INTRODUCTION
Physiology of gut motility has been always a fascinating chapter in gastroenterology. It poses interesting challenges to physiologists. Our understanding of basic gut motility processes is advancing; a major emphasis is placed on elucidating gut regulatory mechanisms. A better appreciation of the importance of the presence of normal function of interstitial cells of Cajal (ICC) transformed this field of research. In 1893, Spanish Nobel Laureate physician and neuropathologist Santiago Ramon y Cajal, was the first to describe cells that are located between the nerve endings and smooth muscle cells in the gastrointestinal (GI) tract. Their location prompted him to call them “interstitial”. They are now known as the ICC.
ICC may be considered to be a specialized population of smooth muscle cells. Both arise from common mesenchymal cells[1-3]. However, whereas smooth muscle cells develop an extensive array of contractile elements, ICC have few contractile elements but contain large numbers of mitochondria, an abundance of endoplasmic reticulum and distinct sets of channels in their membrane. The ICC consist of a fusiform cell body with a thin cytoplasm, a large oval nucleus and dendritic-like processes[4]. Two to five primary dendritic processes divide further into secondary and tertiary processes[5]. Many ICC express Kit, a tyrosine kinase receptor (Kit-ir); this allows them to be recognized by their ability to bind antibodies to Kit[4]. Similarly ICC readily react with antibodies to vimentin whereas nearby smooth muscle cells do not[6]. The presence of ICC is not restricted to the GI tract. They can be found in the bladder[7,8], the ureteropelvic junction[9], the vas deferens[10], the prostate[11], the penis[12,13], the mammary gland, the uterus[14], the pancreas[15], blood vessels[16] such as the portal vein[17] and the vagina[18]. More recently, they have been found in the vermiform appendix in childhood[19]. Some of these cells are thought to have a pacemaker function (such as those in the portal vein, in the lymphatics or prostate) but not those in the arteries, uterus (where the influence is, if any, an inhibitory one) or bladder[20].
The motor activity of the GI tract is critical for life[21]. It is a complex process involving multiple cell types such as enteric neurons that can sense the contents of the GI tract, integrate information and devise a suitable motor pattern, ICC that transduce inputs from enteric motor neurons and generate intrinsic electrical rhythmicity, and smooth muscle cells that can interpret and integrate large arrays of inputs and develop appropriate responses[22]. ICC are a minor component of the tunica muscularis of the GI tract (only about 5% of cells present[23]); however, these cells have very significant physiological roles in GI motility[22].
Many tissues, isolated from different regions of the GI tract, contract rhythmically in the absence of neuronal or hormonal stimulation. When contractions and membrane potential are recorded simultaneously each contraction is seen to be triggered by a long lasting wave of depolarization: because of their low frequency of occurrence and long duration, the waves of depolarization have been termed slow waves[24]. The origin and basis of the generation of slow waves have been debated for many years. It was initially thought that the generation of slow waves reflected some properties of GI smooth muscle cells[25,26], but studies on isolated smooth muscle cells have consistently failed to demonstrate a capability to generate slow wave activity[27]. It has also long been recognized that the generation of slow waves does not rely on the sequential activation of voltage-dependent ion channels as do cardiac pacemaker cells. Rather, many early studies raised the possibility that rhythmical activity relied on the cycling of one or more metabolic processes within cells of the gut wall. Thus Conner and his colleagues proposed that the generation of slow waves involved changes in the activity of the sodium pump[25].
Subsequently Nakayama et al[28] suggested an involvement of glycolytic pathways, again assuming that pace making activity originated in smooth muscle cells. Although ICC were first described in the intestine a century ago by Cajal, they were long viewed as an oddity. Their role in the generation of pacemaker activity in the GI tract was suggested on the basis of histological studies[29]. More recently, studies on mutants that lack subpopulations of ICC revealed their role in the generation of rhythmicity[30].
Critically, whereas isolated smooth muscle cells rarely generate spontaneous electrical activity[27], isolated ICC invariably do[31,32].
