Published online Jun 28, 2010. doi: 10.3748/wjg.v16.i24.2994
Revised: April 23, 2010
Accepted: April 30, 2010
Published online: June 28, 2010
AIM: To investigate the effect of emodin on expression of claudin-4, claudin-5 and occludin, as well as the alveolar epithelial barrier in rats with pancreatitis induced by sodium taurocholate.
METHODS: Experimental pancreatitis was induced by retrograde injection of 5% sodium taurocholate into the biliopancreatic duct. Emodin was injected via the external jugular vein 3 h after induction of acute pancreatitis. Rats from sham operation group and acute pancreatitis group were injected with normal saline (an equivalent volume as emodin) at the same time point. Samples of lung and serum were obtained 6 h after drug administration. Pulmonary morphology was examined with HE staining. Pulmonary edema was estimated by measuring water content in lung tissue samples. Tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) level were measured by enzyme-linked immunospecific assay. Serum amylase and pulmonary myeloperoxidase (MPO) activity were detected by spectrophotometry. Alveolar epithelial barrier was assessed by pulmonary dye extravasation. Expression of claudin-4, claudin-5 and occludin in lung tissue samples was examined by immunohistology, quantitative real-time reverse transcription polymerase chain reaction and Western blotting analysis, respectively.
RESULTS: Pancreatitis-associated lung injury was characterized by pulmonary edema, leukocyte infiltration, alveolar collapse, and elevated serum amylase level. The pulmonary damage, pulmonary pathological scores, serum amylase and MPO activity, TNF-α and IL-6 levels, and wet/dry ratio were decreased in rats after treatment with emodin. Immunostaining of claudin-4, claudin-5 and occludin was detected in lung tissue samples from rats in sham operation group, which was distributed in alveolar epithelium, vascular endothelium, and bronchial epithelium, respectively. The mRNA and protein expression levels of claudin-4, claudin-5 and occludin in lung tissue samples were markedly decreased, the expression level of claudin-4, claudin-5 and occluding was increased, and the pulmonary dye extravasation was reduced in lung tissue samples from rats with acute pancreatitis after treatment with emodin.
CONCLUSION: Emodin attenuates pulmonary edema and inflammation, enhances alveolar epithelial barrier function, and promotes expression of claudin-4, claudin-5 and occludin in lung tissue samples from rats with acute pancreatitis.
- Citation: Xia XM, Wang FY, Wang ZK, Wan HJ, Xu WA, Lu H. Emodin enhances alveolar epithelial barrier function in rats with experimental acute pancreatitis. World J Gastroenterol 2010; 16(24): 2994-3001
- URL: https://www.wjgnet.com/1007-9327/full/v16/i24/2994.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i24.2994
Acute pancreatitis is a common disease with a considerable morbidity and mortality of 20%[1,2]. Its mortality is attributed to inflammation-related complications, such as pancreatitis-associated lung injury, clinically presenting as adult respiratory distress syndrome[2-4]. Intervention can reduce its morbidity and mortality, although its mechanism remains unclear[4].
Pancreatitis-associated lung injury is characterized by significant pulmonary edema, hyperemia and inflammatory infiltration in alveoli[5]. It has been established that pulmonary edema is related to increased permeability and loss of barrier function[2-5]. Although elevated levels of pancreatic enzymes and pro-inflammatory cytokines are attributed to pulmonary vasculature damage and increased endothelial permeability[6], the molecular basis for these damages remains largely undefined. Tight junctions are intimately involved in epithelial and endothelial permeability[7]. Fernandez et al[8] recently demonstrated that claudins, the key components of tight junctions, restrict the paracellular movement of water, proteins, and solutes across cellular barriers including alveolar epithelium. In mammals, the claudin family includes at least 24 members. With small interfering RNA and a blocking peptide, Wray et al[9] described that inhibition of claudin-4 decreases transepithelial electrical resistance in primary rat and human epithelial cells, as well as air space fluid clearance, resulting in pulmonary edema in mice, suggesting that claudin-4 plays an important role in alveolar epithelial barrier function. Moreover, claudin-5 and occludin are also decreased in models of acute lung injury accompanying increased paracellular permeability, indicating that claudin-5 and occludin may also play a role in alveolar epithelial barrier function[10-12]. However, the relation between expression of claudin-4, claudin-5, and occludin in lung tissues of patients with acute pancreatitis and pancreatitis-associated lung injury remains largely undefined.
It was reported that emodin (1,3,8-trihydroxy-6-methyl-anthraquinone), an anthraquinone derivative from the Chinese herb Radix et Rhizoma Rhei, inhibits the production of inflammatory cytokines such as tumor necrosis factor-α (TNF-α)[13]. Our previous study demonstrated that emodin significantly reduces serum TNF-α and interleukin-6 (IL-6) levels, thus attenuating lung injury in rats with acute pancreatitis[14]. The effect of emodin on pulmonary tight junction expression and alveolar epithelial barrier function, however, needs to be further defined.
In the present study, the effect of emodin on pancreatitis-associated lung injury and alveolar epithelial barrier function was assessed by examining pulmonary morphology, myeloperoxidase (MPO) activity (indicator of inflammatory infiltration), expression of claudin-4, claudin-5 and occludin, as well as dye extravasation, in lung tissue samples from rats with acute pancreatitis.
