INTRODUCTION
Improvements in early diagnosis of gastric cancer (GC) have led to an increase in the number of patients who are able to undergo curative resection[1]. However, the prognosis for patients with unresectable or metastatic disease is very poor, and long-term survival, particularly for more than 5 years, is rare. S-1 is a novel oral fluoropyrimidine that was developed in Japan. It has been available for patients with GC in Japan since 1999 and is currently being investigated worldwide. S-1 consists of tegafur, 5-chloro-2,4-dihydroxypyridine (CDHP) and potassium oxonate at a fixed molar ratio of 1:0.4:1. Tegafur is a prodrug of fluorouracil (5-FU), which is the cytotoxic component of this combination. CDHP is a potent reversible inhibitor of dihydropyrimidine dehydrogenase (DPD), the chief catabolic enzyme of 5-FU. Potassium oxonate selectively inhibits orotate phosphoribosyltransferase, the enzyme responsible for 5-FU activation in the gastrointestinal tract, thus reducing the gastrointestinal toxicity of the combination. Many reports have indicated the efficacy of this antitumor agent against gastric cancer.
We report a case of advanced gastric cancer (AGC) with unresectable lymph node metastases treated with S-1.
CASE REPORT
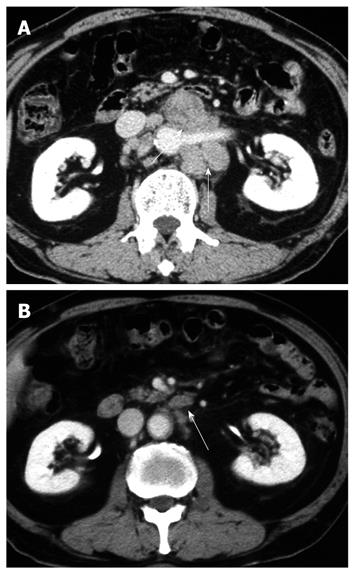
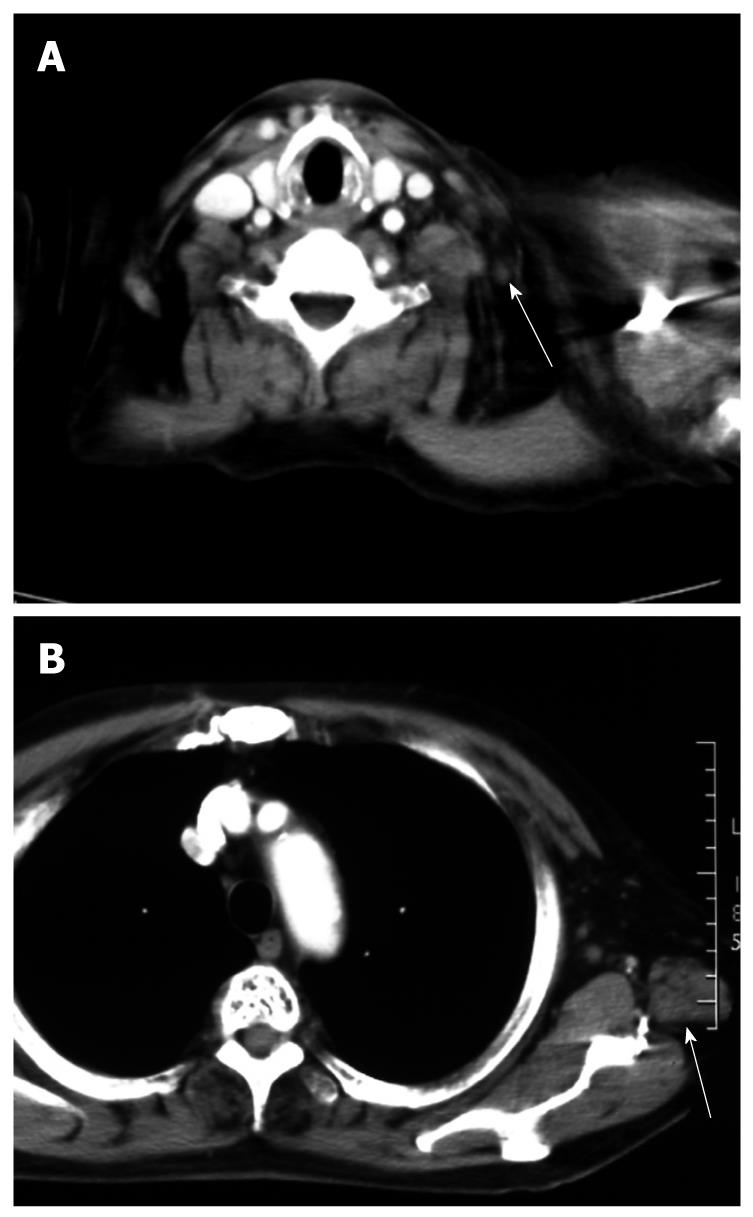
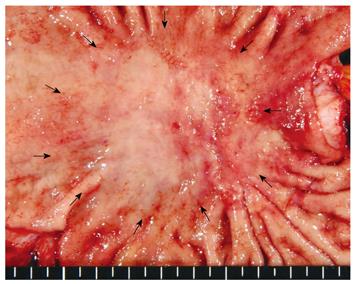
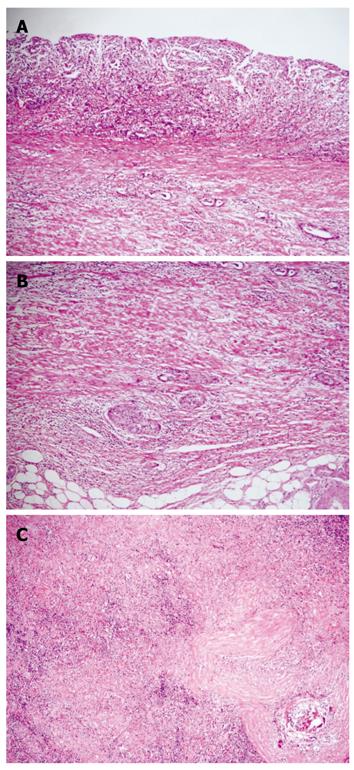
A 71-year-old man visited a local hospital in 2002, complaining of upper abdominal discomfort of one-month duration. Endoscopy revealed a concave lesion in the stomach, and the patient was subsequently referred to our hospital. Endoscopy showed a diffuse concave lesion with ulceration on the lesser curvature in the middle of the stomach. A biopsy specimen showed signet-ring cell carcinoma. Physical examination revealed a few elastic-hard masses in the left supraclavicular area (approximately 7 cm in diameter) and left axillary space (approximately 6 cm in diameter), suggesting lymph node metastases from the GC. Abdominal computed tomography (CT) showed marked swelling of several paraaortic lymph nodes (Figure 1A). We could not find metastasis to the liver, lung, or peritoneum. The patient’s diagnosis was AGC with extensive lymph node metastases (stage IV: cT2 cN3 cP0 cH0 cM1). His general condition was good, with The Eastern Cooperative Oncology Group (ECOG) performance status 0, and biochemical analysis of blood and urine specimens showed no abnormalities. Serum levels of the tumor markers carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) were not elevated (2.1 ng/mL and 12.0 U/mL, respectively). Since curative resection was impossible for this patient, chemotherapy with S-1 was started. As one course, 120 mg (80 mg/m2) of S-1 