STRUCTURAL ORGANIZATION AND IDENTIFICATION OF SPECIFIC POPULATIONS OF ICC
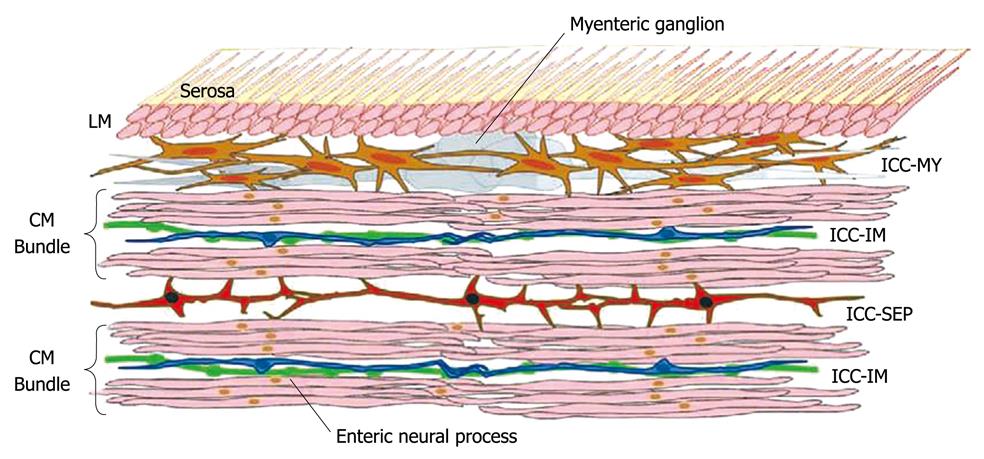
The discovery that ICC express c-Kit, the proto-oncogene that encodes the receptor tyrosine kinase Kit has offered a simple and reliable immunohistochemical method for determining the structure and distribution of ICC networks[30]. ICC are found throughout the GI tract from the esophagus to the internal anal sphincter[33,34]. Hanani et al[35] mentioned that while it is becoming clear that more than one type of ICC exists, based on both morphological and functional data, we still subdivide ICC based on location. Furthermore, Farrugia[36] emphasized the importance of revisiting a classification based solely on location and move towards a classification that is based on function, suggesting a reasonable start, to subdivide ICC into those that have the machinery to, and generate, unitary potential and slow waves and those that do not. Morphological studies now supported by some functional evidence suggest that at least three separate functional groups of ICC exist. In most regions of the GI tract, a network of ICC are located within the intermuscular space at the level of the myenteric plexus (ICC-MY) between the circular and longitudinal muscle layers. ICC-MY are the pacemaker cells in the stomach and small intestine that trigger the generation of slow waves in the tunica muscularis[37].
A second population of ICC (referred to as intramuscular ICC or ICC-IM) are found within the muscle layers of the GI tract and are innervated preferentially by enteric motor nerves[37]. ICC-IM are closely associated with not only enteric motor nerves but also vagal afferent nerves. Vagal afferent nerve fibers, labeled by the injection of neural tracers into the nodose ganglia, can terminate as intramuscular arrays within the musculature and as intraganglionic laminar ending within the myenteric ganglion of the stomach and duodenum. These afferent fibers transmit mechanoreceptive information from the muscle wall[38,39]. Horiguchi et al[40] gave histological evidence that a third population of ICC, ICC-SEP, lies within the septa between the circular muscle bundles, and suggested that it may play a role in conducting electrical information from ICC-MY deep into the distant circular muscle bundles, Figure 1 showing functional organization of ICC in the canine gastric antrum[41].
Figure 1 Diagram showing functional organization of ICC in the canine gastric antrum[41].
ICC: Interstitial cells of Cajal.
Electrophysiological data are presented which indicate that when the normal pathway from ICC-MY is sectioned, electrical stimulation of the cut ends of the muscle bundles can initiate slow waves over considerable distances. In the absence of stimulation, the muscle bundles isolated from ICC-MY can generate rhythmical activity but do so at low frequencies. Thus a distinct population of ICC, ICC-SEP, exists which can transfer pacemaker depolarization from ICC-MY deep into the distant bundles of circular muscle. Although ICC-SEP have the potential to generate pacemaker activity they are not normally the dominant pacemaker centre. As an analogy with the generation of pacemaker activity in the heart, the plexus of ICC-MY, like the sino-atrial node, is the dominant pacemaker centre. ICC-SEP, like Purkinje fibers, have the potential to generate pacemaker activity, but normally function to convey electrical activity from the dominant pacemaker region to more distant tissues[41].
