Published online May 21, 2010. doi: 10.3748/wjg.v16.i19.2407
Revised: February 10, 2010
Accepted: February 17, 2010
Published online: May 21, 2010
AIM: To investigate relapse predictors in chronic hepatitis C (CHC) patients with end-of-treatment response (ETR), after pegylated interferon-α (PegIFN-α) and ribavirin treatment.
METHODS: In a retrospective study we evaluated a spectrum of predictors of relapse after PegIFN-α and ribavirin treatment in 86 CHC patients with ETR. Viral loads were determined with real-time reverse transcription polymerase chain reaction. Hepatitis C virus genotyping was performed by sequencing analysis. Patients with genotype 1 were treated for 48 wk with 180 μg PegIFN-α2a or 1.5 μg/kg PegIFN-α2b once weekly plus ribavirin at a dosage of 1000 mg/d for those under 75 kg or 1200 mg/d for those over 75 kg. Patients with genotypes 2 and 3 were treated for 24 wk with 180 μg PegIFN-α2a or 1.5 μg/kg PegIFN-α2b once weekly plus ribavirin at a dosage of 800 mg/d.
RESULTS: In all ETR patients, binary logistic regression analysis identified absence of complete early virological response (cEVR) (OR 27.07, 95% CI: 3.09-237.26, P < 0.005), serum alkaline phosphatase (ALP) levels prior to therapy < 75 U/L (OR: 6.16, 95% CI: 2.1-18.03, P < 0.001) and body mass index > 26 kg/m2 (OR: 8.27, 95% CI: 2.22-30.84, P < 0.005) as independent predictors of relapse. When cEVR patients were analyzed exclusively, ALP prior to therapy < 75 U/L remained the only predictor of relapse.
CONCLUSION: Lower levels of ALP prior to, during and after therapy seem to be associated with a higher risk of relapse in CHC patients with ETR.
- Citation: Bodlaj G, Hubmann R, Saleh K, Stojakovic T, Biesenbach G, Berg J. Alkaline phosphatase predicts relapse in chronic hepatitis C patients with end-of-treatment response. World J Gastroenterol 2010; 16(19): 2407-2410
- URL: https://www.wjgnet.com/1007-9327/full/v16/i19/2407.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i19.2407
Chronic hepatitis C (CHC) is a major public health problem due to late complications, such as liver failure and hepatocellular carcinoma[1]. The prevalence of CHC has been estimated to be 3% worldwide[2]. Pegylated interferon-α (PegIFN-α) with ribavirin is currently the treatment of choice for CHC[3]. Treatment response depends on patient and viral factors, the viral genotype being the most important[4]. Several studies have defined further predictive factors for response, such as hepatitis C virus (HCV)-RNA serum level, stage of liver disease, body weight, age, sex and race[5-10]. After completion of treatment, HCV may become detectable again considerably reducing sustained virological response (SVR) rates even after achievement of end-of-treatment response (ETR). The relapse rate of genotype 1 seems to be higher than in other genotypes, being as high as 30%-40%[11]. The risk of relapse was also shown to relate to treatment duration, and several efforts have been undertaken to identify those patients who would benefit from extended treatment duration[12].
In this retrospective study we investigate in a cohort of ETR patients whether further predictors of relapse may exist after treatment with PegIFN-α and ribavirin.