Adult male Sprague-Dawely rats, weighing 200-250 g, obtained from Animal Facility of Jinling Hospital (Nanjing, China), were housed under controlled temperature and humidity in a day-night cycle, with free access to standard laboratory foot and water. The study was approved by Animal Studies Ethics Committee of Jinling Hospital.
Acute pancreatitis was induced as previously described[15]. Briefly, animals were anesthetized with intraperitoneal ketamine (80 mg/kg) and acepromazine (2.5 mg/kg). The biliopancreatic duct was cannulated through the duodenum, and the hepatic duct was closed with a small bulldog clamp. Pancreatitis was induced by retrograde injection of 5% sodium taurocholate (Sigma, St. Louis, MO, USA) into the biliopancreatic duct (1 mL/kg body weight), at a constant infusion pressure of 20 mmHg. Rats in sham operation group received retrograde sterile saline infusion.
Effect of emodin on expression of claudin-4, claudin-5 and occludin, as well as on pulmonary dye extravasation, a marker to evaluate alveolar epithelial barrier, was detected in rats with acute pancreatitis. Time course of pulmonary edema and inflammation was recorded. Rats with acute pancreatitis were randomly allocated into pancreatitis group and emodin treatment group. Rats in pancreatitis group were injected with emodin (2.5 mg/kg body weight) via the external jugular vein 3 h after sodium taurocholate infusion. Rats in sham operation group were injected with normal saline (equivalent volume as emodin) at the same time point and served as a control group.
Lung tissue samples were obtained 6 h after emodin injection, and maintained at -80°C until assay. Blood samples were obtained from the inferior cava vein by direct puncture. Lung tissue samples were fixed in 4% neutral phosphate-buffered formalin and embedded in paraffin wax for histology examination. Serum amylase activity was detected to confirm the appropriate induction of pancreatitis.
Serum amylase level was measured by incubating serum with 4,6-ethylidene (G7)-p-nitrophenyl (G1)-1-D-maltoheptoside for 2 min at 37°C, with its absorbance detected once a minute for 2 min at 405 nm by high through universal microplate assay (BMG Lab Technologies, Germany).
Lung tissue sections were stained with hematoxylin and eosin. An experienced pathologist and a pancreatic specialist assessed tissue alterations under light microscope in a blinded fashion and scored them with a grading system[16]. The grading involved measurements of inflammatory infiltration, pulmonary edema and alveolar collapse, each on a scale of 0-3, giving a maximum score of 9.
TNF-α and IL-6 levels in lung tissue samples were measured using a sandwich enzyme-linked immunospecific assay (Jingmei Biotech, Beijing, China) according to its manufacturer’s instructions. Absorbance was measured at 450 nm by high through universal microplate assay. Tissue homogenate was corrected with the protein concentration and expressed as per protein in lung tissue (pg/mg protein).
Sequestration of neutrophils in lung tissue samples was evaluated by measuring tissue MPO activity[15,17]. Briefly, lung tissue samples were homogenized with 0.5% hexadecyltrimethylammonium bromide (Sigma, St. Louis, MO, USA) in 50 mmol/L phosphate buffer (pH 6.0). Homogenate was sonicated for 10 s, freeze-thawed three times, and centrifuged at 14 000 g for 15 min. The resulted suspension was used for assay. The assay mixture contained 20 μL of supernatant, 10 μL of tetramethylbenzidine (final concentration 1.6 mmol/L), and 70 μL of H2O2 (final concentration 3.0 mmol/L). MPO activity was assessed photometrically at 630 nm. The results were corrected with the protein concentration and expressed as the activity of per protein in lung tissue (U/mg protein).
Severity of pulmonary edema was estimated by measuring water content in lung tissue samples. Freshly blotted lung tissue samples were weighed on an aluminum foil, dried for 24 h at 95°C, and reweighed. Difference in wet and dry tissue weights was calculated and expressed as wet/dry ratio.
Alveolar epithelial barrier function was evaluated by measuring Evans blue (Sigma, St. Louis, MO, USA) extravasation[18]. Briefly, Evans blue (20 mg/kg body weight) was injected into the jugular vein of rats, 30 min before duct infusion. Lung tissue samples were obtained 6 h after duct infusion, sectioned and immersed in a formamide solution, homogenized for 2 min. After incubation at room temperature for 24 h, the suspension was centrifuged at 4000 g for 30 min. The amount of dye extracted was determined spectrophotometrically at 620 nm and calculated from a standard curve established with a known amount of Evans blue. Results were corrected by the wet/dry lung tissue ratio and expressed as the dye content per dry weight of lung tissue (μg/g tissue).