was orally administered daily for 28 d, followed by a 14-d rest period; administration occurred primarily at the outpatient clinic. After 2 courses, endoscopy showed no remarkable change in the gastric tumor. However, abdominal CT showed remarkable reductions in the size of regional and paraaortic lymph nodes (Figure 1B), and also in cervical and axillary lymph nodes (Figure 2A and B). This indicated a partial response (PR). The evaluation after 6 courses of S-1 showed that there was no change at the primary site, but CT showed complete disappearance of paraaortic, cervical and axillary lymph nodes. Therefore, this revealed a complete response (CR), and this condition was stable up to the subsequent surgery. The patient did not experience any critical adverse event during S-1 administration, despite developing hematological toxicity; including leucopenia (2100/μL at the minimum), thrombocytopenia, and non-hematological toxicity; including grade 2 hand-foot syndrome and grade 1 stomatitis, according to National Cancer Institute Common Toxicity Criteria Version 2.0. After 19 courses of S-1, the tumor was limited to the primary gastric site, with no metastasis detected from neck to abdomen by CT or positron emission tomography (PET); only the detection of gastric tumor by PET. Therefore, we concluded that curative resection could be achieved. In 2004, distal gastrectomy and lymph node dissection (D2) were performed. We did not find cancer cells in the intraoperative peritoneal lavage, or in the abdominal paraaortic lymph node specimens. Figure 3 shows the resected gastric specimen. A concave lesion of 45 mm × 40 mm with an ulceration scar was located in the lesser curvature of the middle of the stomach (Figure 3). Histopathological examination showed numerous poorly differentiated adenocarcinoma cells in the mucosal layer (Figure 4A). There were only a small number of poorly differentiated adenocarcinoma cells, but severe fibrosis, in the submucosal layer (Figure 4B). The histological effect of chemotherapy on the primary tumor was limited, and according to the Japanese classification of gastric carcinoma (second English edition)[2], it was classified as grade 1a. The only findings in the lymph nodes were remarkable fibrosis and giant cells, with no cancer cells seen; the histological effect on the lymph nodes metastasis was classified as grade 3 (Figure 4C). Therefore, a radical operation was considered to have been performed, and the final pathological stage assigned as Stage IA (pT1, pN0, sP0, sH0, sM0) according to the Japanese classification of gastric carcinoma (second English edition)[2]. There have been no signs of recurrence since the surgery, and the patient has survived for 8 years after initially receiving chemotherapy.
