Published online Jun 28, 2010. doi: 10.3748/wjg.v16.i24.3072
Revised: March 15, 2010
Accepted: March 22, 2010
Published online: June 28, 2010
AIM: To develop the simple, rapid and sensitive dual-label time-resolved fluoroimmunoassay for pepsinogens in human serum.
METHODS: Based on two-site sandwich protocol, monoclonal antibodies (McAbs) against pepsinogen I (PG I) and PG II were co-coated in 96 microtitration wells, and tracer McAbs against PG I and PG II were labeled with europium (Eu) and samarium (Sm) chelate, respectively. Diluted serum samples of Eu3+- and Sm3+-McAbs were added into microtitration wells simultaneously. After washing, fluorescence of bound Sm3+ and Eu3+ tracers was detected.
RESULTS: The detection limit was 0.2 μg/L for PG I and 0.05 μg/L for PG II. The assay range was 5.0-320.0 μg/L for PG I and 1.0-55.0 μg/L for PG II. The average recovery rate was 102.7% for PG I and 98.8% for PG II. Sera from healthy controls and patients with gastric disease were analyzed. The PG detected by dual-label assay was in good agreement with that detected by single-label assay or by enzyme-linked immunosorbent assay.
CONCLUSION: Dual-label assay can provide high-throughput serological screening for gastric diseases.
- Citation: Zhang J, Guo JZ, Xiao HL, Zhu L, Liu HY, Zhang Y, Huang B. Simultaneous detection of different serum pepsinogens and its primary application. World J Gastroenterol 2010; 16(24): 3072-3077
- URL: https://www.wjgnet.com/1007-9327/full/v16/i24/3072.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i24.3072
There is considerable interest in developing methods to simultaneously quantify two or more analyses of one sample in a single assay[1,2], which is advantageous to the confidence level of results, especially in cases when the ratio of compounds gives important information[3,4]. Dual-label has potential applications in various fields such as microbiology, molecular biology, drug analysis, and clinical research. Fluorescence immunoassay, like other immunoassays involving non-isotopic labeling, has been well accepted as a stable, inexpensive, rapid, and sensitive method. However, conventional fluorescent labeling has a limited success in assay of multiple analytes because of its high background, short decay time and broad spectrum, which make it difficult to be distinguished between its emission bands[5,6]. Up to now, fluorescent lanthanide is a favorable choice owing to its narrow emission peak at different wavelengths. Its lifetime ranges 50-1000 μs (over four decades longer than the average background duration) depending on the temperature and the solvent presented[7]. These features can be utilized for optimization of the measurement conditions to get the maximal sensitivity and to minimize the signal spillover. The europium ion (Eu3+), is the lanthanide mainly used in time-resolved fluoroimmunoassay (TRFIA)[8]. Eu3+ and terbium ion (Tb3+) form the most efficient fluorescent chelates, but Tb3+ requires fluorinated aliphatic β-diketone for simultaneous detection[9], rather than β-naphthoyltrifluoroacetone (β-NTA) used in the enhancement solution optimized for Eu3+ detection. β-NTA is also applicable to samarium ion (Sm3+) excitation, which has thus been suggested that Sm3+ can be used as a counterpart to Eu3+ with the same enhancement formulation (enhancement solution or DELFIA inducer) in a dual-label system[10].
Human pepsinogens originating from gastric mucosa can be classified into two immunochemically distinct groups: pepsinogen I (PG I) and PG II[11], which are mostly secreted into the gastric lumen and nearly 1% of them are leaked into the blood circulation. Serum PG levels reflect the morphological and functional status of gastric mucosa. Human pepsinogens have a diagnostic value for various gastroduodenal disorders, especially for peptic ulcer and atrophic gastritis, which have been widely discussed[12,13]. The PG I/PG II ratio can provide even better information on the extent of chronic gastritis than gastric intubation[14].
Since PG I and PG II serve as useful predictors in early diagnosis of gastric cancer and in mass screening of populations at a high risk of gastric cancer[15,16], a reliable and sensitive method is needed to detect PG I and PG II in human sera. In our previous study[17], a fast and highly sensitive TRFIA was developed to measure serum PG I and PG II. The present study was to evaluate the dual-label TRFIA for simultaneous detection of PG I and PG II in human serum.
