NF-kappaB pathway. Toxicol Appl Pharmacol. 2009;235:57-67. |
| 15. | Mabley JG, Haskó G, Liaudet L, Soriano F, Southan GJ, Salzman AL, Szabó C. NFkappaB1 (p50)-deficient mice are not susceptible to multiple low-dose streptozotocin-induced diabetes. J Endocrinol. 2002;173:457-464. |
| 16. | Dixit VP, Jain P, Joshi SC. Hypolipidaemic effects of Curcuma longa L and Nardostachys jatamansi, DC in triton-induced hyperlipidaemic rats. Indian J Physiol Pharmacol. 1988;32:299-304. |
| 17. | Joshi H, Parle M. Nardostachys jatamansi improves learning and memory in mice. J Med Food. 2006;9:113-118. |
| 18. | Rao VS, Rao A, Karanth KS. Anticonvulsant and neurotoxicity profile of Nardostachys jatamansi in rats. J Ethnopharmacol. 2005;102:351-356. |
| 19. | Bagchi A, Oshima Y, Hikino H. Neolignans and Lignans of Nardostachys jatamansi Roots1. Planta Med. 1991;57:96-97. |
| 20. | Lyle N, Bhattacharyya D, Sur TK, Munshi S, Paul S, Chatterjee S, Gomes A. Stress modulating antioxidant effect of Nardostachys jatamansi. Indian J Biochem Biophys. 2009;46:93-98. |
| 21. | Lyle N, Gomes A, Sur T, Munshi S, Paul S, Chatterjee S, Bhattacharyya D. The role of antioxidant properties of Nardostachys jatamansi in alleviation of the symptoms of the chronic fatigue syndrome. Behav Brain Res. 2009;202:285-290. |
| 22. | Bae GS, Park HJ, Kim DY, Song JM, Kim TH, Oh HJ, Yun KJ, Park RK, Lee JH, Shin BC. Nardostachys jatamansi protects against cerulein-induced acute pancreatitis. Pancreas. 2010;39:520-529. |
| 23. | Kim EK, Kwon KB, Koo BS, Han MJ, Song MY, Song EK, Han MK, Park JW, Ryu DG, Park BH. Activation of peroxisome proliferator-activated receptor-gamma protects pancreatic beta-cells from cytokine-induced cytotoxicity via NF kappaB pathway. Int J Biochem Cell Biol. 2007;39:1260-1275. |
| 24. | Takasu N, Komiya I, Asawa T, Nagasawa Y, Yamada T. Streptozocin- and alloxan-induced H2O2 generation and DNA fragmentation in pancreatic islets. H2O2 as mediator for DNA fragmentation. Diabetes. 1991;40:1141-1145. |
| 25. | Eldor R, Yeffet A, Baum K, Doviner V, Amar D, Ben-Neriah Y, Christofori G, Peled A, Carel JC, Boitard C. Conditional and specific NF-kappaB blockade protects pancreatic beta cells from diabetogenic agents. Proc Natl Acad Sci USA. 2006;103:5072-5077. |
| 26. | Iwakiri R, Nagafuchi S, Kounoue E, Nakamura M, Kikuchi M, Nakano S, Niho Y. Immunohistochemical study of insulitis induced by multiple low doses of streptozocin in CD-1 mice. Diabetes Res Clin Pract. 1990;9:75-82. |
| 27. | Blackwell TS, Christman JW. The role of nuclear factor-kappa B in cytokine gene regulation. Am J Respir Cell Mol Biol. 1997;17:3-9. |
| 28. | Richmond A. Nf-kappa B, chemokine gene transcription and tumour growth. Nat Rev Immunol. 2002;2:664-674. |
| 29. | Adcock IM, Brown CR, Kwon O, Barnes PJ. Oxidative stress induces NF kappa B DNA binding and inducible NOS mRNA in human epithelial cells. Biochem Biophys Res Commun. 1994;199:1518-1524. |
| 30. | Schmidt KN, Amstad P, Cerutti P, Baeuerle PA. The roles of hydrogen peroxide and superoxide as messengers in the activation of transcription factor NF-kappa B. Chem Biol. 1995;2:13-22. |
| 31. | Giannoukakis N, Rudert WA, Ghivizzani SC, Gambotto A, Ricordi C, Trucco M, Robbins PD. Adenoviral gene transfer of the interleukin-1 receptor antagonist protein to human islets prevents IL-1beta-induced beta-cell impairment and activation of islet cell apoptosis in vitro. Diabetes. 1999;48:1730-1736. |
| 32. | Téllez N, Montolio M, Biarnés M, Castaño E, Soler J, Montanya E. Adenoviral overexpression of interleukin-1 receptor antagonist protein increases beta-cell replication in rat pancreatic islets. Gene Ther. 2005;12:120-128. |
| 33. | Arnush M, Heitmeier MR, Scarim AL, Marino MH, Manning PT, Corbett JA. IL-1 produced and released endogenously within human islets inhibits beta cell function. J Clin Invest. 1998;102:516-526. |
| 34. | Corbett JA, McDaniel ML. Intraislet release of interleukin 1 inhibits beta cell function by inducing beta cell expression of inducible nitric oxide synthase. J Exp Med. 1995;181:559-568. |
| 35. | Lee JH, Song MY, Song EK, Kim EK, Moon WS, Han MK, Park JW, Kwon KB, Park BH. Overexpression of SIRT1 protects pancreatic beta-cells against cytokine toxicity by suppressing the nuclear factor-kappaB signaling pathway. Diabetes. 2009;58:344-351. |
| 36. | Heitmeier MR, Scarim AL, Corbett JA. Interferon-gamma increases the sensitivity of islets of Langerhans for inducible nitric-oxide synthase expression induced by interleukin 1. J Biol Chem. 1997;272:13697-13704. |
| 37. | Kharroubi I, Ladrière L, Cardozo AK, Dogusan Z, Cnop M, Eizirik DL. Free fatty acids and cytokines induce pancreatic beta-cell apoptosis by different mechanisms: role of nuclear factor-kappaB and endoplasmic reticulum stress. Endocrinology. 2004;145:5087-5096. |
| 38. | Lv N, Kim EK, Song MY, Choi HN, Moon WS, Park SJ, Park JW, Kwon KB, Park BH. JANEX-1, a JAK3 inhibitor, protects pancreatic islets from cytokine toxicity through downregulation of NF-kappaB activation and the JAK/STAT pathway. Exp Cell Res. 2009;315:2064-2071. |
| 39. | Bremner P, Heinrich M. Natural products as targeted modulators of the nuclear factor-kappaB pathway. J Pharm Pharmacol. 2002;54:453-472. |