Published online May 21, 2010. doi: 10.3748/wjg.v16.i19.2341
Revised: January 10, 2010
Accepted: January 17, 2010
Published online: May 21, 2010
The development of laparoscopic surgery has generated the new field of study, laparoscopic anatomy. This article reviews the reported literature on laparoscopic anatomy and explores how it has evolved along with advances in abdominal surgery. In addition, the principal concerns in current laparoscopic anatomy research are discussed, including: (1) types of special adjacent anatomical structures; and (2) special surgical planes and anatomical landmarks. Understanding of systematic laparoscopic anatomy can provide the junior surgeons a clear procedural approach, and would benefit laparoscopic surgeons in training.
- Citation: Li LJ, Zheng XM, Jiang DZ, Zhang W, Shen HL, Shan CX, Liu S, Qiu M. Progress in laparoscopic anatomy research: A review of the Chinese literature. World J Gastroenterol 2010; 16(19): 2341-2347
- URL: https://www.wjgnet.com/1007-9327/full/v16/i19/2341.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i19.2341
The current surgery relies heavily on the use of laparoscopy due to its minimally invasive nature. In the field of abdominal surgery, the traditional approach of open surgery and related surgical anatomy are well established, while laparoscopic surgery and correlative laparoscopic anatomy are still under study.
Since laparoscopic surgery produces less trauma, and yields rapid recovery and superior cosmetic effects, it has become increasingly popular among both patients and physicians. However, laparoscopic surgery is not yet advanced enough to overcome its disadvantage of the lack of tactile sense. This impedes the sensitivity of applied laparoscopy to define the important anatomic features, structure locations, and boundary and texture of excision target tissues. Hand-assisted laparoscopy was developed to give surgeons more control and sensitivity while using laparoscopic instrumentation and digital visualization tools, which has been applied widely in abdominal surgery. Unfortunately, the lap-disc sealing and access device is costly and not extensively used in operations.
A second compelling disadvantage in the laparoscopic procedure is the loss of hand flexibility of the surgeon due to limited availability of space in the patient’s body, thus increasing the technical difficulty of the procedure. However, a systematical knowledge of laparoscopic anatomy can help surgeons to address this challenge. Clarified anatomical structures in the high-resolution and high-magnified view are of great significance in miniscule anatomical procedures, with little bleeding in the laparoscopic field of sight in the correct surgical plane while dissection was performed. Thus, gaining a thorough understanding of the laparoscopic anatomy is crucial to a successful laparoscopic surgery.
In order to provide guidance for laparoscopic surgeons in anatomy and technique, we have reviewed and summarized the current laparoscopic anatomical studies on abdominal surgery published in the literature. Although there are variations in anatomy among individual patients and in procedures used among surgeons, many commonalities exist and can be generalized to aid in the application of laparoscopy.
Systematic literature search was performed on PubMed, Medline, ScienceDirect, SpringerLink, CMCI, VIP, and CNKL to find relevant articles using the terms “laparoscopy”, “anatomy”, and “minimally invasive”. Papers both in English and Chinese concerning anatomical characteristics in laparoscopic procedures in the fields of abdominal surgery were identified. The search results were classified as: (1) anatomical descriptions; (2) anatomical variations; (3) surgical planes and landmarks; and (4) measurements of certain structures. Papers dealing with different types of laparoscopic procedures were summarized according to the above classifications to identify the key points of each procedure.