Diethylenetriaminepentaacetate (DTPA), bovine serum albumin (BSA), Tris and Triton X-100 were purchased from Sigma (St. Louis, MO, USA). PD-10 column and sepharoseCL-6B column were from the Pharmacia Company (Chalfont St Giles, UK). Q2 anion exchange chromatography, DEAE-52 chromatography, and gel filtration HPLC were purchased from Bio-Rad Company (Hercules, USA). Pure water was produced by Barnstead Equipment (Dubuque, Iowa, USA). Ninety six -well polystyrene microtitre plates were obtained from Nunc International (Roskilde, Denmark). Eu-labeling reagent 1244-302 and Sm-labeling reagent 1244-303, both including N′-[p-isothiocyanatobenzyl]-diethylenetriamine-N1, N2, N3, N4-tetraacetic acid, were purchased from Perkin-Elmer (Waltham, Massachusetts, USA). β-NTA was synthesized in our laboratory. Two counterparts of monoclonal antibodies (McAbs) to human PG I and PG II respectively for capture and detection were obtained from Chinese Institute of Cancer with a purity of over 95% (Beijing, China). Enzyme-linked immunosorbent assay (ELISA) kits for detection of PG I and PG II were from Biohit Plc(Helsinki, Finland). AutoDELFIA1235, from Perkin-Elmer (Waltham, Massachusetts, USA), was used to measure Eu3+ and Sm3+ fluorescence in microtiter wells. All other reagents used were of analytical grade.
Labeling buffer contained 50 mmol/L Na2CO3-NaHCO3 (pH 8.5), and 0.155 mol/L NaCl. Elution buffer contained 50 mmol/L Tris-HC1 (pH 7.8), 0.9% NaC1, and 0.05% NaN3. Assay buffer contained 50 mmol/L Tris-HCl (pH 7.8), 0.9% NaC1, 0.2% BSA, 0.05% NaN3, 20 μmol/L DTPA, and 0.1% Tween-20. Washing solution was a Tris-HCl buffered saline solution (pH 7.8) containing 0.9% NaCl, 0.2% Tween-20, and 0.05% NaN3. Enhancement solution was a 0.1 mol/L acetate-phthalate buffer (pH 3.2) containing 0.1% triton X-100, 15 μmol /L βNTA, and 50 μmol/L tri-n-octylphosphine oxide.
Serum samples, collected from healthy volunteers who had no upper abdominal complaints and evidence of gastroduodenal disorder and liver diseases, were stored at -20°C. Blood samples were collected from patients at endoscopic and histological examinations. This study was conducted with the approval of the Ethics Committee of Jiangyuan Hospital Affiliated to Jiangsu Institute of Nuclear Medicine.
Five micrograms of the capture McAbs to PG I and PG II in 200 μL of 50 mmol of Na2CO3-NaHCO3 buffer (pH 9.6) was co-immobilized in each well and incubated overnight at room temperature. After washing, 200 μL of 1 g/L BSA in 50 mmol of Na2CO3-NaHCO3 buffer (pH 9.6) was added to block the coated surface for 2 h. After the blocking solution was removed, the plates were dried in a high vacuum, and then stored at -20°C in a sealed plastic bag with desiccant.
McAbs to human PG I (PG I McAbs) and PG II (PG II McAbs) were labeled with Sm3+- and Eu3+-chelates, respectively. The buffer for McAbs was replaced with the labeling buffer. Five hundred micrograms of PG I McAbs was gently mixed in 200 μL of labeling buffer with 250 μg of Sm 3+-chelates in 100 μL of the same buffer. After an 18-h incubation with continuous gentle shaking at room temperature, free Sm3+-chelates and aggregated McAbs were separated from Sm3+-McAbs conjugates using a 1 cm × 40 cm column packed with sepharose CL-6B (lower 20 cm), eluted with a descending elution buffer, and collected with 1.0 mL per fraction. The concentration of Sm3+-conjugates in collected fraction was measured with fluorescence, and diluted with an enhancement solution (1:1000). The fluorescence in microtitration wells (200 μL per well) was detected by comparing the signal of samples to that of stock standards diluted at 1:100 in an enhancement solution. The fractions from the first peak with the highest Sm3+ count were pooled and characterized. PG II McAbs were labeled with Eu. The labeled McAbs were rapidly lyophilized under high vacuum after dilution with an elution buffer containing 0.2% BSA as a stabilizer, and stored at -20°C. No loss of immuno-reactivity was observed during a 6-mo storage period.