We reviewed the records of all CHC patients with an ETR who started PegIFN-α and ribavirin treatment between April 2005 and August 2007 at our clinic. Exclusion criteria were age < 18 years; any bone or bowel diseases, chronic renal failure, use of medication that could cause an elevation of liver enzymes; biliary obstruction confirmed sonographically; hepatitis B virus or human immunodeficiency virus co-infection; daily ethanol ingestion greater than 20 g for women and 40 g for men; drug abuse; history of previous antiviral treatment. Finally, 86 patients (62 men and 24 women, mean age 42.5 ± 11.3 years, range 18-67 years) were included. Genotypes 1, 2 and 3 were present in 52 (60.5%), 9 (10.5%) and 25 (29%) patients, respectively. Patients with genotype 1 were treated for 48 wk with 180 μg PegIFN-α2a or 1.5 μg/kg PegIFN-α2b once weekly plus ribavirin at a dosage of 1000 mg/d for those under 75 kg or 1200 mg/d for those over 75 kg. Patients with genotypes 2 and 3 were treated for 24 wk with 180 μg PegIFN-α2a or 1.5 μg/kg PegIFN-α2b once weekly plus ribavirin at a dosage of 800 mg/d. Patients with extended treatment duration were not included in this study. A complete early virological response (cEVR) was considered present, when HCV-RNA by polymerase chain reaction (PCR) was negative 12 wk after initiation of therapy. Patients, who did not achieve a cEVR showed at least a partial early virological response (pEVR), defined as a 2-log-decline of HCV-RNA at 12 wk after the initiation of therapy compared to baseline viral load. ETR was defined as negative HCV-RNA at the end of treatment, and SVR was defined as persistent absence of HCV-RNA by PCR at 6 mo, thereafter.
Alkaline phosphatase (ALP), alanine aminotransferase, aspartate aminotransferase and γ-glutamyl transferase were assessed by routine colorimetric assays on the Modular Clinical Analyzer (Roche, Vienna, Austria). Diagnostic ranges were 1-1200 U/L, 4-600 U/L, 4-800 U/L and 3-1200 U/L, respectively. Normal ranges of ALP were 40-129 U/L for males and 35-104 U/L for females. The precision of the ALP assay was > 99.3%.
Viral loads were determined with the Cobas TaqMan HCV assay (Quantification range 10 to 6.9 × 107 IU/mL; Roche). HCV genotyping was performed by sequencing with the TruGene™ HCV 5’NC Genotyping Kit (Siemens Medical Solutions Diagnostics, Bad Nauheim, Germany).
Liver biopsy was available in 33 patients. Stages of liver fibrosis and inflammatory activity were classified according to the Metavir score[7].
Statistical analysis was performed using the Statistical Package for Social Sciences (version 10, SPSS Inc., Chicago, Illinois, USA). Values are expressed as mean ± SD or as median (range). Differences between groups were compared by Student’s t-test or Mann-Whitney U test where appropriate. Fisher’s exact test was used to assess possible differences in categorical variables between patients with SVR and relapse. Possible correlations between variables were analyzed using the Spearman rank test. Binary logistic regression analysis was performed to identify independent predictors of relapse. Significance tests were 2-sided, and P-values < 0.05 were considered significant.
The clinical characteristics of the ETR patients are summarized in Table 1. Relapse occurred in 26 patients (30.2%), 17 patients (65.4%) with genotype 1, three patients (11.5%) with genotype 2 and 6 patients (23.1%) with genotype 3. The frequency of patients with genotype 1 was not significantly different between the relapse and the SVR group (Table 1). Median ALP prior to therapy was significantly higher, whereas mean body mass index (BMI), stage of liver fibrosis and viral load prior to therapy were significantly lower in patients with SVR compared to those with relapse. The frequency of cEVR was significantly higher in the SVR group compared to the relapse group (Table 1).
