Published online May 28, 2010. doi: 10.3748/wjg.v16.i20.2566
Revised: March 25, 2010
Accepted: April 1, 2010
Published online: May 28, 2010
The involvement of the small bowel in systemic forms of amyloidosis may be diffuse or very rarely focal. Some cases of focal amyloidomas of the duodenum and jejunum without extraintestinal manifestations have been reported. The focal amyloidomas consisted of extensive amyloid infiltration of the entire intestinal wall thickness. Radiological barium studies, ultrasound and computed tomography (CT) patterns of diffuse small bowel amyloidosis have been described: the signs are non-specific and may include small-bowel dilatation, symmetric bowel wall thickening, mesenteric infiltration, and mesenteric adenopathy. No data are available about the positron emission tomography (PET)/CT and magnetic resonance imaging (MRI) patterns of intestinal amyloidosis. We report two cases of small bowel amyloidosis: the former characterized by focal deposition of amyloid proteins exclusively within blood vessel walls of the terminal ileum, the latter characterized by diffuse intestinal involvement observed on MRI and PET/CT studies.
- Citation: Mainenti PP, Segreto S, Mancini M, Rispo A, Cozzolino I, Masone S, Rinaldi CR, Nardone G, Salvatore M. Intestinal amyloidosis: Two cases with different patterns of clinical and imaging presentation. World J Gastroenterol 2010; 16(20): 2566-2570
- URL: https://www.wjgnet.com/1007-9327/full/v16/i20/2566.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i20.2566
Systemic amyloidosis is a rare disease characterized by the extracellular deposition of amyloid protein in one or more organs[1]. Involvement of the small bowel is frequent in systemic amyloidosis[2], and protein deposition is mainly seen in the mucosa, the submucosal connective tissues, the muscularis propria and within blood vessel walls. The deposition of amyloid into the blood vessel walls causes bowel ischemia and muscle atrophy leading, eventually, to mucosal erosion or ulceration[3].
The involvement of small bowel may be diffuse or very rarely localized, presenting as focal amyloidoma[4-6]. Focal amyloidomas of the small bowel without extraintestinal manifestations are rare and typically present as multiple polypoid lesions or large amyloid tumors of the duodenum and jejunum[5-7].
To the best of our knowledge, a case of focal deposition of amyloid proteins exclusively within blood vessel walls of the terminal ileum has never been described in the literature.
Radiological barium studies, ultrasound (US) and computed tomography (CT) pattern of diffuse small bowel amyloidosis have been described: the signs are non-specific and may include small-bowel dilatation, symmetric bowel wall thickening, mesenteric infiltration, and mesenteric adenopathy[4,8,9].
On the contrary, the positron emission tomography (PET)/CT and magnetic resonance imaging (MRI) patterns of intestinal amyloidosis have never been described in the literature.
We report two cases of small bowel amyloidosis: the former characterized by focal deposition of amyloid proteins exclusively within blood vessel walls of the terminal ileum, the latter characterized by diffuse intestinal involvement observed on PET/CT and MRI studies.
A 72-year-old woman with a medical history of hypertension and of multiple episodes of acute onset of abdominal pain accompanied by intestinal sub-occlusion presented in our hospital.
Two months before our observation, the patient had undergone conventional colonoscopy with retrograde ileoscopy, which disclosed mucosal ulcerations of the terminal ileum and of the ileo-cecal valve. The biopsy specimen showed active inflammatory infiltration without evidence of granulomas.
On admission the patient had acute abdominal pain and intestinal sub-occlusion. She was afebrile with stable vital signs. Findings on physical examination were significant for distended abdomen with discrete tenderness in the right iliac fossa.
Laboratory studies including complete blood count, serum electrolytes, liver function tests, coagulation and tumour markers were as follows (with normal values): hematocrit, 34% (37%-54%); haemoglobin, 11.5 g/dL (12-16); white blood cell count, 16 300 cells/mm3 (4800-10 800); alkaline phosphatase, 117 U/L (35-104); γ-glutamyl transpeptidase, 81 U/L (5-36); albumin, 3.5 g/dL (3.6-5.2). The remaining values were unremarkable.