Western blotting analysis was performed as previously described[19]. Total protein (20 μg) was separated from each sample by electrophoresis on a 4%-20% SDS-polyacrylamide gel and electroblotted onto polyvinylidene difluoride membranes. Membranes were blocked in a blocking solution, incubated overnight with primary antibodies, and developed with a horseradish peroxidase-conjugated secondary antibody (Kangchen Biotech, Shanghai, China) diluted at 1:1000. Primary antibody (Zymed Laboratories, South San Francisco, CA, USA) was diluted as follows: claudin-4 at 1:100, claudin-5 at 1:100, and occludin at 1:300. The immune complexes were then visualized on X-ray film using chemiluminecent HRP substrate (Millipore, Boston, MA, USA). Additional immunoblots were performed using GAPDH antibody (Abcam, OFW, UK) as the primary antibody to evaluate equal loading.
Lung tissue sections (4 μm) were dewaxed in graded alcohols, and washed with tap water. Endogenous peroxidase activity was blocked with 3% (v/v) H2O2, and antigen was retrieved with microwave in 0.01 mol/L citrate buffer. The sections were then washed with phosphate-buffered saline (PBS, 0.1 mol/L). Mouse anti-rat claudin-4 and claudin-5, and rabbit anti-rat occludin polyclonal antibodies (Zymed Laboratories, South San Francisco, CA, USA) were diluted at 1:100 and incubated overnight at 4°C. The sections were washed 4 times with PBS, 5 min once. Power vision two-step histostaining reagent (ImmunoVision Technologies, Norwell, MA, USA) was used for detection of claudin and occludin expression. All sections were developed using diaminobenzidine and counterstained with hematoxylin.
Total RNA was extracted with a TRIzol kit (Invitrogen Carlsbad, CA, USA) and converted to cDNA with a first strand cDNA synthesis kit (Fermentas, Burlington, Canada). Quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) was performed using SYBR Green SuperMix-UDG (Invitrogen Carlsbad, CA, USA). The primer sequences used for PCR are as follows: claudin-4 (forward 5'-CCTTTCCCATACGGTCTTGCT-3', reverse 5'-CCCGTACCTTCCACAGACTG-3'), claudin-5 (forward 5'-TACTCAGCACCAAGGCGAACCAC-3', reverse 5'-GCGGCTTCCCACATCGGTC-3'), occludin (forward 5'-AGTACATGGCTGCTGCTGATG-3', reverse 5'-CCCACCATCCTCTTGATGTGT-3'), GAPDH (forward 5'-CAGTGCCAGCCTCGTCTCATA-3', reverse 5'-TGCCGTGGGTAGAGTCATA-3'). Amplification was performed at 50°C for 2 min (UDG incubation), at 95°C for 2 min, followed by 40 cycles of denaturing at 95°C for 15 s and annealing at 60°C for 30 s. All reactions were performed in triplicate. Melting curve analysis was performed to ensure the specificity of quantitative PCR. Data analysis was performed as previously described[20], with GAPDH used as a reference gene.
Data were expressed as mean ± SD. ANOVA was used to analyze differences between experimental and control groups. Student-Newman-Kleus method was used for multiple pair-wise comparisons. All statistical analyses were carried out using the SPSS version 11.5 for Windows (Chicago, IL, USA). P < 0.05 was considered statistically significant.
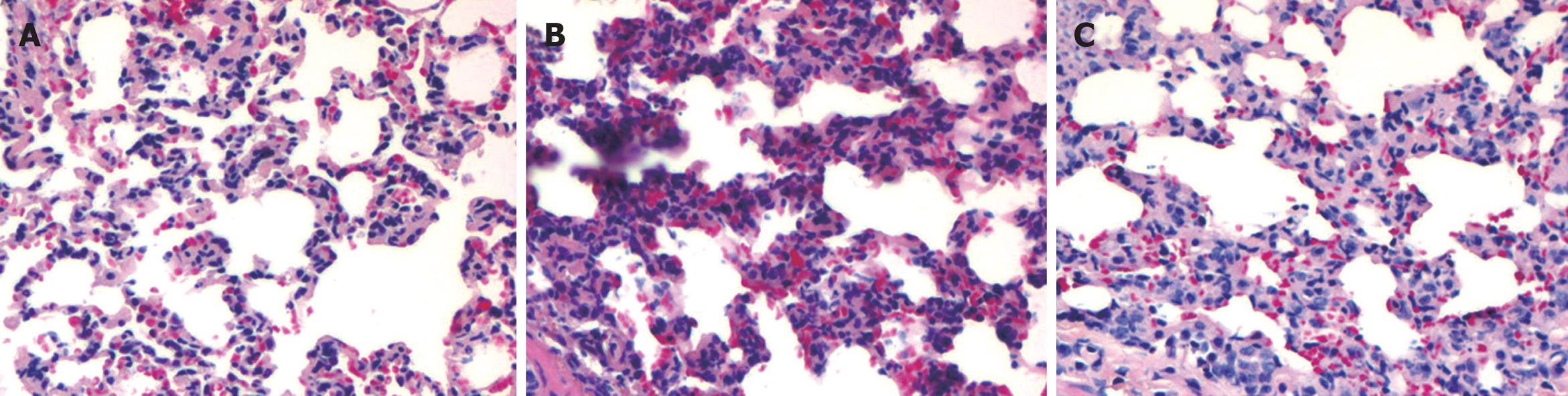
The appropriate induction of pancreatitis-associated lung injury was demonstrated by histology and elevated serum amylase activity (Figure 1 and Table 1). Lung injury was characterized by pulmonary edema, leukocyte infiltration, and alveolar collapse. Pulmonary pathological scores and serum amylase activity were significantly lower after treatment with emodin.