PHYSIOLOGICAL FUNCTIONS OF ICC
Peristaltic motor activity is a motor pattern orchestrated by complex sequencing of neural excitation and inhibition in cooperation with intrinsic muscular control mechanisms, including those residing in ICC[42]. Peristalsis is defined as waves of contraction propagating along the GI tract for various distances as a means of mixing and propelling its content distally. Both the type of neural activity and the type of intrinsic myogenic control mechanism differ widely throughout the GI tract[42]. Physiological activation of peristalsis will in most cases involve the stretching of a segment of stomach, intestine, or colon and it will occur by neural pathways that contain additional mechanisms to those required for the ascending excitatory reflex[43]. When peristaltic motor activity occurs, in particular in stomach and proximal small intestine, the waves of contraction always have rhythmicity to it. This rhythmicity is determined by electrical slow wave activity in the musculature, referred to as pace maker activity[44].
New reagents, coupled with immunohistochemical techniques and new electrophysiological experimental approaches opened the door to recent progress in identification of the important roles of ICC as pacemakers, in propagation of slow waves and as mediators of inputs from enteric motor neurons[45]. Other functions, such as mechanosensors have also been proposed, but little physiological evidence supporting this function has been published[45].
Laboratory approaches used for ICC study
Isolated ICC have been examined using conventional patch clamp recording techniques. This approach, which has been applied to ICC-MY, allows a description of the specific populations of ion channels present in their membrane[31,32,46] and an analysis of the cellular mechanisms which regulate the channels[47,48]. Simple intracellular recording from smooth muscle cells in isolated segments of GI tissues and isolated segments of urethra, after blocking smooth muscle L-type Ca2+ channels, record primarily the activity of the ICC in the tissues. The properties of ICC-MY can be determined in situ using sharp electrodes, allowing one to monitor the behavior of populations of interconnected ICC-MY and to determine how pacemaker potentials generate signals in adjacent smooth muscle layers[47,49,50]. A third method used to study the properties of ICC IM involves recording from small isolated segments of circular muscles; if dissected appropriately the preparations are isopotential and contain up to 2000 smooth muscle cells linked to up to 200 ICC-IM. The membrane potential of both smooth muscle cells and ICC-IM, can be varied over a limited range and the effects of nerve stimulation can be analyzed[51-53]. Finally, the use of mutant mice in which specific sets of ICC are either absent of dramatically reduced in numbers has allowed an evaluation of the physiological properties of tissues, with and without different sets of ICC[24].
COMMON GI MOTILITY PROBLEMS
ICC in the human esophagus and cardia
ICC in human esophagus has a myoid ultrastructure with abundant smooth endoplasmic reticulum, numerous mitochondria, intermediate filaments, scattered caveolae, and discontinuous basal lamina. They are most frequent in the esophageal part of the lower esophageal sphincter (LES) but rare in the gastric part. They are in close contact with nerve terminals and make specific junctions with smooth muscle cells[59,60].
Achalasia: Achalasia is a disorder of esophageal motility that has been well documented for over 300 years[61]. Achalasia is characterized by relaxation failure of the LES and lack of peristaltic contraction of the esophageal body[62]. The mechanism of LES relaxation is complex, requiring the coordinated interaction of nerves, smooth muscle, ICC and hormones. The LES is a functional and anatomic barrier between the stomach and esophagus. It consists of a thickening of the circular smooth muscle layer of the esophagus at the gastroesophageal junction. It is anatomically asymmetric, and this is reflected in the physiology of the sphincter as demonstrated by ultrasound and pharmacologic manometric studies[63]. The LES is tonically contracted. Initiation of a peristaltic wave in the esophagus is accompanied by a decrease in LES pressure as a result of smooth muscle relaxation. This allows the swallowed bolus to enter the stomach[61].
ICC involvement in achalasia is debated. Electron microscope studies of the muscle coat of the LES in seven patients with achalasia showed that muscle wall components (nerve endings, smooth muscle cells, ICC and connective tissue) were modified. ICC ultrastructure was altered, namely clear cytoplasm, fewer mitochondria, and scarce smooth endoplasmic reticulum[64].