Surgically resected stomach tissues were free from the invaded part. PG I and PG II were purified by DEAE-52 chromatography, gel filtration HPLC, and Q2 anion exchange chromatography, as previously described[18]. The purity of PG I was over 98% and that of PG II was over 95.0%. Calibrators were prepared by diluting them with the assay buffer containing 0, 5, 10, 50, 100, 300 μg/L of highly purified PG I and 0, 5, 10, 20, 30, 50 μg/L of highly purified PG II, respectively.
Dual-label TRFIA was performed to detect PG I and PG II simultaneously in serum with a one-step “sandwich-type” protocol. In brief, 25 μL of calibrators (samples) and 200 μL of 50-fold diluted Eu3+ and Sm3+ tracer McAbs solution in assay buffer were pipetted into the coated microtitration wells. The plates were incubated with continuous shaking for 2 h at 25°C. After washed 6 times, 200 μL of enhancement solution was added into each well. The plates were shaken for 5 min before fluorescence reading. All procedures were automatically performed by autoDELFIA1235 with the software designed in our laboratory. Calibration curve was plotted and concentrations in unknown samples were measured using Multicalc software program, where a spline algorithm on logarithmically transformed data was employed. ELISA was performed with a kit following its instructions.
Data about PG I or PG II were expressed as mean ± SD. The limit of detection was defined by the concentration of PG I or PG II corresponding to the fluorescence of the zero calibrators plus two SD. The average intra- or inter-assay coefficient of variation (CV) was calculated for the precision of the assay. The recovery rate was evaluated by comparing the measured and theoretical values. Regression analysis was used to display the linearity and correlations. Differences in patients with gastric disease and healthy controls were analyzed using paired t-test. P < 0.05 was considered statistically significant. Analysis of data was performed using SPSS 13.0 (Chicago, IL, USA).
The calibrators covered a range of 10-300 μg/L of PG I and 2-50 μg/L of PG II. The serum samples from healthy controls and patients with chronic atrophic gastritis and peptic ulcer were incubated for different periods of time (60, 90, 120, 150 min) at different temperatures (25°C, 37°C). Both calibrators and serum samples reached a plateau value around 120 min at 25°C and around 60 min at 37°C, respectively. In this study, the incubation time was 120 min and the temperature was 25°C for the assay on autoDELFIA1235.
The calibration curves of PG I and PG II were linear over the concentration. The equation was y = 46.4x + 383.2 for the calibration curve of PG I and y = 2198.2x + 5189.1 for the calibration curve of PG II, where y indicates the response counts (cps), x indicates the concentration (μg/L). With 25 μL of serum samples, the measurement range of PG I, ED20, ED50, and ED80 was 5.0-320.0, 19.87 ± 4.3, 64.32 ± 6.2, and 176.0 ± 12.9 μg/L, respectively, and the measurement range of PG II, ED20, ED50, and ED80 was 1.0-55.0, 3.546 ± 2.2, 9.746 ± 4.7, and 23.79 ± 6.3 μg/L, respectively.
The limit of detection was 0.2 μg/L for PG I and 0.05 μg/L for PG II. The average intra-assay CV of the calibrators was 4.6% for PG I and 5.3% for PG II. The intra- and inter-assay CV of serum-based controls was summarized in Table 1. The results showed that the assay had a good precision not only for the calibrators but also for the clinical samples.
| PG I (μg/L) | PG II (μg/L) | |||
| mean ± SD | CV (%) | mean ± SD | CV (%) | |
| Serum pool 1 | ||||
| Within-run (n = 10) | 43.2 ± 1.36 | 3.2 | 5.23 ± 4.62 | 4.8 |
| Between-run (n = 6) | 42.8 ± 2.28 | 5.1 | 5.65 ± 6.45 | 6.7 |
| Serum pool 2 | ||||
| Within-run (n = 10) | 105.0 ± 1.88 | 2.3 | 11.7 ± 3.66 | 3.9 |
| Between-run (n = 6) | 103.8 ± 2.73 | 3.6 | 10.5 ± 4.55 | 5.1 |
| Serum pool 3 | ||||
| Within-run (n = 10) | 198.2 ± 5.31 | 6.3 | 22.0 ± 3.88 | 4.6 |
| Between-run (n = 6) | 186.7 ± 4.35 | 5.4 | 21.2 ± 5.71 | 8.3 |
The cross-reactivity between anti-PG I antibody to PG II and anti-PG II antibody to PG I was detected. No interference between them was found. The result showed that the specificity of the assay was good.