Pulmonary edema was evaluated by measuring the water content in lung tissue samples and expressed as wet/dry ratio, which was significantly decreased after treatment with emodin (Table 1).
In the present study, the effect of emodin on pulmonary inflammation and MPO activity was evaluated. The TNF-α and IL-6 levels and MPO activity were decreased after treatment with emodin (Table 2).
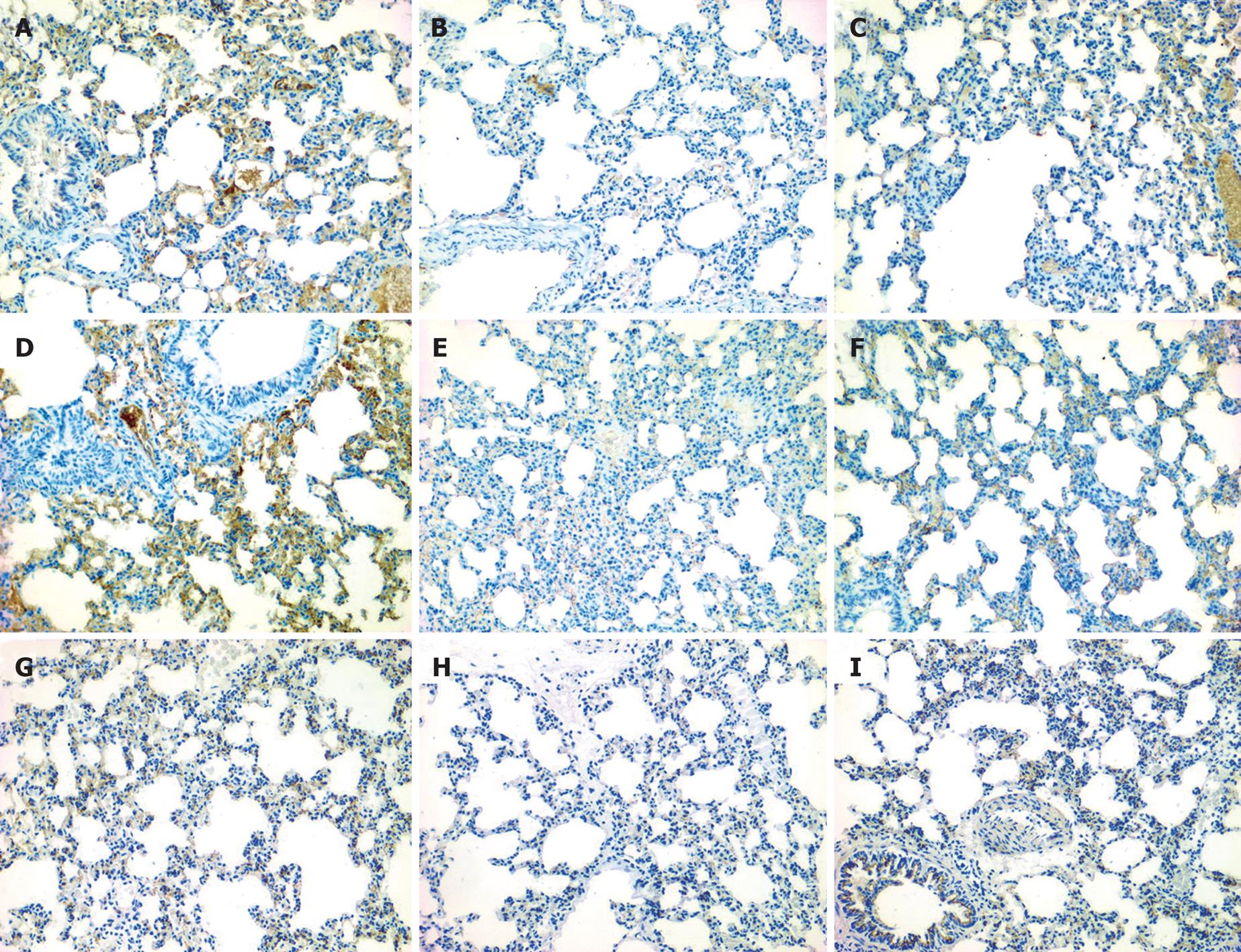
The expression levels of claudin-4, claudin-5, and occludin were markedly lower in experimental group than in control group (data not shown). Immunolocalization of claudin-4, claudin-5 and occludin in lung tissue samples was investigated with immunohistochemical staining. Moderate immunostaining of claudin-4, claudin-5, and occludin was detected in control group, which was distributed in alveolar epithelium, vascular endothelium, and bronchial epithelium, respectively (Figure 2A, D and G). Immunostaining of claudin-4, claudin-5, and occludin was markedly decreased in experimental group (Figure 2B, E and H), and moderately elevated after treatment with emodin (Figure 2C, F and I).