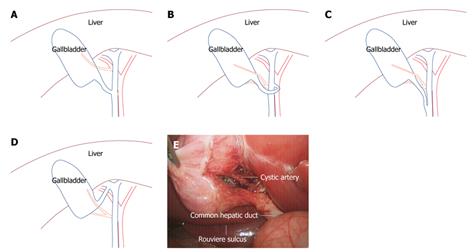
Studies on laparoscopic anatomy of laparoscopic cholecystectomy (LC) mainly focused on Calot’s triangle, including the cystic artery and cystic duct[1,2]. The cystic artery normally has 1-3 branches into the gallbladder, originates from the proper hepatic artery, and is located within Calot’s triangle. The variation of the common structure included origination from the right hepatic artery (Figure 1A), or from gallbladder bed of an aberrant type. The cystic duct is normally connected directly to the right wall of the hepatic duct; variations included connection to the left wall of the hepatic duct through an anterior or posterior approach (Figure 1B). Other structural differences reported in the literature were as follows: cystic duct location in parallel with the common hepatic duct (Figure 1C), presentation as an aberrant bile duct, adhesion and atresia, and an even connection to the right hepatic duct (Figure 1D).
Anatomical landmarks in the LC were mainly reported as Rouviere’s sulcus, cystic lymph nodes and arteries[3]. The Rouviere’s sulcus is a useful demarcation for the division between the S5 and S6 of the liver. When the gallbladder ampulla is stretched to the head side, the Rouviere’s sulcus can be exposed. The Rouviere’s sulcus was reported as an accurate landmark for the common hepatic duct plane, as they are on the same transversal level, above which the dissection of Calot’s triangle is safe. When the fascial strip in Calot’s triangle was flattened, the cystic artery could be identified by its visible pulsation and the presence of an adjacent, cystic lymph node. The cystic artery has been reported to run through the level of the gallbladder neck; exposure of the cystic artery may help surgeons locate the gallbladder neck at the same level. In addition, identification of the cystic lymph node may help define the cystic duct and the cystic artery structures (Figure 1E).
The key concerns in LC anatomy research are how to protect the common bile duct and right hepatic duct, which are injury-prone during the laparoscopic procedure. It is necessary to clarify the anatomy of Calot’s triangle and ensure that the superior Rouviere’s sulcus is on a safe surgical plane.
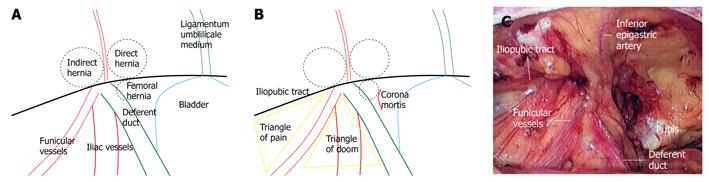
Studies on the laparoscopic anatomy of herniorrhaphy focused primarily on inguinal hernias. Currently, there are two main approaches for laparoscopic inguinal herniorrhaphy (LIH), i.e. the transabdominal pre-peritoneal and the totally extra-peritoneal. Using laparoscopy, the presence of a direct hernia, indirect hernia or femorocele is readily found according to the position of the hernial ring, as shown in Figure 2A. There are marked differences in anatomical structure between oblique hernia and direct hernia. Thus, the size of mesh used in the operation should be decided according to each individual patient’s anatomic data[4].
In the reported LIH procedures, meshes were always secured by either staples or sutures. Three potentially hazardous regions (Figure 2B) were identified and were seriously considered. (1) The so-called triangle of doom, wherein the medial margin is the deferent duct and the lateral margin contains the funicular vessels, should not be subject to staples or sutures because the iliac artery resides in this region; (2) An unexpected source of hemorrhage is identified as the corona mortis (translated as the crown of death), wherein lies the aberrant obturator artery and vein. Branches and distribution of the vascular connection between the obturator system and the external iliac or inferior epigastric systems, are located over the superior pubic ramus[5]; and (3) The triangle of pain, wherein the superior margin is the iliopubic tract (inguinal ligament), and the medial margin is the funicular vessel, is the region where the femoral branch of the genitofemoral nerve and the lateral femoral cutaneous nerve reside. The occurrence of post-operative pain was reported to have originated from staples or sutures placed in this region. It is reported that 15% of the lateral femoral cutaneous nerve was within 0.5 cm of the iliopubic tract or in the vertical plane of the anterior superior iliac spine[6]. To avoid post-operative pain, some surgeons opted against all use of the stapler.