A reduced number of contacts between nerves and ICC were reported. Specific changes in smooth muscle cells were also documented, whereas the nerve endings had abnormal ultrastructure. Alterations in older patients were more pronounced[65]. Since the LES components specifically altered in achalasia are the nerve endings and ICC, they are regarded as principally responsible for abnormal motility[65].
Achalasia is uncommon among the pediatric population. It is usually sporadic and affects mainly teenagers[66]. A rare familial form combining early onset achalasia of cardia, alacrymia (absence of tears), and ACTH insensitivity, are known as Allgrove’s syndrome[67,68] or “Triple A” syndrome[69]. These forms are inherited on the autosomal recessive mode[70]. Massive loss of neural elements and neuronal nitric oxide synthase as well as a marked fibrotic process of the muscle layers of the cardia have been observed in “Triple A” syndrome[71]. ICC in the cardia are also markedly diminished or are completely absent while ICC (and neural structures) are preserved in the pylorus[59].
Gastroesophageal reflux: Gastroesophageal reflux (GERD) is a common condition and its prevalence varies in different parts of the World[72]. Typical symptoms of heartburn and acid regurgitation are encountered in 15%-20% of the general population[62]. The major mechanism for GERD is transient relaxation of the LES[73]. The role of the ICC in inhibitory transmission in the LES is still being discussed[62].
In W/Wv mutant mice (lack of ICC) LES pressure was lower than wild-type mice but a normal swallow still induced LES relaxation, arguing against the role of ICC in inhibitory transmission[74]. Another study demonstrated that in W/Wv animals, cholinergic and nitrergic neurotransmission is greatly reduced pleading for the role of ICC in mediating neural inputs[37]. However, enteric neurons, varicose processes, and the ability to release neurotransmitters are not reduced, and smooth muscle cells demonstrate responsiveness to exogenous transmitters[37].
Loss of ICC during development or in pathologic conditions would significantly compromise the ability of GI muscles to generate typical motor reflexes[75].
Esophagitis itself may be at the origin of an alteration of normal function of the Cajal cells: in advanced stages of GERD, inflammatory changes in the esophageal wall will also involve the ICC. That way, the more severe the esophagitis, the more severe is the ICC impairment. This destruction leads to loss of effective contraction of esophagus, maintaining reflux and thus aggravating the symptoms[76].
ICC in the human stomach and pylorus
Gastroparesis: The pathogenesis of gastroparesis is complicated and poorly understood. This lack of understanding remains a major impediment to the development of effective therapies for this condition. Most of the scientific information available on the pathogenesis of gastroparesis has been derived from experimental studies of diabetes in animals. These studies suggest that the disease process can affect nerves (particularly those producing nitric oxide, but also the vagus nerve), ICC and smooth muscle[77]. It is broadly defined as disordered gastric emptying, and is a commonly encountered clinical problem[78]. Delayed gastric emptying can be secondary to muscular, neural, humoral causes or use of anticholinergic and opiate medicines. In the absence of an identified cause, gastroparesis is termed as idiopathic[79]. Gastroparesis has a broad range of clinical presentations ranging from dyspeptic symptoms to nausea, vomiting, abdominal pain, malnutrition, frequent hospitalizations and incapacitation[80], chronic abdominal pain and vomiting leading to dehydration, electrolyte imbalance, nutritional impairment and weight loss[81].
The ICC are fundamental in the generation of gastric slow waves[79]. A decrease in ICC density ranging from 60%-100% depending on the area investigated was demonstrated in histologic studies of the stomach of type 1 diabetic patients[55]. The number of immunopositive cells for c-kit was significantly decreased in the corpus and antrum of the gastroparesis patients compared with control tissues[62]. The loss of intramuscular ICC and associated nerves in the gastric fundus could explain the low basal gastric tone and increased compliance of the stomach. The hypomotility of the antrum can also be explained by the absence of slow wave generation by the ICC[23].
Infantile hypertrophic pyloric stenosis: Infantile hypertrophic pyloric stenosis (IHPS) is common in infants, characterized by marked delayed gastric emptying and hypertrophy of the inner (circular) muscle layer of the pylorus[59]. IHPS has been known for more than a century[82] but it remains a puzzling disorder[83]. The genetic susceptibility to development of IHPS seems to be multifactorial[84]. Hypertrophy of the pyloric musculature develops after birth[85] and produces the characteristic palpable pyloric “olive.” The pyloric lumen is however not fully occluded[86] and can be intubated relatively easily[87], suggesting that the obstruction of the gastric outlet in IHPS is not merely due to a mechanical obstruction by the hypertrophied musculature. The extent of muscle hypertrophy appeared to be unrelated to the age or duration of symptoms[88].