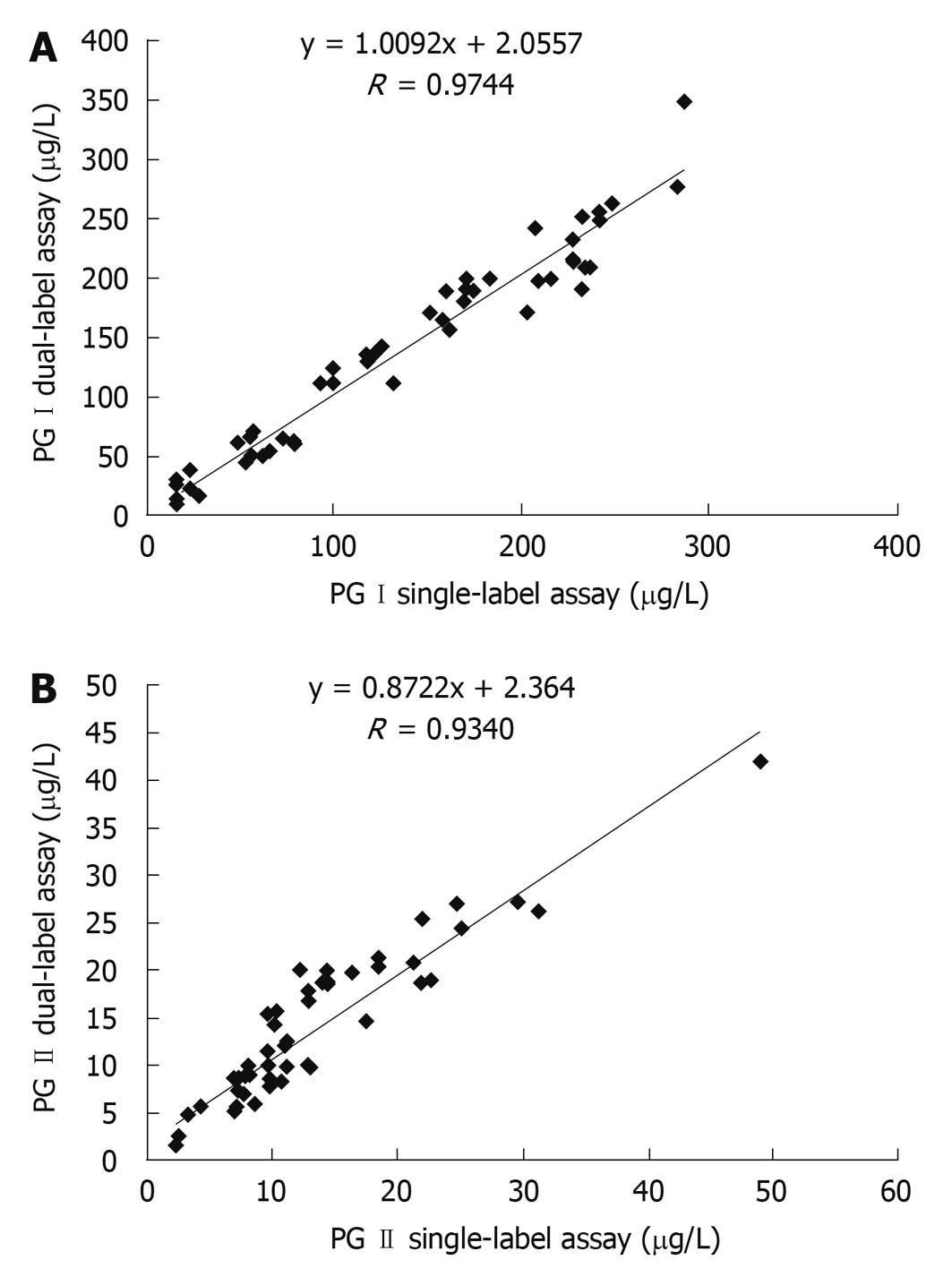
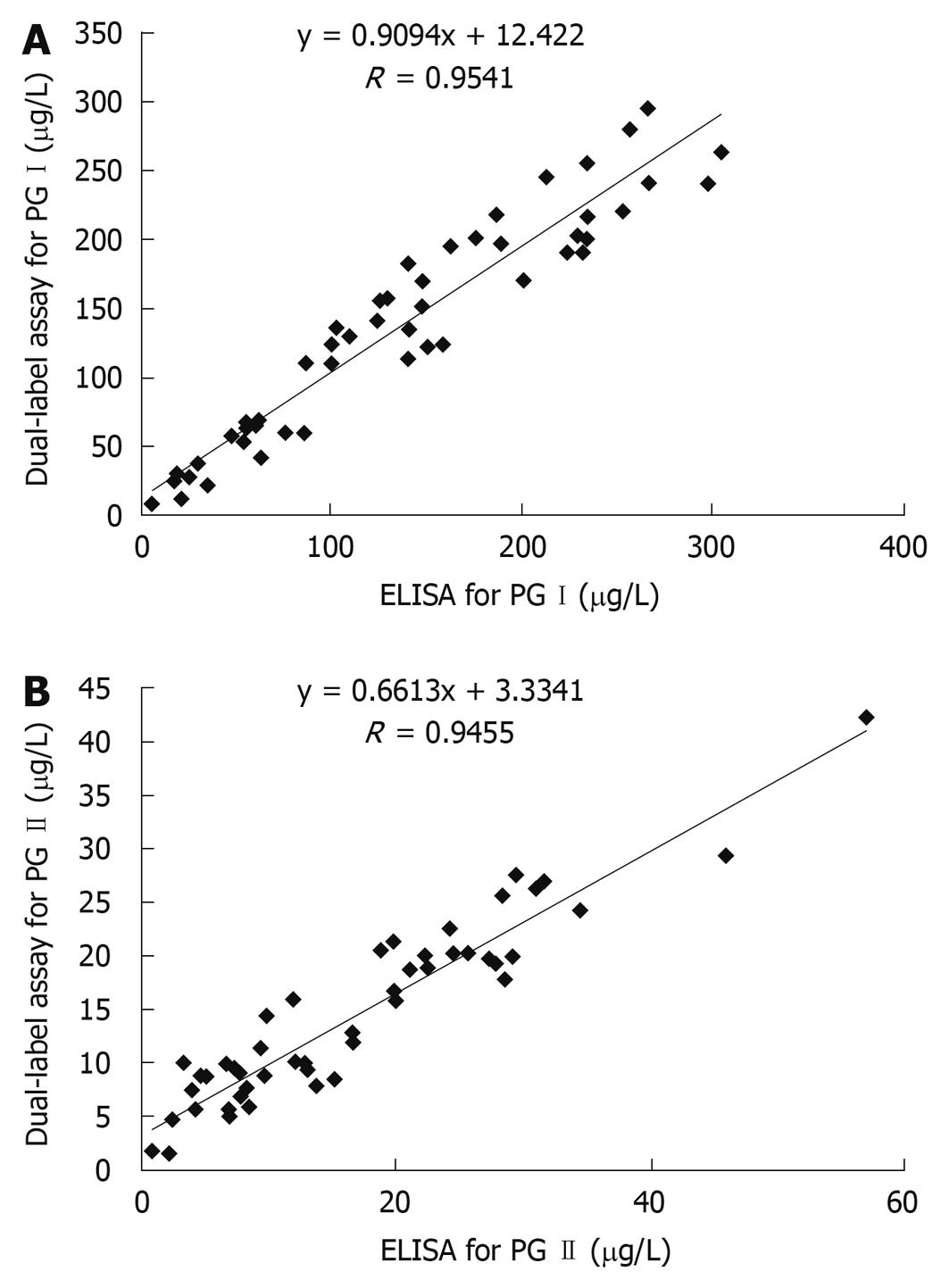
The correlations between dual-label and single-label assay are shown in Figure 1. The correlation ratio between single-label and dual-label assay for PG I and PG II was 0.9744 and 0.9340, respectively. The correlations between dual-label assay and ELISA for PG I and PG II are shown in Figure 2. The correlation ratio between ELISA and dual-label for PG I and PG II was 0.9541 and 0.94550, respectively. The results indicate that the dual-label assay is in agreement with ELISA or with single-label assay.