Methods for distinguishing these important structures are a main focus throughout the laparoscopic anatomy literature[7-9]. In live procedures, the structures could be totally exposed after the peritoneum was completely removed from the abdominal wall, as shown in Figure 2C.
The obvious hazards in LIH are the potential for injury of the deferent duct and unexpected hemorrhea. The major technique to protect the deferent duct in this procedure is skeletonization of the spermatic cord. In a nutshell, surgeons should take heed of one “cord”, one “crown” and two “triangles”.
Studies on the laparoscopic anatomy of colonic resection have focused on the left colon and rectum. Locating the root of the inferior mesenteric artery (IMA) and protecting the autonomic nerve and ureter are of importance in total mesorectal excision. The reports identified a particular landmark and a surgical plane.
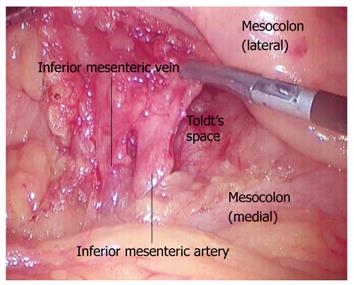
IMA is located between the horizontal portion of the duodenum and the bifurcation of the abdominal aorta; the pulsation of the aorta made the identification easy. The landmark used to find the root of IMA was the convex stalk on the surface of the abdominal aorta. The superior sigmoid colon mesentery was lifted to the left side, straightening out the IMA to form an included angle, and the margins were defined as the abdominal aorta and the root of IMA[10] (Figure 3).
The important surgical plane that is yet to be defined is known as Toldt’s space. During embryonic development, the mid-gut mesentery after succeeding in the volvulus combined with the peritoneal wall layer to form Toldt’s fascia. Toldt’s space appeared and remained consistently between the lateral left mesocolon and pre-renal fascia, as well as between the medial left mesocolon and pre-aortic fascia. The space also appeared between the mesorectum and parietal layer of the pelvic fascia.
On the medial side, Toldt’s space in the angle of the abdominal aorta and IMA was a vessel free area (Figure 3). IMA ran along the surface of the cephalic abdominal aorta, down to the pelvic cavity and pre-sacral space[11,12]. On the lateral side, an obvious yellow-white borderline was visible. The yellow portion is a part of the sigmoid mesocolon, and the white is a lateral abdominal wall. Dissecting from the medial and lateral space frees the colorectal mesentery completely. The surgical planes are between the colorectal mesenteries and the continuous pre-renal fascia.
Dissecting in the Toldt’s space might protect autonomic nerves and the ureter because they are all located beneath the pre-renal fascia. The superior hypogastric plexus closely associates with the back of the IMA. No obvious branches of autonomic nerves were found in the loose space between the visceral layer fascia (mesorectum) and the wall layer fascia (parietal pelvic fascia) at the back of the rectum[13].
In summary, the root of IMA and Toldt’s space, which guided most total dissections of lymph nodes and protected the autonomic nerve, appear to be the most important anatomic structures in laparoscopic colorectal resection.
Laparoscopy was often used to treat benign gastric diseases during the early stages. Recently, laparoscopic surgery has been increasingly used in radical gastrectomy, and more researches in laparoscopic anatomy have appeared[14,15], focusing on identifying crucial surgical planes and anatomical landmarks.