Various neurotransmitters[89-91] and the neuronal isoform of NO synthase[92] are reduced or lacking in the hypertrophic musculature. The increased thickness of the pyloric muscular coats appears to be due to hypertrophy, rather than to hyperplasia, of the smooth muscle cells[93]. ICC, identified either by electron microscopy[94] or by Kit-ir[95,96] were consistently lacking in the hypertrophic circular muscle layer. However, Kit-ir cells, similar to Kit-ir ICC observed in controls, were observed in the innermost part of the hypertrophic pylorus and in the antrum, indicating that the lack of Kit-ir is restricted to the hypertophic pyloric musculature[95].
The lack of ICC in IHPS may interfere with the propagation of slow waves and may be, at least partly, involved in antro-pyloric incoordination[59]. Homozygous transgenic mice carrying inactivated genes (‘‘knock-out’’) coding for the neuronal NO synthase developed hypertrophy of the pylorus[97]. The link between the lack of ICC, the lack of inhibitory nitrergic neurotransmission, and the hypertrophy of the smooth musculature in IHPS remains to be elucidated[59].
Small intestine and colon
Hirschsprung’s disease: Hirschsprung’s disease (HD) is characterized by the lack of intrinsic enteric nervous system (ENS) in the distal part of the GI tract (‘‘aganglionosis’’). The affected segment extends cranially from the anus and encompasses a variable portion of the gut. Functionally, the lack of propulsive movements may lead either to an early obstructive syndrome in infancy or to a severe constipation[98]. Lack of slow wave activity in the aganglionic segment has been identified[99]. Kit immunohistochemistry identified ICC in HD. However, the cellular density of Kit+ ICC appeared markedly reduced in the aganglionic segment[100]. ICC-MP were rather abundant in the (aganglionic) space between the muscle layers. Kit+ ICC were specially scarce in the inner part of the circular musculature and in the submuscular plexus. However, the presence of some ICC-SMP was confirmed by electron microscopy.
In contrast, another study reported a distribution of Kit+ ICC in HD comparable to controls and claimed that Kit1 ICC-MP form “normal” networks in aganglionic segments when studied by confocal microscopy on whole mount preparations[101].
Differences in interpretation may be less significant than it appears as there is an agreement in the literature to acknowledge the presence of a number of interconnecting ICC-MP in aganglionic segments but there is no objective criterion to assess the “normality” of networks. Considering the very close relationships of ICC with intrinsic nerves and glial cells in the normal gut, a normal arrangement of ICC appears quite unlikely in the absence of both intrinsic nerves and glial cells as encountered in aganglionic segments. In the embryonic chicken[1] or mouse[102] gut experimentally deprived of neural crest derivatives, ICC develop in the absence of ENS, confirming the mesenchymal nature of ICC. But it has not been established if ICC fully develop morphologically and functionally in such conditions.
HD is a heterogenous, multigenic disease and reviewing its genetic aspects is beyond the scope of this paper. Several systems regulating neural crest migration have recently been identified[103]. Some genes are expressed by the neural crest, others by the mesenchyme of the gut. Kit has previously been considered as a possible candidate in the search for genes involved in hereditary forms of HD[104] but the absence of linkage between HD and the region of the Kit gene has been more recently reported[105]. The genetic defects leading to aganglionosis in the HD patients enrolled in all studies on ICC published so far have not been assessed. Subtle differences may explain the discrepancies observed between studies, and a link between some specific genetic defect leading to aganglionosis and the differentiation of ICC in HD patients cannot be ruled out.
Intestinal neural dysplasia: A clinical condition that resembles HD was first described by Meier-Ruge[106] in 1971 as a malformation of the enteric plexus. In 1983, Fadda et al[107] subclassified intestinal neural dysplasia (IND) into two clinically and histologically distinct subtypes. Type A occurs in less than 5% of cases, is characterized by congenital aplasia or hypoplasia of the sympathetic innervations, and presents acutely in the neonatal period with episodes of intestinal obstruction, diarrhea and bloody stools. The clinical picture of Type B resembles HD and is characterized by malformation of the parasympathetic submucous and myenteric plexuses and accounts for over 95% of cases of isolated IND. IND occurring in association with HD is of Type B[108]. IND have been reported to be associated with loss or deficiency of ICC networks[109].