Figure 1 Abdominal computed tomography (CT).
A: Marked swelling of paraaortic lymph nodes before treatment (arrow); B: After two courses of S-1, abdominal CT showed remarkable reductions (arrow).
Figure 2 CT shows a Virchow’s (A) and an axillary lymph node (B) which were reduced remarkably in size after treatment.
Figure 3 Resected specimen shows a concave lesion of 45 mm × 40 mm (arrows) with an ulceration scar which was located in the lesser curvature in the middle of the stomach.
Figure 4 Histopathological examination.
A: Histopathological examination showed numerous poorly differentiated adenocarcinoma cells in the mucosa; B: There were only a small number of poorly differentiated adenocarcinoma cells, but severe fibrosis, in the submucosal layer; C: The only findings in the lymph nodes were remarkable fibrosis and giant cells, with no cancer cells seen.
DISCUSSION
There have been some series that reported high response rates with the use of newer combinations of chemotherapy regimens for unresectable or metastatic GC[3]. However, standard regimens have not yet been established worldwide. S-1-based chemotherapy has shown survival benefits in recent randomized controlled trials. Boku et al[4] reported that S-1 monotherapy showed a significant non-inferiority benefit compared to continuously infused 5-FU in unresectable or metastatic GC (P < 0.001). Moreover, Koizumi et al[5] reported the results of a randomized controlled trial of S-1 vs S-1 with CDDP. The overall survival for S-1 with CDDP was superior to S-1 alone. The 1- and 2-year survival rates were 54.1% and 23.6%, respectively; whereas a 2-year survival rate of less than 10% was shown in the 5-FU+CDDP arm of a Japanese randomized controlled trial with a 10-year follow-up[6].
Although recent phase III clinical trials indicated longer survival times[7,8], few patients with unresectable GC have survived longer than 5 years. During the last 10 years at our hospital, only 9 of more than 500 patients treated with chemotherapy for metastatic or recurrent GC have survived for 5 years. Yoshida et al[9] reported a 5-year survival rate of 2% for registered Japan Clinical Oncology Group (JCOG) clinical trials and mentioned certain characteristics of these survivors. Most of the 5-year survivors had good performance status, macroscopically non-scirrhous-type tumors, only one metastatic site, a paraaortic node metastasis as the only unresectable factor, and achieved a CR after the initial chemotherapy. However, it is unclear whether such long survivals are because of biological non-aggressiveness or due to a good response to chemotherapy. There have been only five cases reported in the literature of AGC with neck lymph node metastasis in which there was no residual tumor following chemotherapy and surgical resection[10-14]. Ohyama et al[10] reported a case of a patient treated with combination chemotherapy, including 5-FU, leucovorin, cisplatin, and etoposide, in which there was a good response. Chemotherapy was followed by curative resection, including subtotal gastrectomy and inguinal, neck, and abdominal lymph node dissection. Pathological examination showed cancer cells only in the abdominal lymph nodes, but not in the stomach or neck lymph nodes. Iwazawa et al[11] reported a case of AGC with cervical lymph node metastasis, in which a CR for cervical lymph nodes was achieved with S-1 alone treatment, and a curative resection of the residual tumor was performed. However, further outcomes of these cases were not described. It remains unknown whether adjuvant surgery for residual tumor after effective chemotherapy is beneficial for GC patients with distant metastasis or not. In our experience, if there is a long-maintained disappearance of inoperable metastasis, adjuvant surgery is considered beneficial.
In our case, there was no confirmation of the neck and axillary lymphadenopathy from imaging examinations before treatment began, and therefore no evidence that this lymphadenopathy was due to metastasis from GC. This is a limitation of our report and we assume responsibility for not obtaining all available evidence of metastasis from GC, although surgeons agreed on the non-operability of this patient in the preoperative conference. However, in patients with histologically diffuse type GC that is greater than 3 cm in size and which has invaded the sub-mucosal layer, 7.5% have lymph node metastasis beyond the perigastric site[15]. Thus, our assumption that cervical and axillary lymphadenopathy was due to GC metastases was probably correct.
Peer reviewers: Cuneyt Kayaalp, MD, Professor, Department of General Surgery, Staff Surgeon of Gastrointestinal Surgery, Turgut Ozal Medical Center, Inonu University, Malatya 44315, Turkey; Giuseppe Sica, MD, PhD, Department of Surgery, University Hospital Tor Vergata, Viale Oxford 81, 00133 Rome, Italy
S- Editor Wang JL L- Editor Logan S E- Editor Ma WH