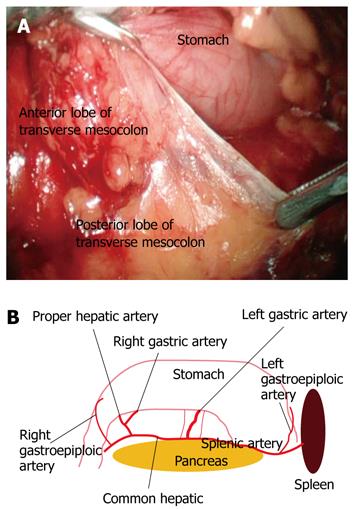
In LRG, there appeared to be two surgical planes that were important to define. The first was found between the posterior layer of the dorsal mesogastrium and the pancreas. The derivation of the posterior layer of the dorsal mesogastrium formed the gastrosplenic ligament, pancreatic fascia, pancreaticoduodenal fascia and the anterior lobe of the transverse mesocolon, which was continuous with each other as integrity. The surgical plane could be fixed in the space between the anterior and posterior lobe of the transverse mesocolon (Figure 4A). The plane could be reached more easily for dissection from the shortest distance of connecting fascia from the transverse colon to the descending part of the duodenum. The second surgical plane was identified in the space between the retropancreatic and pre-renal fascia, a safe plane for retropancreatic lymph node dissection. The pre-renal fascia was the post-boundary for ensuring a safe operational plane.
Because of restricted laparoscopic sight and instrument manipulability, the procedure was performed from the caudal end to head. Therefore, the main vessels were identified in the space of the first surgical plane mentioned above. The paries posterior gastricus was gradually turned over to the head side following the opening of the first surgical plane, where each important vessel could be identified with the guidance of the following landmarks (Figure 4B). The pancreas was the largest central landmark. When dissecting in the gastropancreatic ligament near the pancreas head, the gastroduodenal artery and right gastroepiploic artery could be identified. Likewise, when dissecting in the splenocolic ligament near the pancreas tail, the left gastroepiploic vessels could be exposed. After the pre-pancreas fascia was absolutely dissociated, two folds were formed by the pre-pancreas fascia and its related vessels. The hepatopancreatic fold was the landmark used to find the common hepatic artery and proper hepatic artery at the inferior margin of the pancreatic neck. The gastropancreatic fold was used to locate the left gastric artery.
In LRG, lymph nodes dissection was complicated, which could be achieved by recognizing and skeletonizing the related vessels. In summary, one center (pancreas), two ligaments (gastropancreatic and plenocolic ligaments), and two folds (hepatopancreatic and gastropancreatic folds) are the key landmarks identified in the literature used to find each vessel. These landmarks could be readily defined if the surgical plane (space between posterior layer of dorsal mesogastrium and pancreas) was appropriately identified.
Anatomic studies of laparoscopic liver resection were mainly carried out by autopsy. The research priorities were placed on the first and the secundum porta hepatis[16,17]. Laparoscopic procedures were mostly performed as hepatic left lateral lobectomies. Therefore, the important structure of the left triangle ligament was defined as the peritoneal reflection of the diaphragm and the left lateral lobe. Superior hepatic arteries, branches originating from the left gastric artery and inferior phrenic artery, could be found after the left triangle ligament was opened (taking up 80%). The trunk of the superior hepatic arteries crossing the left hepatic veins of the superior posterior margin was an important landmark for locating the hepatic vein, as well as the secundum porta hepatis[18]. The falciform ligament was found as the anterior pathway to reach the secundum porta hepatis by laparoscopy. The left, inferior phrenic artery and middle segment artery of the liver formed a vessel that arched and gave off 6-12 branches to the falciform ligament, finally draining into the left inferior phrenic vein. The falciform ligament provides significant collateral circulation to the liver and is an important landmark for laparoscopic liver surgery[19].