| SVR (n = 60) | Relapse (n = 26) | P-value | |
| Age (yr) | 41.3 ± 11.3 | 45.4 ± 10.9 | NS |
| Male/female | 43/17 | 19/7 | NS |
| Body mass index (kg/m2) | 24.9 ± 4.0 | 28.7 ± 5.5 | < 0.010 |
| Systolic blood pressure (mmHg) | 119 ± 14 | 119 ± 12 | NS |
| Diastolic blood pressure (mmHg) | 73 ± 10 | 75 ± 10 | NS |
| Genotype 1, n (%) | 35 (58.3) | 17 (65.4) | NS |
| HCV-RNA prior to therapy (IU/mL) | 480 500 (2430-23 000 000) | 806 000 (107 000-8 400 000) | < 0.050 |
| PegIFN α2a/PegIFN α2b | 32/28 | 14/12 | NS |
| cEVR, n (%) | 58 (96.7) | 17 (65.4) | < 0.001 |
| Histological fibrosis stage | 1 (0-4) | 3 (1-4) | < 0.050 |
| Histological activity | 1 (1-3) | 2 (1-3) | NS |
| Aspartate aminotransferase (U/L) | 40 (15-416) | 47.5 (24-156) | NS |
| Alanine aminotransferase (U/L) | 72.5 (17-560) | 70 (32-214) | NS |
| γ-glutamyl transferase (U/L) | 39 (3-693) | 48 (11-183) | NS |
| Alkaline phosphatase (U/L) | 84 (40-232) | 67 (48-129) | < 0.005 |
| Cholinesterase (kU/L) | 7.22 ± 2.18 | 7.50 ± 2.37 | NS |
| Lactate dehydrogenase (U/L) | 190 ± 42 | 193 ± 34 | NS |
| Total bilirubin (mg/dL) | 0.77 ± 0.57 | 0.68 ± 0.24 | NS |
| C-reactive protein (mg/dL) | 0.2 (0-1.8) | 0.1 (0-1.0) | NS |
| Fibrinogen (mg/dL) | 256 ± 52 | 273 ± 54 | NS |
In all ETR patients, binary logistic regression identified absence of cEVR (OR 27.07, 95% CI: 3.09-237.26, P < 0.005), ALP prior to therapy < 75 U/L (OR: 6.16, 95% CI: 2.1-18.03, P < 0.001) and BMI > 26 kg/m2 (OR: 8.27, 95% CI: 2.22-30.84, P < 0.005) as independent predictors of relapse. When only cEVR patients were analyzed, ALP prior to therapy < 75 U/L (OR: 7.79, 95% CI: 1.96-30.98, P < 0.005) remained the only predictor of relapse.
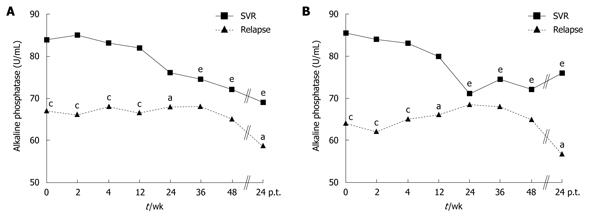
Prior to therapy, all ALP levels in patients with relapse were within the normal range.
During PegIFN-α and ribavirin treatment, ALP levels showed variations: in patients with SVR there was a significant decline, whereas in patients with relapse there was a slight non-significant variation (Figure 1A). Similar results were obtained when patients with genotype 1 were analyzed, only (Figure 1B).
The results of our study indicate that serum levels of ALP are related to the risk of relapse in CHC patients with ETR after treatment with PegIFN-α and ribavirin.
Elevated levels of ALP are found in bone, bowel and bile duct diseases. As our study did not include patients with bone or bowel diseases, the observed significant difference in pre-treatment ALP between patients with relapse and those with SVR might be caused mainly by differing amounts of ALP from the liver or the bile ducts as the primary source of disease. Bile duct inflammation has been reported in up to 95% of HCV patients and was shown to correlate with serum ALP[13]. Since immune response and inflammation play key-roles in the elimination of HCV, the higher pre-treatment ALP levels in patients with SVR may possibly reflect a higher degree of inflammation with a more sustained response to therapy. However, the histological activity score in our patients failed to show this, as there was no significant difference between patients with SVR and relapse, which could be due to our limited sample size. The observed decline in ALP during PegIFN-α and ribavirin treatment may possibly be due to a decrease of intrahepatic inflammation, which initially could have been higher in those patients with SVR. PegIFN-α and ribavirin may also influence bone metabolism and levels of bone-derived ALP, however, other studies were unable to confirm these findings[14-16]. Any pathophysiological reasoning for the association of ALP levels and risk of relapse of CHC is speculative at present.