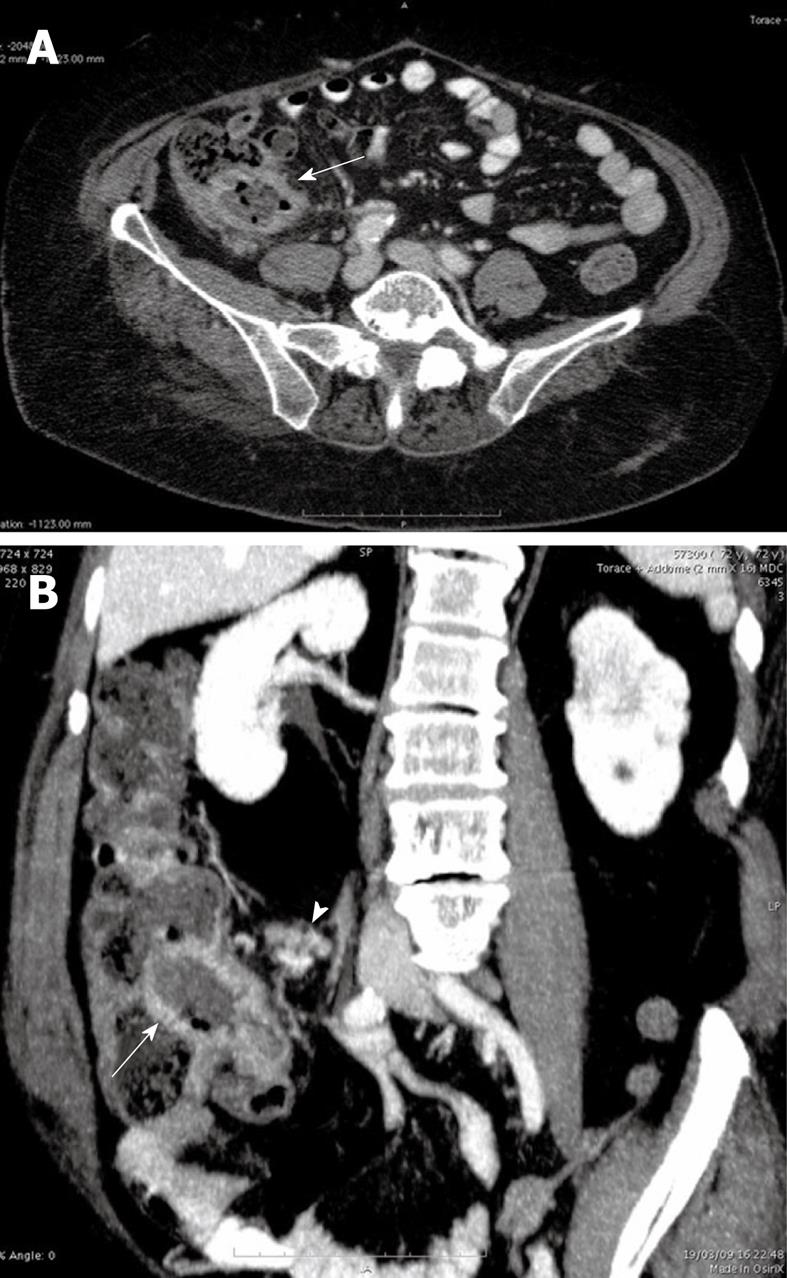
An US examination of the abdomen showed thickened (20 mm) hypoechoic walls of the terminal ileum with loss of parietal stratification and mild pseudoaneurysmal dilatation of the lumen. Moreover multiple ileo-cecal lymph nodes with a diameter ranging between 10 and 20 mm were disclosed.
To better evaluate the US intestinal findings, a 64 multidetector CT scan of the abdomen and pelvis was performed 75 s after the intravenous bolus administration of 150 cc iodinated non ionic contrast agent. The CT images confirmed the findings of US (Figure 1A and B). The mesenteric vessels and the collateral branches were patent.
The US and CT findings suggested the following diagnostic hypothesis: an intestinal lymphoma, considering the pseudoaneurysmal dilatation of the intestinal lumen and the dimension of ileo-cecal lymph nodes or less probably a terminal ileitis. Features of intestinal ischemia were not present.