The serum samples were analyzed by dual-label assay (Table 2). The normal range of serum PG I in healthy controls was 60.1-240.3 μg/L. The range of serum PG II was lower than 27.2 μg/L. The cut-off point was 5.9 for the PG I/PG II ratio.
| Diagnosis | n | Age (yr) | PG I (μg/L) | PG II (μg/L) | PG I/PG II ratio |
| Healthy controls | 500 | 41.5 ± 18.8 | 150.3 ± 45.1 | 10.4 ± 8.4 | 14.5 ± 4.3 |
| Patients with duodenal ulcer | 112 | 43 ± 14.2 | 252.9 ± 84.5d | 18.4 ± 16.0d | 13.7 ± 8.0 |
| Patients with gastric ulcer | 44 | 37 ± 11.2 | 221.2 ± 91.7d | 15.6 ± 12.4b | 14.2 ± 7.1 |
| Patients with atrophic gastritis | 21 | 48 ± 9.7 | 89.5 ± 51.2d | 12.9 ± 9.39 | 6.9 ± 6.2b |
| Patients with superficial gastritis | 76 | 35 ± 7.4 | 175.3 ± 45.8a | 12.7 ± 10.1 | 13.8 ± 5.3 |
| Patients with gastric cancer | 126 | 55 ± 12.2 | 157.1 ± 81.9 | 15.6 ± 14.4b | 13.4 ± 7.8 |
The serum PG level was lower or higher than its normal range in dyspeptic patients including those with gastric cancer. The distributions of PG I and PG II value and PG I/PG II ratio in patients with gastric cancer and in those with duodenal ulcer are shown in Table 3. The difference in PG I value was relatively small. The serum PG I level was remarkably higher in peptic ulcer patients, especially in those with active duodenal ulcer than in healthy controls. The increased PG I level would be a high risk factor for duodenal ulcer, and a remarkably low serum PG I level could exclude the diagnosis of peptic ulcer[19].
| n | PG I (μg/L) | PG II (μg/L) | PG I/PG II ratio | |||||
| < 60 | 60-240 | > 240 | ≤ 27 | > 27 | ≤ 6 | > 6 | ||
| Patients with gastric cancer | 126 | 17 (13.5) | 88 (69.8) | 21 (16.7) | 109 (86.5) | 17 (13.5) | 25 (19.8) | 101 (80.2) |
| Patients with duodenal ulcer | 112 | 0 | 69 (61.6) | 43 (38.4) | 100 (89.2) | 12 (10.8) | 3 (2.7) | 109 (97.3) |
Eu3+ chelate is the most commonly used label in time-resolved fluorometry-based analysis because of its higher fluorescence yield and lower background than other lanthanide complexes. Tb3+ chelate usually has a longer decay time and a higher fluorescence yield than Sm3+ chelate, and their fluorescence is less sensitive to aqueous quenching. However, the relatively shorter emission wavelength of Tb3+ chelate (545 nm) makes it more prone to interference (e.g. phosphorescence) derived from plastic or glass materials. Additionally, it is required to use an aliphatic β-diketone to enhance the fluorescence of Tb3+ in immunoassay for DELFIA-type of multiple analytes[9]. Considering these factors, we selected Eu3+ and Sm3+ as labels in the present study.
As the Sm photoluminescence yield is lower than that of Eu, Sm3+ is usually used as a tracer in assays not requiring a great sensitivity. The detection limit for PG I in dual-label assay is 0.2 μg/L, whereas that of single-label assay is 0.05 μg/L[17]. The sensitivity and precision for PG I can be improved significantly by increasing Sm3+ label yield. The labeling reaction between PG I McAbs and Sm labeling reagent can be prolonged with a suitable excess of the Sm labeling reagents, which may help to get a higher Sm3+ label yield. Despite this, the detection sensitivity for PG I with a limit of 0.2 μg/L is more than adequate for measuring the PG I concentration in clinical samples.
Direct passive absorption of two or more binders is still the routine method for multi-analyte immunoassay (MAIA)[20]. When only the sandwich-type configuration is employed in MAIA, it is necessary to prepare an activated surface binder (e.g. antibody) in order to get a high sensitivity. In this study, the anti-PG I and anti-PG II antibodies were co-coated simultaneously in a strip well, which was found to be beneficial and economical for a suitable fluorescence by adjusting the concentration of coated antibodies. The total concentration of the co-coated antibodies which can achieve favorable results was no more than 5 μg/mL (1000 ng per well) in this study.