Our study is the first to focus on ETR-patients treated with PegIFN-α and ribavirin. Most patients in our study exhibited ALP levels within the normal range with only 5% of patients with SVR exceeding the upper limit of the normal range. The limits of normal ranges are different in males and females, but gender distribution was almost equal in both patient groups.
In previous studies, genotype 1 was associated with increased relapse rates compared to the other genotypes[11]. In our study, the relapse rate in genotype 1 patients was not significantly higher probably due to the limited number of patients studied.
The strongest predictor of relapse in our study was absence of cEVR. Nearly all patients with SVR and almost two thirds of patients with relapse exhibited a cEVR. When cEVR is achieved, treatment duration is usually not extended. As prolongation of treatment has been shown to reduce relapse rates, ALP may possibly help to identify those patients, who would benefit from extended treatment duration[12].
Limitations of our study comprise its retrospective nature, limited sample size and lack of histological examinations in all patients. As ALP is elevated in a variety of diseases, we cannot exclude that some subclinical diseases with minor ALP elevations confounded our data. Despite those limitations, median ALP was higher in our SVR patients compared to those with relapse at all time points.
In conclusion, lower levels of ALP prior to, during and after therapy with PegIFN-α and ribavirin seem to be associated with a higher risk of relapse in patients with ETR. Further studies are required to clarify whether our observation could help to identify those patients who would benefit from extended therapy despite the presence of cEVR.
Chronic hepatitis C (CHC) is a major public health problem due to late complications, such as liver failure and hepatocellular carcinoma. Pegylated interferon-α (PegIFN-α) together with ribavirin is currently the treatment of choice for CHC. After achievement of end-of-treatment response (ETR) in CHC patients, the occurrence of relapse reduces sustained virological response (SVR) rates.
Treatment response in CHC patients depends on patient and viral factors, the viral genotype being the most important. Several studies have defined further predictive factors for response, such as hepatitis C virus (HCV)-RNA serum level, stage of liver disease, body weight, age, sex and race. In this study the authors investigated in a cohort of ETR patients, whether further predictors of relapse may exist after treatment with PegIFN-α and ribavirin.
Previous studies investigated predictors of relapse in HCV infected individuals after PegIFN-α and ribavirin therapy. Those studies examined responding as well as non-responding patients, and predictive factors for response, such as HCV-RNA serum level, stage of liver disease, body weight, age, sex and race were identified. After completion of treatment, recurrence of the virus is still a major issue and a quest has been initiated to determine whether there may be predictors of relapse, in order to identify those patients who might benefit from extended treatment duration up front. The result of this study would suggest that alkaline phosphatase (ALP) might be such a predictor in ETR patients. This study is the first to focus on ETR patients, exclusively.
The authors suggest that apart from monitoring the already known predictors of relapse, ALP monitoring should be included in the work-up of HCV infected patients in preparation for anti-HCV treatment. Furthermore, ALP as an inexpensive clinical parameter should be monitored through-out the treatment period. Their results warrant larger studies to be conducted in a prospective fashion, in order to elucidate the clinical value of ALP monitoring in HCV patients undergoing treatment.
It’s a very interesting paper and I propose it to be published. Sure AP is not the only important tool to guarantee a SVR but can be useful to speak with patient’s about this possibility.