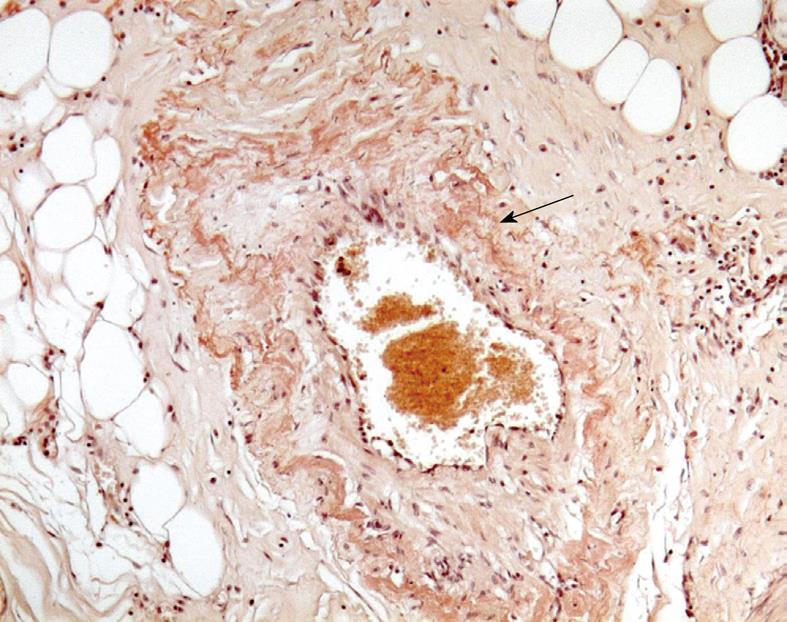
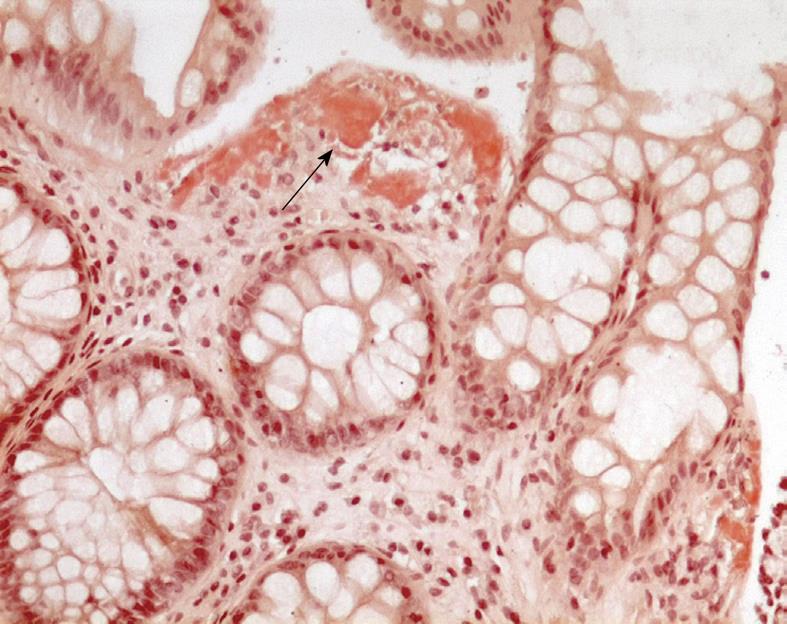
The patient underwent laparotomy with ileo-caecal resection. The biopsy specimen was consistent with a deposition of amyloid within blood vessels of the terminal ileum walls after staining with Congo red (Figure 2).
The patient was discharged from the hospital in a stable condition 5 d after surgery. Further workup showed (1) a monoclonal spike in the serum immunoelectrophoreses; (2) the immunoglobulin κ light-chain urine concentration was 71 mg/L (normal, < 10 mg/L) and the immunoglobulin λ light-chain urine concentration was 26 mg/L (normal, < 5 mg/L); and (3) 8% plasma cells in the bone marrow aspirate. There was no clinical, laboratory, or radiographic evidence of extraintestinal involvement of amyloidosis. These examinations suggested the diagnosis of primary systemic amyloidosis (AL) with focal intestinal manifestation.
In March 2009, a 78-year-old man with a medical history of multiple myeloma and diabetes mellitus presented with diffuse abdominal pain, nausea, lack of appetite and diarrhoea. The patient had a recent episode of rectorrhage.
On admission the patient was afebrile with stable vital signs. Findings on physical examination were significant for moderately distended abdomen with mild diffuse tenderness.