Samples with a relatively high PG I or PG II were analyzed at various dilutions. The diluting buffer was identical to the calibrator buffer. The percentage of expected value was 96.3%-101.7% for PG I and 98.1%-109.6% for PG II. No hook effect of dual-label assay was found at a relatively high PG I or PG II concentration. Recovery was identified by supplementing PG I calibrators at 20 μg/L and 100 μg/L, and PG II calibrators at 5 μg/L and 50 μg/L. The average recovery rates for PG I and PG II were 102.7% and 98.8%, respectively, showing that the analytical accuracy is satisfactory for clinical use.
Different analytes (i.e. t-PSA/f-PSA, AFP/CEA) can be detected at present by dual-label TRFIA[3,21] and the corresponding instruments are commercially available. In this study, the human PG detected by dual-label TRFIA was similar to that detected by ELISA and single-label TRFIA. The sensitivity, measurement range and stability of dual-label TRFIA were substantially better than those of ELISA. Unlike radioimmunoassay or ELISA[22,23], dual-label TRFIA can measure the concentration of PG I and PG II, as well as the ratio of PG I/II, thus reducing the random handling errors and increasing the clinical confidence level of PG I/ PG II ratio. Direct labeling of immunoreagents with lanthanide chelates and lack of overlapping between Eu3+ and Sm3+ chelates allow a rapid assay. In addition, 25 μL of samples is enough for the simultaneously detection of PG I and PG II.
In summary, dual-label TRFIA can serve as a high-throughput tool for the detection of serum PG and has good prospects of clinical application.
Non-invasive serum pepsinogen (PG) test provides much information on intestinal metaplasia, atrophic gastritis, as well as Helicobacter pylori infection and peptide ulcer, which has a significant clinical value for the mass screening of patients at a high risk of gastric cancer. A reliable and effective detection method covering a wide concentration range with good sensitivity for PG is required. Furthermore, simultaneous determination of PG I and PG II can improve the confidence level of the PG I/PG II ratio.
In this study, the authors found that multi-analyte immunoassay could increase the throughput and reduce the overall cost per test. A simple, rapid and sensitive dual-label time-resolved fluoroimmunoassay (TRFIA) for pepsinogens in human serum was developed.
TRFIA is a sensitive technique used in analysis of trace substances. Compared with traditional methods, such as radioimmunoassay or enzyme-linked immunosorbent assay (ELISA), dual-label assay can reduce random handling errors and increase the confidence level of PG I and PG II (especially for the PG I/PG II ratio). Only 25 μL of serum samples is enough for each test, which is useful for mass screening.
The PG detected by dual-label TRFIA was in good agreement with that detected by single-label assay or by ELISA. The analytical accuracy, precision and stability are satisfactory for its use in clinical practice. Dual-label TRFIA may serve as a high-throughput tool for the detection of serum PG and has good prospects of clinical application.
The analysis of pepsinogens in serum/plasma of patients is a well established method to identify subjects at a higher risk of developing gastric cancer. The described method for analyzing Pep-I and Pep-II simultaneously seems to have similar parameters in relation to sensitivity and specificity as EIA or ELISA. The manuscript is well written.