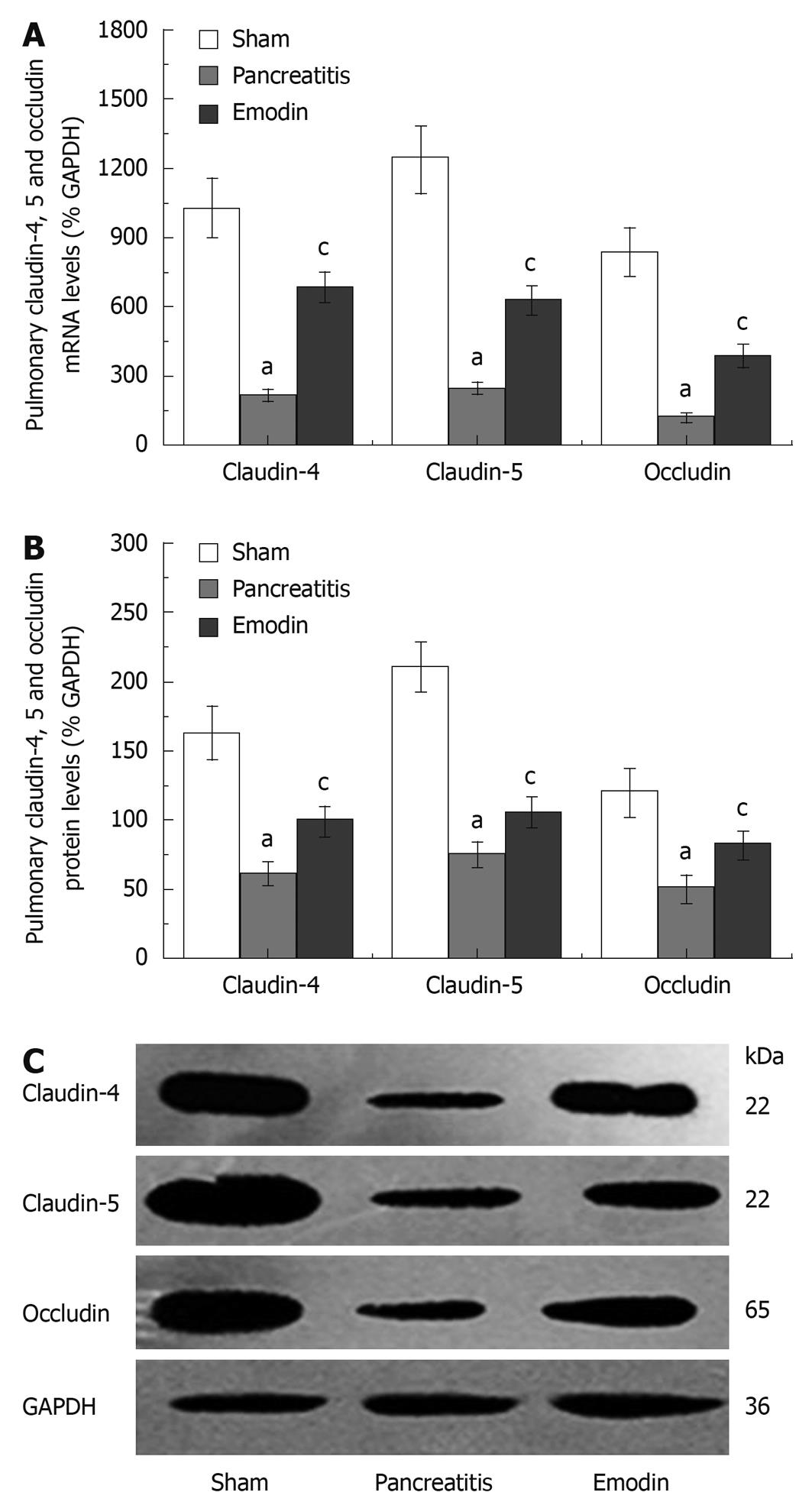
RT-PCR analysis showed that emodin could increase the expression levels of claudin-4, claudin-5, and occludin mRNA in rats with acute pancreatitis (Figure 3A).
Western blotting analysis showed that the expression levels of claudin-4, claudin-5, and occludin were significantly higher in emodin treatment group than in pancreatitis group (Figure 3B and C).