Laparoscopic pancreaticoduodenectomy (LPD) is recognized as an extremely difficult laparoscopic operation due to the emergency status of the laparoscopic surgery. Chinese surgeons[20] developed a method of retroperitoneal laparoscopic dissection to expose the pancreatic body and tail. The retro-pancreatic space was reached using testis (ovarian) vessels as anatomical landmarks. The peritoneum was a landmark for the belly side of the para-renal space, and the splenic artery was a landmark of the upper pancreatic edge. The left renal vein was a landmark of the lower pancreatic edge, the left diaphragm colon ligament (or splenic lower pole) was a landmark for the pancreatic tail, and the intersection point between the right edge of the inferior mesenteric vein and the inferior margin of the pancreas were landmarks for the transition part of the pancreatic neck and body. Lu et al[21] developed the duodenal wall approach, in which the lateral wall of the duodenum was dissected and the root of the mesenteric vessel could be seen at the horizontal level of the duodenum; and the posterior pancreatic segment of the visible portal vein could be located by searching along the superior mesenteric vein.
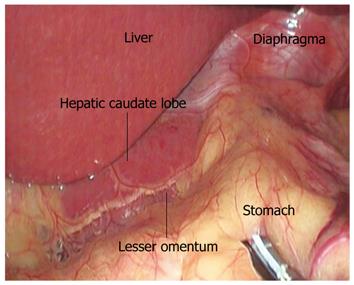
Laparoscopic fundoplication, routinely used in laparoscopic anti-reflux surgery (LS), is a classical approach for the treatment of gastroesophageal reflux disease. Its related laparoscopic anatomy emphasized the anatomic landmark and surgical plane. The procedure is initiated by opening the lesser omentum above the hepatic caudate lobe, the landmark for finding the right crura of diaphragma. The space between the right crura of diaphragma and the esophagus is the surgical plane for dissociating the esophagus[22] (Figure 5). Special anatomical variations, such as an aberrant left hepatic artery, can complicate the procedure[23].
Anatomic studies on laparoscopic splenectomy focused on the splenic vessels. In most cases, splenic arteries and veins run together along the superior edge of the pancreatic body and tail; the arteries are located above the veins. In some reported cases, the splenic arteries ran along the superior edge of the pancreatic body tail, and the splenic arteries and veins were either found at the back of the pancreas, or the arteries were absolutely absent around the pancreatic body and tail. It was required that two (in most cases), three, and four splenic, lobar branches should be clamped[24,25].
Researches on laparoscopic anatomy have not been extensively carried out or reported throughout the world. Perusal and a summary of all of the relevant studies indicated that China-based scholars did most of the research work on laparoscopic anatomy. Some works were initially carried out by autopsy, and the results were provided to surgeons for guidance in live procedures. Procedures that were already extraordinarily familiar by surgeons, such as LC and LIH, were initiated in live surgeries.
Studies on the laparoscopic anatomy of abdominal surgery mainly focused on the following aspects: (1) Simulating laparoscopic surgery on cadavers, or relevantly studying endoscopic anatomy through clinical laparoscopic surgery; (2) Obtaining relevant data by measuring the length, distance, and diameter of the tissue under the study; (3) Calculating the case percentage of special adjacent anatomical structures; and (4) Finding relevant anatomical planes, landmarks and providing relevant intraoperative pictures.
Due to the various surgical approaches, the portion of our study that focused on certain laparoscopic anatomy also varied. During laparoscopic procedure, the size and boundary of target tissues and the distance between two structures in laparoscopy sight were estimated by surgeons based on their experience. These data could not be obtained accurately by the naked eye using the existing laparoscopic technology. Therefore, some data in the autopsy studies could only be used as a reference during live laparoscopic procedures. The key point for laparoscopic surgeons was to understand the special, adjacent anatomical structure and the location of the correct anatomical plane and anatomical landmark. Gaining a definite understanding will guide future prospective studies of laparoscopic anatomy.
Why could there be less bleeding in the laparoscopic sight if manipulation occurs in certain surgical planes? Each surgical plane and natural space in the human body, are formed by stratum membranosum, like the membranous layer of the superficial fascia present in many regions of the body[26]. The basic research on surgical planes in laparoscopic anatomy should focus on vessel-less stratum membranosum.