Peer reviewer: Dr. Thomas Wex, PhD, Clinic of Gastroenterology, Hepatology and Infectious Diseases, Otto-von-Guericke University Magdeburg, Leipziger Str. 44, Magdeburg, 39120, Germany
S- Editor Tian L L- Editor Wang XL E- Editor Zheng XM
| 2. | Ohkuma H, Abe K, Kosaka Y, Maeda M. Simultaneous assay of pepsinogen I and pepsinogen II in serum by bioluminescent enzyme immunoassay using two kinds of Luciola lateralis luciferase. Anal Chim Acta. 1999;395:265-272. |
| 3. | Mitrunen K, Pettersson K, Piironen T, Björk T, Lilja H, Lövgren T. Dual-label one-step immunoassay for simultaneous measurement of free and total prostate-specific antigen concentrations and ratios in serum. Clin Chem. 1995;41:1115-1120. |
| 5. | Blake C, Al-Bassam MN, Gould BJ, Marks V, Bridges JW, Riley C. Simultaneous enzyme immunoassay of two thyroid hormones. Clin Chem. 1982;28:1469-1473. |
| 6. | Dean KJ, Thompson SG, Burd JF, Buckler RT. Simultaneous determination of phenytoin and phenobarbital in serum or plasma by substrate-labeled fluorescent immunoassay. Clin Chem. 1983;29:1051-1056. |
| 8. | Hemmilä I, Dakubu S, Mukkala VM, Siitari H, Lövgren T. Europium as a label in time-resolved immunofluorometric assays. Anal Biochem. 1984;137:335-343. |
| 9. | Hemmilä I. Time-resolved fluorometric determination of terbium in aqueous solution. Anal Chem. 1985;57:1676-1681. |
| 10. | Bador R, Déchaud H, Claustrat F, Desuzinges C. Europium and samarium as labels in time-resolved immunofluorometric assay of follitropin. Clin Chem. 1987;33:48-51. |
| 11. | Samloff IM. Pepsinogens, pepsins, and pepsin inhibitors. Gastroenterology. 1971;60:586-604. |
| 12. | Kitahara F, Kobayashi K, Sato T, Kojima Y, Araki T, Fujino MA. Accuracy of screening for gastric cancer using serum pepsinogen concentrations. Gut. 1999;44:693-697. |
| 13. | Miki K, Ichinose M, Shimizu A, Huang SC, Oka H, Furihata C, Matsushima T, Takahashi K. Serum pepsinogens as a screening test of extensive chronic gastritis. Gastroenterol Jpn. 1987;22:133-141. |
| 14. | Yoshihara M, Sumii K, Haruma K, Kiyohira K, Hattori N, Tanaka S, Kajiyama G, Shigenobu T. The usefulness of gastric mass screening using serum pepsinogen levels compared with photofluorography. Hiroshima J Med Sci. 1997;46:81-86. |
| 15. | Ohata H, Oka M, Yanaoka K, Shimizu Y, Mukoubayashi C, Mugitani K, Iwane M, Nakamura H, Tamai H, Arii K. Gastric cancer screening of a high-risk population in Japan using serum pepsinogen and barium digital radiography. Cancer Sci. 2005;96:713-720. |
| 16. | Hattori Y, Tashiro H, Kawamoto T, Kodama Y. Sensitivity and specificity of mass screening for gastric cancer using the measurment of serum pepsinogens. Jpn J Cancer Res. 1995;86:1210-1215. |
| 17. | Huang B, Xiao H, Zhang X, Zhu L, Liu H, Jin J. Ultrasensitive detection of pepsinogen I and pepsinogen II by a time-resolved fluoroimmunoassay and its preliminary clinical applications. Anal Chim Acta. 2006;571:74-78. |
| 18. | Xiao ZJ, Yang XZ, Jiang MJ, Huang XQ. Purification and Characterization of the Pepsinogen with 67 ku Mass of Molecular. Shengwuhuaxue Yu Shengwuwuli Jinzhan. 1996;23:342-345. |
| 19. | Samloff IM, Stemmermann GN, Heilbrun LK, Nomura A. Elevated serum pepsinogen I and II levels differ as risk factors for duodenal ulcer and gastric ulcer. Gastroenterology. 1986;90:570-576. |
| 20. | Pettersson K, Alfthan H, Stenman UH, Turpeinen U, Suonpää M, Söderholm J, Larsen SO, Nørgaard-Pedersen B. Simultaneous assay of alpha-fetoprotein and free beta subunit of human chorionic gonadotropin by dual-label time-resolved immunofluorometric assay. Clin Chem. 1993;39:2084-2089. |
| 21. | Matsumoto K, Yuan J, Wang G, Kimura H. Simultaneous determination of alpha-fetoprotein and carcinoembryonic antigen in human serum by time-resolved fluoroimmunoassay. Anal Biochem. 1999;276:81-87. |
| 22. | Huang SC, Miki K, Furihata C, Ichinose M, Shimizu A, Oka H. Enzyme-linked immunosorbent assays for serum pepsinogens I and II using monoclonal antibodies--with data on peptic ulcer and gastric cancer. Clin Chim Acta. 1988;175:37-50. |