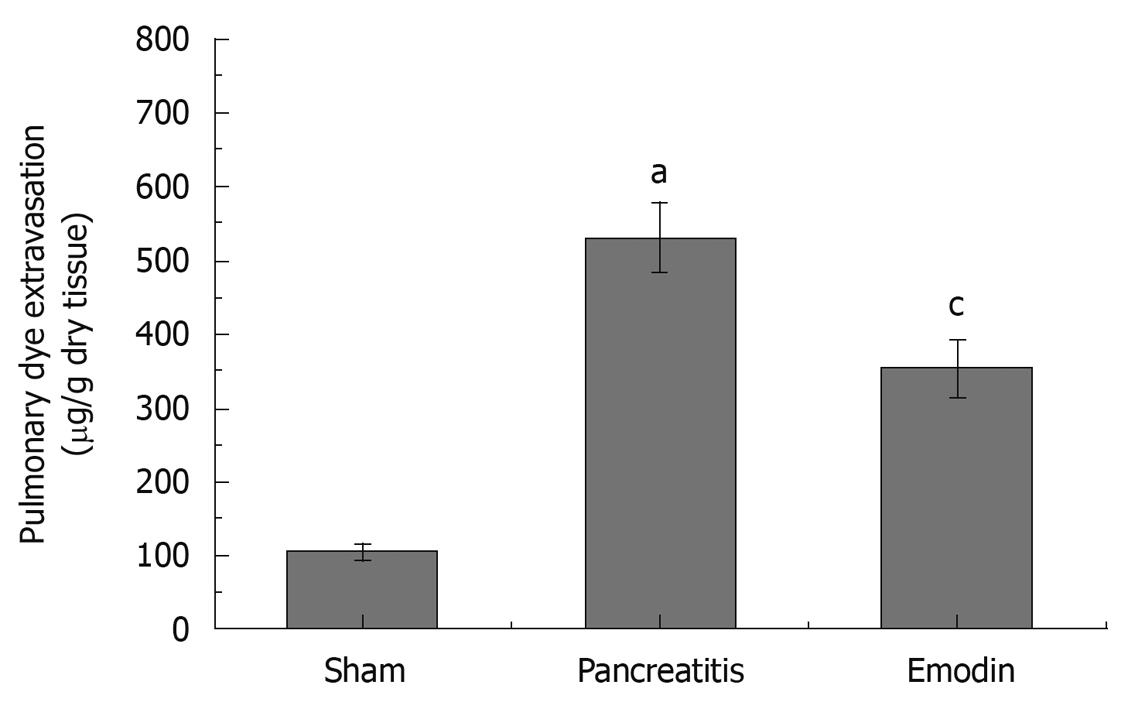
The pulmonary dye extravasation, as a marker of local paracellular permeability, was significantly reduced in rats with acute pancreatitis after treatment with emodin, indicating that emodin can augment alveolar epithelial barrier function (Figure 4).
In the present study, we identified the down-regulation of claudin-4, claudin-5, and occludin in rats with acute pancreatitis induced by sodium taurocholate. Intravenous administration of emodin promoted the down-regulation of tight junctions, enhanced alveolar epithelial barrier function, attenuated pulmonary edema and inflammatory infiltration in rats with acute pancreatitis.
Among the systemic complications of severe acute pancreatitis, pulmonary complication, also known as pancreatitis-associated lung injury, is the most frequent and serious[6]. Pancreatitis-associated lung injury is characterized by significant pulmonary edema, hyperemia and inflammatory infiltration in alveoli[5]. Increased interstitial edema cuts down the transport of carbon dioxide through the alveolar barrier, causing respiratory distress syndrome. It has been recently reported that claudins, the key components of tight junctions, restrict paracellular movement of water, proteins, and solutes across cellular barriers including pulmonary vascular endothelium and alveolar epithelium[8]. Disruption of claudins impairs barrier function and increases paracellular permeability, which may allow noxious contents to enter pulmonary interstitium and alveoli, further aggravating pulmonary edema and inflammation[5-9].
Recently, several studies have demonstrated the localization and function of claudin-4 in pulmonary cellular barriers[9-11]. In human airway epithelia, elevated claudin-4 level is associated with increased transepithelial electrical resistance, indicating that claudin-4 plays a role in alveolar epithelial barrier function[10]. Although increased claudin-4 expression has been found in a mice model of acute lung injury, inhibition of claudin-4 can lead to pulmonary edema in mice by decreasing transepithelial electrical resistance and air space fluid clearance, suggesting that claudin-4 plays an important role in alveolar epithelial barrier function, and early increased claudin-4 expression may represent a mechanism by which pulmonary edema is limited[9]. Similar to claudin-4, claudin-5 also plays a role in cellular barrier function. Recombinant claudin-5 protects brain microvascular endothelial cell cultures against increased paracellular permeability induced by VEGF, showing that claudin-5 is a key determinant of blood-brain barrier function[21]. It has been recently reported that expression of pulmonary claudin-5 is decreased in models of carrageenan-induced acute lung inflammation, associated with the decreased pulmonary paracellular permeability, suggesting that claudin-5 may play role in alveolar epithelial barrier function[11].
Occludin shares a very similar membrane location with claudin. Based on the staining feature of claudins and occludin along the endothelial cell borders, Persidsky et al[22] speculated that claudins form the primary “makeup” of the tight junctions, and occludin further enhances tight junction tightness. In ethanol abused rats, which is decreased mRNA and protein expression of occludin has also been observed in lung tissues, associated with increased bronchoalveolar epithelial permeability[12]. Azithromycin-induced processing of occludin is accompanied by increased transepithelial electrical resistance[10], suggesting that occludin alteration may be related with alveolar barrier function.