On admission, laboratory studies including complete blood count, serum electrolytes, liver function tests, coagulation and tumour markers were as follows (with normal values): hematocrit, 27% (37%-54%); red blood cell count, 3 780 000 cells/mm3 (4 200 000-5 600 000); haemoglobin, 8.4 g/dL (12-17.5); white blood cell count, 2333 cells/mm3 (4800-10 800); glucose, 177 mg/dL (60-110); total protein, 5.6 g/dL (6.5-8.2); albumin, 3.3 g/dL (3.6-5.2). The remaining values were unremarkable.
Urinalysis showed 100 mg/dL of proteinuria. Serum immunoelectrophoreses showed a monoclonal spike. In the urine, the immunoglobulin λ light-chain concentration was 929 mg/L (normal, < 5 mg/L) and the immunoglobulin κ light-chain concentration was 37.8 mg/L (normal, < 10 mg/L).
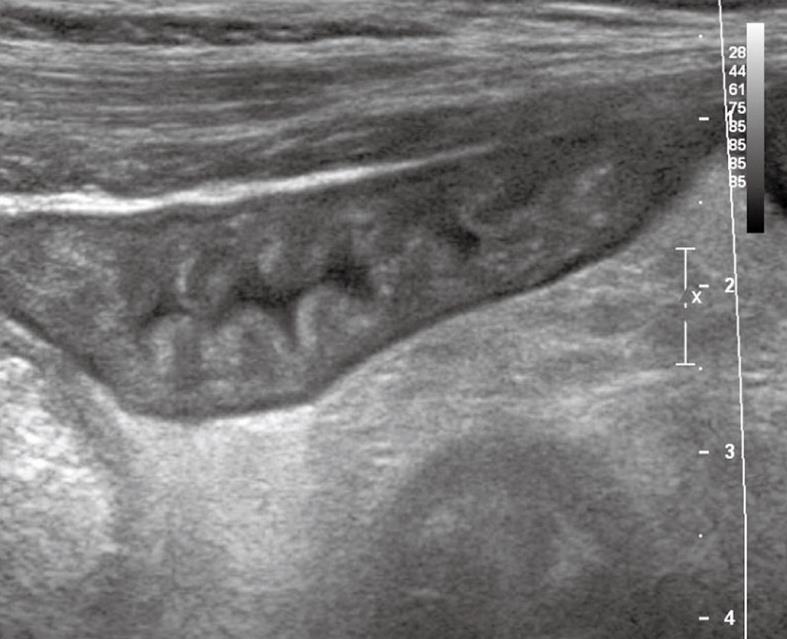
An US examination of the abdomen showed thickened and stratified intestinal walls with hypertrophy of mucosal and sub-mucosal strata and increased number of plicae which were forming a polyp-like shape (Figure 3).
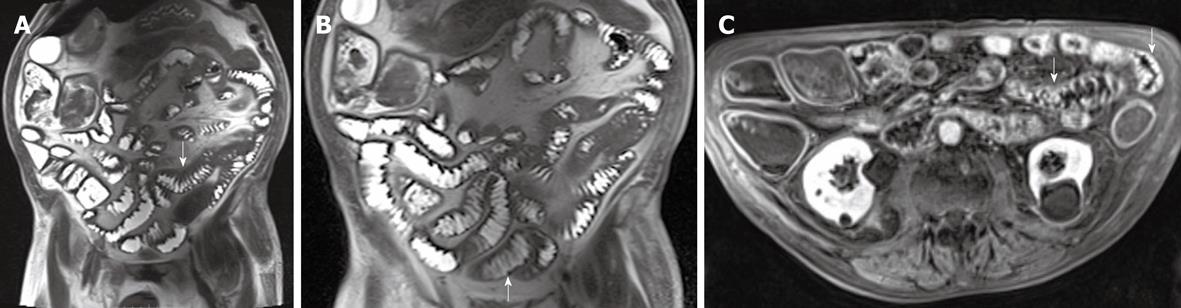
To better evaluate the US intestinal findings, an MRI of the small bowel previous gastro-intestinal preparation with 750 mL of polyethylene glycol solution as an oral contrast agent was performed using a 3.0 T MR device and the following acquisition protocol: axial and coronal T2-weighted half-Fourier single-shot turbo spin-echo sequences and true fast imaging in the steady-state precession sequences, fast low-angle shot T1-weighted sequences with fat suppression, followed by “spoiled” 3D T1-volume interpolated breath-hold examination acquired before and 70 s after intravenous administration of gadopentetate dimeglumine (0.1 mmol/kg).