Peer reviewer: Dr. Bernardo Frider, MD, Professor, Department of Hepatology, Hospital General de Agudos Cosme Argerich, Alte Brown 240, Buenos Aires 1155, Argentina
S- Editor Wang JL L- Editor Webster JR E- Editor Lin YP
| 1. | El-Serag HB. Hepatocellular carcinoma and hepatitis C in the United States. Hepatology. 2002;36:S74-S83. [Cited in This Article: ] |
| 2. | Heintges T, Wands JR. Hepatitis C virus: epidemiology and transmission. Hepatology. 1997;26:521-526. [Cited in This Article: ] |
| 3. | National Institutes of Health Consensus Development Conference Statement: Management of hepatitis C: 2002--June 10-12, 2002. Hepatology. 2002;36:S3-S20. [Cited in This Article: ] |
| 4. | Xie Y, Xu DZ, Lu ZM, Luo KX, Jia JD, Wang YM, Zhao GZ, Zhang SL, Zhang DZ. Impact of virus genotype on interferon treatment of patients with chronic hepatitis C: a multicenter controlled study. Hepatobiliary Pancreat Dis Int. 2004;3:369-374. [Cited in This Article: ] |
| 5. | Lindsay KL. Introduction to therapy of hepatitis C. Hepatology. 2002;36:S114-S120. [Cited in This Article: ] |
| 6. | Soresi M, Tripi S, Franco V, Giannitrapani L, Alessandri A, Rappa F, Vuturo O, Montalto G. Impact of liver steatosis on the antiviral response in the hepatitis C virus-associated chronic hepatitis. Liver Int. 2006;26:1119-1125. [Cited in This Article: ] |
| 7. | Myers RP, Patel K, Pianko S, Poynard T, McHutchison JG. The rate of fibrosis progression is an independent predictor of the response to antiviral therapy in chronic hepatitis C. J Viral Hepat. 2003;10:16-22. [Cited in This Article: ] |
| 8. | Reddy KR, Hoofnagle JH, Tong MJ, Lee WM, Pockros P, Heathcote EJ, Albert D, Joh T. Racial differences in responses to therapy with interferon in chronic hepatitis C. Consensus Interferon Study Group. Hepatology. 1999;30:787-793. [Cited in This Article: ] |
| 9. | Medina J, García-Buey L, Moreno-Monteagudo JA, Trapero-Marugán M, Moreno-Otero R. Combined antiviral options for the treatment of chronic hepatitis C. Antiviral Res. 2003;60:135-143. [Cited in This Article: ] |
| 10. | Fukutomi T, Fukutomi M, Iwao M, Watanabe H, Tanabe Y, Hiroshige K, Kinukawa N, Nakamuta M, Nawata H. Predictors of the efficacy of intravenous natural interferon-beta treatment in chronic hepatitis C. Med Sci Monit. 2000;6:692-698. [Cited in This Article: ] |
| 11. | Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL Jr, Häussinger D, Diago M, Carosi G, Dhumeaux D. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. [Cited in This Article: ] |
| 12. | Brouwer JT, Nevens F, Bekkering FC, Bourgeois N, Van Vlierberghe H, Weegink CJ, Lefebvre V, Van Hattum J, Henrion J, Delwaide J, Hansen BE, Schalm SW, For The Benelux Study Group On Treatment Of Chronic Hepatitis C. Reduction of relapse rates by 18-month treatment in chronic hepatitis C. A Benelux randomized trial in 300 patients. J Hepatol. 2004;40:689-695. [Cited in This Article: ] |
| 13. | Banner BF, Allan C, Smith L, Savas L, Bonkovsky HL. Effect of interferon therapy on bile duct inflammation in hepatitis C. Virchows Arch. 1996;428:253-259. [Cited in This Article: ] |
| 14. | Trombetti A, Giostra E, Mentha G, Negro F, Rizzoli R. Lack of evidence for ribavirin-induced bone loss. Hepatology. 2002;36:255-257. [Cited in This Article: ] |
| 15. | Oreffo RO, Romberg S, Virdi AS, Joyner CJ, Berven S, Triffitt JT. Effects of interferon alpha on human osteoprogenitor cell growth and differentiation in vitro. J Cell Biochem. 1999;74:372-385. [Cited in This Article: ] |
| 16. | Lee J, Kim JH, Kim K, Jin HM, Lee KB, Chung DJ, Kim N. Ribavirin enhances osteoclast formation through osteoblasts via up-regulation of TRANCE/RANKL. Mol Cell Biochem. 2007;296:17-24. [Cited in This Article: ] |