In the present study, we identified the localization of claudin-4, claudin-5, and occludin in lung tissue samples from rats with acute pancreatitis, and found that claudin-4 and claudin-5 were uniformly and continuously distributed along the alveolar epithelium and vascular endothelium in normal lung tissue samples, which are consistent with the reported findings[9-11]. Furthermore, occludin was uniformly and continuously distributed along the alveolar epithelium, vascular endothelium, and bronchiolar epithelium, which is in line with the reported results[10,12]. In this study, RT-PCR and Western blotting showed that the expression of claudin-4, claudin-5 and occludin was down-regulated in lung tissue samples from rats with acute pancreatitis. Aggravated pulmonary edema and increased paracellular permeability (marked by extravasation of Evans blue) were in parallel with the down-regulation of claudin-4, claudin-5 and occludin expression, which is consistent with the findings in previous studies[9-12], suggesting that claudin-4, claudin-5 and occludin may play a role in alveolar barrier function.
In the present study, emodin significantly promoted the expression of claudin-4, claudin-5 and occludin at mRNA transcription and protein synthesis level, and decreased pulmonary edema and paracellular permeability. Based on the previous and present studies, we speculate that emodin may contribute, in part at least, to the expression of claudin-4, claudin-5 and occludin by increasing the alveolar barrier function.
Emodin has long been used for anti-inflammatory purposes. Many studies have demonstrated that emodin intervention can significantly decrease TNF-α and IL-6 levels, or MPO activity in lung tissues[13,23,24], and the mechanism of emodin underlying cytokine inhibition is involved in NF-κB activity suppression[23-26]. Moreover, emodin also has antioxidant effects, promotes generation of ATP and antioxidant components, such as glutathione, α-tocopherol, and superoxide dismutase[27], and exhibits a promising free radical scavenging activity[28]. It has been shown that emodin markedly reduces serum amylase, TNF-α and IL-6 levels, attenuates lung damage in rats with acute pancreatitis[14,29,30], which is in line with the present study. Considering that MPO activity is a marker of local leukocyte sequestration[30], the results of our present study suggest that emodin ameliorates pancreatitis-associated lung injury by inhibiting the production of cytokines and the infiltration of leukocytes in lungs.
In conclusion, emodin can attenuate pulmonary edema and inflammation, enhance alveolar epithelial barrier function, and promote expression of claudin-4, claudin-5 and occludin in lung tissues.
The mortality associated with acute pancreatitis is attributed to inflammation-related complications such as pancreatitis-associated lung injury. Interventions with emodin are likely to reduce its morbidity and mortality of the disease.
Claudins and occludin, the key components of tight junctions, restrict the paracellular movement of water, proteins, and solutes across cellular barriers including alveolar epithelium. Emodin, an anthraquinone derivative from the Chinese herb Radix et Rhizoma Rhei, has been used for anti-inflammatory purposes. Weather emodin has effects on pulmonary tight junction expression and alveolar epithelial barrier has not been defined.
A recent report has highlighted the importance of tight junction components in a model of ethanol-induced lung injury, or carrageenan-induced acute lung inflammation. This is the first study to report that down-regulation of pulmonary claudin-4, claudin-5 and occludin is parallel with pancreatitis-associated lung injury. Emodin can promote expression of claudin-4, claudin-5 and occluding in lungs, enhance alveolar epithelial barrier, and inhibit pulmonary inflammation.
The results of this study may improve our understanding of the pathogenesis of pancreatitis-associated lung injury. The present study also provides evidence for emodin in treatment of lung injury.
This is a largely observational study representing an incremental advance in treatment of acute pancreatitis with emodin.
Peer reviewer: Minoti V Apte, Associate Professor, Pancreatic Research Group, South Western Sydney Clinical School, University of New South Wales, Liverpool, NSW 2170, Australia
S- Editor Wang YR L- Editor Wang XL E- Editor Ma WH
| 1. | Steer M. Pancreatitis severity: who calls the shots? Gastroenterology. 2002;122:1168-1172. |
| 2. | Van Acker GJ, Perides G, Weiss ER, Das S, Tsichlis PN, Steer ML. Tumor progression locus-2 is a critical regulator of pancreatic and lung inflammation during acute pancreatitis. J Biol Chem. 2007;282:22140-22149. |
| 3. | Mentula P, Kylänpää ML, Kemppainen E, Jansson SE, Sarna S, Puolakkainen P, Haapiainen R, Repo H. Early prediction of organ failure by combined markers in patients with acute pancreatitis. Br J Surg. 2005;92:68-75. |