Anatomical landmarks are descriptions of neighboring structures crucial to identifying the proper target tissue for resection. Although individual patients vary in their anatomical structure, certain commonalities exist. These commonalities become obvious through the numerous cases and procedures reported. Laparoscopic surgeons must rely on landmarks in each procedure; it is crucial in laparoscopy that detours must be minimized, otherwise an unexpected injury is likely to occur.
Sometimes, the surgical planes and anatomical landmarks can assist each other. In an appropriate plane, it would be easy to identify certain landmarks. Take LRG for example; if the space between the posterior layer of dorsal mesogastrium and pancreas could be found correctly, the landmarks of one center, two ligaments and two folds would be immediately obvious. Likewise, a landmark can help find readily a surgical plane. In LAS, the hepatic caudate lobe was the landmark for finding the right crura of diaphragma, the surgical plane for dissociating the esophagus.
Currently, laparoscopy is usually performed using the approach of natural orifice transluminal endoscopic surgery and a single entry port. These techniques require advanced skills of surgeons and a detailed knowledge of laparoscopic anatomy.
In the field of abdominal surgery, laparoscopic surgery has become increasingly popular. Systematic laparoscopic anatomy can provide laparoscopic surgeons with a clear idea to design their operation, and is particularly important for practicing novices. Laparoscopic anatomy is a basic curriculum for laparoscopic surgeons, and will certainly become an important new subject as the use of laparoscopic procedures increases.
Peer reviewers: Basil Ammori, MD, Department of Surgery, Salford Royal Hospital, Stott Lane, Salford, Greater Manchester, M6 8HD, United Kingdom; Ming-Te Huang, Professor, Department of Surgery, Taipei Medical University-Shuang Ho Hospital, Taipei County, 23561, Taiwan, China
S- Editor Wang YR L- Editor Ma JY E- Editor Zheng XM
| 1. | Balija M, Huis M, Nikolic V, Stulhofer M. Laparoscopic visualization of the cystic artery anatomy. World J Surg. 1999;23:703-707; discussion 707. |
| 2. | Ding YM, Wang B, Wang WX, Wang P, Yan JS. New classification of the anatomic variations of cystic artery during laparoscopic cholecystectomy. World J Gastroenterol. 2007;13:5629-5634. |
| 3. | Singh K, Ohri A. Anatomic landmarks: their usefulness in safe laparoscopic cholecystectomy. Surg Endosc. 2006;20:1754-1758. |
| 4. | Liu JL, Zhou HX, Yu XF, Bao SY, Shuai J, Wu HX, Bi JG, Zhong CN. Anatomic study of groin hernia in laparoscopic herniorrhaphy. Zhongguo Linchuang Jiepouxue Zazhi. 2005;23:620-622. |
| 5. | Hong HX, Pan ZJ, Chen X, Huang ZJ. An anatomical study of corona mortis and its clinical significance. Chin J Traumatol. 2004;7:165-169. |
| 6. | Marks SC Jr, Gilroy AM, Page DW. The clinical anatomy of laparoscopic inguinal hernia repair. Singapore Med J. 1996;37:519-521. |
| 7. | Brick WG, Colborn GL, Gadacz TR, Skandalakis JE. Crucial anatomic lessons for laparoscopic herniorrhaphy. Am Surg. 1995;61:172-177. |
| 8. | Katkhouda N, Mouiel J. Laparoscopic treatment of inguinal hernias. A personal approach. Endosc Surg Allied Technol. 1993;1:193-197. |