The MR images showed diffuse intestinal wall symmetrical thickening with increased number and hypertrophy of the folds causing “jejunalization” of the ileum; wall stratification was not observed. After intravenous administration of contrast agent, diffuse enhancement of intestinal walls was observed (Figure 4A-C). The wall thickening associated with the increased number and hypertrophy of the folds suggested a malabsorption disease. The enhanced images did not show any sign indicative of a specific pathology. As a consequence it was not possible to perform a definitive diagnosis exclusively on the basis of MR findings.
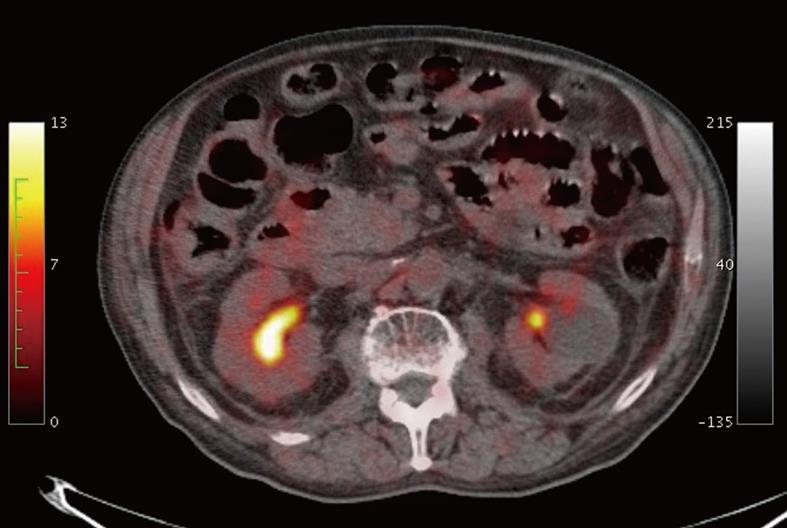
Moreover, a PET/CT scan was performed to re-stage the multiple myeloma as requested by the haematologist. After a fast of 6 h prior to examination and previous normalization of the blood glucose levels with an oral hypoglycemic agent, PET/CT examination was carried out 60 min after intravenous administration of 370 MBq of 18FDG. The PET/CT images did not show a focal or diffuse increase in tracer uptake of small bowel walls (Figure 5).
Finally, the US, MRI and PET/CT findings suggested a malabsorption disease. As a result, considering that the patient was affected by myeloma, the diagnosis of primary systemic AL involving diffusely the gastro-intestinal tract was evaluated as the most probable. To test this diagnostic hypothesis we performed a biopsy of the rectal mucosa: the histological examination of the specimen disclosed diffuse infiltration by amyloid after staining with Congo red (Figure 6).
Focal amyloidomas of the duodenum and jejunum have been described as extensive amyloid deposits involving the entire wall thickness, with destruction of the mucosa and deep infiltration into the muscularis propria and with luminal narrowing of blood vessels[4-7].
The peculiarity of case report 1 is related to the focal deposition of amyloid proteins exclusively within intramural blood vessel walls of the terminal ileum. In our patient arteriolar amyloid deposition may have caused ischemia, resulting pathologically in submucosal edema and mucosal erosion and ulceration and small bowel wall thickening, and clinically in acute onset of abdominal pain with intestinal sub-occlusion.
The thickened walls of the terminal ileum with mild pseudoaneurysmatic dilatation of the lumen associated to multiple ileo-cecal lymph nodes ranging between 10 and 20 mm suggested to us an intestinal lymphoma or less probably an atypical presentation of a terminal ileitis. The hypothesis of intestinal ischemia related to focal amyloid deposition was not evaluated.
Although a specific US or CT pattern has never been reported, the presence of a focal pseudoaneurysmal dilatation of the small bowel lumen associated to wall thickening may represent a radiological sign useful for considering intestinal amyloidosis in the differential diagnosis[4,8,9]. Amyloid may form deposits within the neural myenteric plexus, which determine the hypotonia of the gastro-intestinal wall and promote the pseudoaneurysmal dilatation[9]. On the contrary, the segmental bowel loop pseudoaneurysmal dilatation of intestinal lymphoma is highly suggestive of underlying celiac disease, likely as a reflection of the generalized hypotonia known to exist in the presence of this condition[10].