| 4. | Mota R, Sánchez-Bueno F, Berenguer-Pina JJ, Hernández-Espinosa D, Parrilla P, Yélamos J. Therapeutic treatment with poly(ADP-ribose) polymerase inhibitors attenuates the severity of acute pancreatitis and associated liver and lung injury. Br J Pharmacol. 2007;151:998-1005. |
| 6. | Browne GW, Pitchumoni CS. Pathophysiology of pulmonary complications of acute pancreatitis. World J Gastroenterol. 2006;12:7087-7096. |
| 7. | Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207-235. |
| 8. | Fernandez AL, Koval M, Fan X, Guidot DM. Chronic alcohol ingestion alters claudin expression in the alveolar epithelium of rats. Alcohol. 2007;41:371-379. |
| 9. | Wray C, Mao Y, Pan J, Chandrasena A, Piasta F, Frank JA. Claudin-4 augments alveolar epithelial barrier function and is induced in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2009;297:L219-L227. |
| 10. | Asgrimsson V, Gudjonsson T, Gudmundsson GH, Baldursson O. Novel effects of azithromycin on tight junction proteins in human airway epithelia. Antimicrob Agents Chemother. 2006;50:1805-1812. |
| 11. | Mazzon E, Cuzzocrea S. Role of TNF-alpha in lung tight junction alteration in mouse model of acute lung inflammation. Respir Res. 2007;8:75. |
| 12. | Zhang YL, Li QQ, Guo W, Huang Y, Yang J. Effects of chronic ethanol ingestion on tight junction proteins and barrier function of alveolar epithelium in the rat. Shock. 2007;28:245-252. |
| 13. | Lee J, Jung E, Lee J, Huh S, Hwang CH, Lee HY, Kim EJ, Cheon JM, Hyun CG, Kim YS. Emodin inhibits TNF alpha-induced MMP-1 expression through suppression of activator protein-1 (AP-1). Life Sci. 2006;79:2480-2485. |
| 14. | Li Z, Xia X, Zhang S, Zhang A, Bo W, Zhou R. Up-regulation of Toll-like receptor 4 was suppressed by emodin and baicalin in the setting of acute pancreatitis. Biomed Pharmacother. 2009;63:120-128. |
| 15. | Pereda J, Sabater L, Cassinello N, Gómez-Cambronero L, Closa D, Folch-Puy E, Aparisi L, Calvete J, Cerdá M, Lledó S. Effect of simultaneous inhibition of TNF-alpha production and xanthine oxidase in experimental acute pancreatitis: the role of mitogen activated protein kinases. Ann Surg. 2004;240:108-116. |
| 16. | Lichtenstein A, Milani R Jr, Fernezlian SM, Leme AS, Capelozzi VL, Martins MA. Acute lung injury in two experimental models of acute pancreatitis: infusion of saline or sodium taurocholate into the pancreatic duct. Crit Care Med. 2000;28:1497-1502. |
| 17. | Lau HY, Wong FL, Bhatia M. A key role of neurokinin 1 receptors in acute pancreatitis and associated lung injury. Biochem Biophys Res Commun. 2005;327:509-515. |
| 18. | Muhs BE, Patel S, Yee H, Marcus S, Shamamian P. Inhibition of matrix metalloproteinases reduces local and distant organ injury following experimental acute pancreatitis. J Surg Res. 2003;109:110-117. |
| 19. | Hietaranta A, Mustonen H, Puolakkainen P, Haapiainen R, Kemppainen E. Proinflammatory effects of pancreatic elastase are mediated through TLR4 and NF-kappaB. Biochem Biophys Res Commun. 2004;323:192-196. |
| 20. | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-408. |
| 21. | Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc Natl Acad Sci USA. 2009;106:1977-1982. |
| 22. | Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD. Blood-brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol. 2006;1:223-236. |
| 23. | Wu Y, Tu X, Lin G, Xia H, Huang H, Wan J, Cheng Z, Liu M, Chen G, Zhang H. Emodin-mediated protection from acute myocardial infarction via inhibition of inflammation and apoptosis in local ischemic myocardium. Life Sci. 2007;81:1332-1338. |
| 24. | Ding Y, Zhao L, Mei H, Zhang SL, Huang ZH, Duan YY, Ye P. Exploration of Emodin to treat alpha-naphthylisothiocyanate-induced cholestatic hepatitis via anti-inflammatory pathway. Eur J Pharmacol. 2008;590:377-386. |
| 25. | Li HL, Chen HL, Li H, Zhang KL, Chen XY, Wang XW, Kong QY, Liu J. Regulatory effects of emodin on NF-kappaB activation and inflammatory cytokine expression in RAW 264.7 macrophages. Int J Mol Med. 2005;16:41-47. |
| 26. | Kumar A, Dhawan S, Aggarwal BB. Emodin (3-methyl-1,6,8-trihydroxyanthraquinone) inhibits TNF-induced NF-kappaB activation, IkappaB degradation, and expression of cell surface adhesion proteins in human vascular endothelial cells. Oncogene. 1998;17:913-918. |
| 27. | Du Y, Ko KM. Effects of emodin treatment on mitochondrial ATP generation capacity and antioxidant components as well as susceptibility to ischemia-reperfusion injury in rat hearts: single versus multiple doses and gender difference. Life Sci. 2005;77:2770-2782. |
| 28. | Shia CS, Hou YC, Tsai SY, Huieh PH, Leu YL, Chao PD. Differences in pharmacokinetics and ex vivo antioxidant activity following intravenous and oral administrations of emodin to rats. J Pharm Sci. 2010;99:2185-2195. |
| 29. | Zhang XP, Li ZF, Liu XG, Wu YT, Wang JX, Wang KM, Zhou YF. Effects of emodin and baicalein on rats with severe acute pancreatitis. World J Gastroenterol. 2005;11:2095-2100. |
| 30. | Li ZF, Xia XM, Huang C, Zhang S, Zhang J, Zhang AJ. Emodin and baicalein inhibit pancreatic stromal derived factor-1 expression in rats with acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2009;8:201-208. |