| 9. | Kraus MA. Laparoscopic identification of preperitoneal nerve anatomy in the inguinal area. Surg Endosc. 1994;8:377-380; discussion 380-381. |
| 10. | Chengyu L, Xiaoxin J, Jian Z, Chen G, Qi Y. The anatomical significance and techniques of laparoscopic rectal surgery. Surg Endosc. 2006;20:734-738. |
| 11. | Li GX, Ding ZH, Zhang C, Huang XC, Zhong SZ. Anatomical observations on left colectomy related fascia planes in laparoscopies. Zhongguo Linchuang Jiepouxue Zazhi. 2006;24:298-301. |
| 12. | Pan K, Xia LG, Xie YL, Zhong KL, Li MW, Lin LW. Studies of the applied anatomy of laparoscopic radical resection of rectal carcinoma. Zhonghua Putong Waike Zazhi. 2006;21:598-599. |
| 13. | Zhang C, Li GX, Yu J, Huang XC, Ding ZH, Zhong SZ. Clinical anatomy on ureter protection in laparoscopic total mesorectal excision. Jiepouxue Zazhi. 2006;29:360-361. |
| 14. | Li GX, Zhang C, Yu J. Laparoscopic assisted D2 distal radical gastrectomy: the art of anatomy. Waike Lilun Yu Shijian. 2007;12:533-338. |
| 15. | Wu T, Li GX, Ding ZH, Liu XG, Zhong SZ. Anatomic features of dorsal mesogastrium and interfascial space in laparoscopic surgery for gastric cancer. Zhongguo Linchuang Jiepouxue Zazhi. 2007;25:251-254. |
| 16. | Li XP, Zhou J, Xu DC, Li CL. Study of the operation pathway of the porta hepatic on laparoscopic surgery. Zhongguo Linchuang Jiepouxue Zazhi. 2004;22:230-233. |
| 17. | Li XP, Xu DC. Applied anatomy of vessels of the secundum porta hepatic on laparoscopic surgery. Zhongguo Linchuang Jiepouxue Zazhi. 2006;24:393-394. |
| 18. | Li XP, Xu DC. Study of the structures of left triangle ligament and its clinical significance in the operation pathway of laparoscopic liver surgery. Zhongguo Linchuang Jiepouxue Zazhi. 2005;23:540-541. |
| 19. | Li XP, Xu DC, Tan HY, Li CL. Anatomical study on the morphology and blood supply of the falciform ligament and its clinical significance. Surg Radiol Anat. 2004;26:106-109. |
| 20. | Liu XG, Ran L, Wu T, Ding ZH, Zhong SZ. Applied anatomy of superior mesenteric vessels during laparoscopic pancreatoduodenectomy. Zhongguo Linchuang Jiepouxue Zazhi. 2007;25:172-175. |
| 21. | Lu BY, Huang YB, Liu ZJ, Cai XY, Lu WQ, Huang F, Jin XJ, Li JJ. The application of duodenal-wall approach in laparoscopic pancreaticoduodenectomy: experience of 17 cases. Linchuang Waike Zazhi. 2008;16:659-662. |
| 22. | Zhang W, Liu S, Jiang DZ, Zheng XM, Shen HL, Shan CX, Qiu M. Identification of anatomic landmark and technological key points in laparoscopic antireflux surgery. Fuqiangjing Waike Zazhi. 2009;14:35-37. |
| 23. | Klingler PJ, Seelig MH, Floch NR, Branton SA, Freund MC, Katada N, Hinder RA. Aberrant left hepatic artery in laparoscopic antireflux procedures. Surg Endosc. 2004;18:807-811. |
| 24. | Wang LC, Hu SY, Zhang GY, Zhang HF, Chen B, Liu CZ. Research of the clinical application on anatomy of splenic lobial artery in iaparoscopic splentomy. Fuqiangjing Waike Zazhi. 2006;11:367-370. |
| 25. | Xu JH, Lu BY, Cai XY, Lu WQ, Huang YB, Huang F. Anatomical basis and techniques of total laparoscopic splensctomy. Weichuang Yixue. 2006;1:65-67. |
| 26. | Abu-Hijleh MF, Roshier AL, Al-Shboul Q, Dharap AS, Harris PF. The membranous layer of superficial fascia: evidence for its widespread distribution in the body. Surg Radiol Anat. 2006;28:606-619. |