The diagnosis of localized intestinal amyloidosis is (1) potentially problematic because it is possible that the pathology may occur in the setting of subclinical extraintestinal disease or may represent an early manifestation of systemic amyloidosis; and (2) really crucial because the prognosis for patients with focal amyloidosis is generally good, whereas that for patients with systemic disease is usually poor[1]. Therefore, since the clinical presentation is often not specific and an abdominal CT scan may be required, it is important that radiologists consider the possible diagnosis of amyloidosis in the presence of focal pseudoaneurysmal dilatation of the small bowel lumen associated with wall thickening.
Case report 2 is the first describing the MRI findings of diffuse intestinal amyloidosis characterized by (1) wall symmetrical thickening with increased number and hypertrophy of the folds causing “jejunalization” of the ileum; (2) the absence of wall stratification; and (3) diffuse non-specific wall enhancement and the PET/CT findings characterized by the absence of a focal or diffuse increase of tracer uptake in diffuse thickened small bowel walls.
MRI signs, like the US and CT patterns, seem to be non-specific and are common to other malabsorption diseases, however they may suggest the clinician should consider diffuse intestinal amyloidosis in the differential diagnosis. On the other hand, the lack of pathological tracer uptake on PET/CT images is highly indicative of the absence of active inflammatory and/or neoplastic cells in the intestinal walls and represents a really important finding suggesting the storage nature of the disease characterized by the intramural deposition of an amorphous substance such as amyloid. Considering that a PET/CT scan may be performed in the staging or restaging protocol of patients with multiple myeloma, the radiologist and the nuclear medicine physician should suggest the coexistence of primary systemic AL when they observe absence of pathological tracer uptake in a diffuse intestinal wall thickening.
In conclusion, since the clinical presentation of focal or diffuse intestinal amyloidosis is frequently non-specific and requires an US and/or CT and/or MRI scan and occasionally a PET/CT scan, it is important that radiologists become confident with imaging findings of intestinal amyloidosis in order to make a correct differential diagnosis allowing a proper diagnostic and therapeutic management.
Peer reviewers: Xiao-Peng Zhang, Department of Radiology, Peking University School of Oncology, Beijing Cancer Hospital and Institute, No. 52 Haidian District, Beijing 100142, China; Marc Basson, MD, PhD, MBA, Department of Surgery, Michigan State University, 1200 East Michigan Avenue, Suite #655, Lansing, MI 48912, United States
S- Editor Tian L L- Editor O’Neill M E- Editor Zheng XM
| 1. | Scott PP, Scott WW Jr, Siegelman SS. Amyloidosis: an overview. Semin Roentgenol. 1986;21:103-112. |
| 2. | Tada S, Iida M, Yao T, Kawakubo K, Yao T, Fuchigami T, Okada M, Fujishima M. Gastrointestinal amyloidosis: radiologic features by chemical types. Radiology. 1994;190:37-42. |
| 3. | Gilat T, Revach M, Sohar E. Deposition of amyloid in the gastrointestinal tract. Gut. 1969;10:98-104. |
| 4. | Kim SH, Han JK, Lee KH, Won HJ, Kim KW, Kim JS, Park CH, Choi BI. Abdominal amyloidosis: spectrum of radiological findings. Clin Radiol. 2003;58:610-620. |
| 5. | Peny MO, Debongnie JC, Haot J, Van Gossum A. Localized amyloid tumor in small bowel. Dig Dis Sci. 2000;45:1850-1853. |
| 6. | Hamaya K, Kitamura M, Doi K. Primary amyloid tumors of the jejunum producing intestinal obstruction. Acta Pathol Jpn. 1989;39:207-211. |
| 7. | Saindane AM, Losada M, Macari M. Focal amyloidoma of the small bowel mimicking adenocarcinoma on CT. AJR Am J Roentgenol. 2005;185:1187-1189. |
| 8. | Araoz PA, Batts KP, MacCarty RL. Amyloidosis of the alimentary canal: radiologic-pathologic correlation of CT findings. Abdom Imaging. 2000;25:38-44. |
| 9. | Kala Z, Válek V, Kysela P. Amyloidosis of the small intestine. Eur J Radiol. 2007;63:105-109. |
| 10. | Lohan DG, Alhajeri AN, Cronin CG, Roche CJ, Murphy JM. MR enterography of small-bowel lymphoma: potential for suggestion of histologic subtype and the presence of underlying celiac disease. AJR Am J Roentgenol. 2008;190:287-293. |