Chronic intestinal pseudo-obstruction: Chronic intestinal pseudoobstruction (CIPO) is characterized by defective GI propulsion together with symptoms and signs of bowel obstruction in the absence of any lesions or mechanical obstacle[110]. It is generally a serious, even life-threatening, condition with frequent need for long-term parenteral nutrition. CIPO can either be restricted to the intestine, can involve other parts of the GI tract, or can be part of a multisystemic disorder[59]. CIPO can be secondary to a number of identified disorders or can be “idiopathic”[111]. Very little is known about the etiology of idiopathic CIPO. Pathological features of CIPO are pleiomorphic. A number of alterations of the ENS (‘‘neuropathic” forms)[112] and “myopathic” forms, limited to the musculature of the GI tract or involving also the musculature of the urinary system[113,114], have been described.
Slow transit constipation: Functional constipation encompasses a group of functional disorders that exhibit persistent difficult, infrequent, or seemingly incomplete defecation and infrequent, lumpy, or hard stools[115,116]. This symptom is very common and may occur in up to 20% of populations, depending on demographic factors, sampling, and the definitions employed[115,117]. The term constipation is probably better viewed as a sort of semantic umbrella, covering pathophysiologic subtypes, among which 2 major groups may now be identified: slow transit constipation (STC) and pelvic floor dysfunction[118].
STC is thought to have, as a primary defect, slower than normal movement of contents from the cecum to the rectum[119]. This is a very prevalent motility problem, but its mechanisms are unclear[62]. Although STC may not be a congenital disease, the frequent onset in adolescence and strong female predominance suggest that STC could be a result of a sex modified multifactorial disorder of the GI tract with a genetic basis[120].
ICC volume was significantly lower in the STC patien+cross all colonic regions[121]. Expression of c-kit mRNA and c-kit protein was significantly decreased in the colon of STC, suggesting that the c-kit signal pathway may play an important role in ICC reduction in STC[122]. Shafik et al[123] concluded that a disorder of the ICC, which generate electric activity, may have a role in inducing diminished or absent colonic motor activity, a point that should be further investigated.
TUMORS OF GI TRACT
GI stromal tumors (GISTs) have been recognized as a biologically distinctive tumor type, different from smooth muscle and neural tumors of the GI tract. They constitute the majority of GI mesenchymal tumors[124].
GISTs exhibit considerable phenotypic heterogeneity[125]. Their origin remains unclear, although origin in smooth muscle cells has been proposed[126,127]. CD34-ir is often present in GIST[125,128-130], a property shared with various other solid tumors[131,132].
Kit-ir may be a suitable marker for GIST[133], possibly superior to CD34-ir[134]. Mutations (usually activating) of the proto-oncogene Kit have been identified in GIST[133,135-137]. GIST with Kit mutation appear to have a poorer prognosis[133,135-138]. Therefore Kit mutations may merely be part of the oncogenic process rather than an indication of the origin of these tumors.
Recent studies suggesting that ICC in the human gut were both Kit-ir and CD34-ir raised the idea that Kit+ CD34+ GIST may derive from ICC[128,133,137].
The majority of GISTs occurs in the stomach (60%-70%), small intestine (20%-30%) and only 10% or less in the esophagus, colon and rectum, and they affect mainly middle aged patients. Similar tumors, sometimes known as extra-GIST, may arise in the omentum, mesentery, or retroperitoneum and at least one case of pancreatic tumor was described[139,140]. The presence of ICC in normal pancreas was demonstrated recently[15].
The symptoms may vary from none or slight abdominal discomfort to brisk GI hemorrhage, perforation or obstruction.
Imatinib mesylate, a synthetic tyrosine kinase inhibitor developed for the use in the management of interferon resistant chronic myeloid leukemia, was shown to be effective against a number of other tyrosine kinases including c-kit and platelet derived growth factor and now it is considered to be the drug of choice for metastatic and inoperable GISTs[124,141].