INTRODUCTION
Inflammatory bowel disease (IBD) includes two chronic pathologies characterised by alternating phases of active inflammation and quiescence: ulcerative colitis (UC) and Crohn’s disease (CD)[1]. Although the pathogenesis of IBD has not been well established, it has been suggested that some frequently observed clinical manifestations such as abdominal pain, nausea, vomiting, ileus, or diarrhoea, can be attributed to deranged gastrointestinal motility associated with inflammation[2]. The hypothesis that immune abnormalities induced by Th1/Th2 imbalances play a core role in the pathogenesis of IBD has been widely accepted[3,4]. Th1 cells predominantly secrete interferon-γ (IFN-γ), interleukin 2 (IL-2) and tumor necrosis factor α (TNF-α), while Th2 cells secrete interleukins 4, 5 and 10 (IL-4, IL-5, IL-10). Cytokines can be divided into pro-inflammatory and anti-inflammatory cytokines and growth factors. Pro-inflammatory cytokines such as IL-1, IL-2, IL-8, IL-12, TNF-α, TNF-β and IFN-γ are produced by monocytes and macrophages and are involved in cell-mediated immune responses. Anti-inflammatory cytokines such as IL-4, IL-5, IL-10 and IL-13 are produced mainly by T cells and are involved in the humoral immune response.
Communication between the periphery and the brain takes place via neural and humoral pathways. Cytokines released by activated immune cells are mediators of inter- and intra-cellular communication[5-11] and are part of a chemical signalling system that regulates development, tissue repair, haemopoiesis, inflammation and specific and nonspecific immune responses. Potent cytokine polypeptides (such as interleukins 1, 6, 8 and TNF-α) have pleiotropic activities and functional redundancy and exist in a complex, intermingled network in which one cytokine can influence the production of and response to many other cytokines[12]. Of note, modulation of cytokines and their antagonists also exhibits therapeutic potential in several chronic and acute diseases[10,11]. The immune system is regulated in part by the central nervous system (CNS), acting principally via the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic nervous system (SNS)[13-15]. IL-6 is a pleiotropic cytokine whose expression in physiological conditions is important for host responses to a number of infections; in addition, it exerts antigen-specific immune responses and has both pro- and anti-inflammatory effects[16,17]. In CD, IL-6 is present at high levels in both serum and intestinal tissues[18]. Increased levels of IL-6 receptor (IL-6R) and gp130 expression have been also demonstrated in peripheral lymphocytes of Crohn’s patients, along with increased serum soluble IL-6R[19].
To explore the role of IL-6 in the initiation and development of IBD with respect to the neuroimmunomodulation and regulation of immunological homeostasis at the organismic level, we used real-time reverse transcription polymerase chain reaction (RT-PCR) and immunohistochemistry (IHC) to evaluate the mRNA expression and cellular localisation of IL-6 in brain and colon tissues in the inflammatory and quiescent phases of IBD in an animal model with trinitrobenzene sulfonic acid (TNBS)-induced IBD.
MATERIALS AND METHODS
Reagents
All chemicals were purchased from Sigma Chemical, unless otherwise stated.
Animals
Eighty female Wistar rats (Laboratory Animal Service of Jilin University, Changchun, China), 8-10 wk old and weighing 180-200 g, were maintained under specific pathogen-free conditions according to our institutional guidelines for animal care. This study was carried out in accordance with guidelines established by a consensus statement entitled the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes.
Experimental model
After a seven-day acclimation period, 80 rats were divided randomly into 8 cages (one cage for 10) and fed in the same condition. Among them, 4 cages were experimental group and the others were controls. Rats were fasted overnight, and colitis was induced in the TNBS group by the method originally described by Morris et al[20], with some modifications made by our group. Briefly, rats were anaesthetised with ethylether and given a 5% TNBS (Sigma, R&D, USA) and ethanol mixture (2:1) at a dose of 0.3 mL/100 g inserted through the anus to a distance of 8 cm. Rats from the non-colitis group were given an intracolonic dose of 0.25 mL phosphate buffered saline (PBS) instead of TNBS. Animals were maintained in a vertical position for 30 s and returned to their cages.
Assessment of experimental model
Macroscopic scoring: At 3, 7, 14, 21, and 28 d after induction of colitis, rats were exsanguinated under ether anaesthesia, and the small and large intestines and the brain were excised. Samples of the descending colon were obtained for paraffin section and measurement of myeloperoxidase activity (see below). The remaining descending colon, sigmoid colon, and rectum were opened longitudinally and fixed onto a board with pins, and the macroscopic appearance was scored by two blinded investigators on a 0-4 scale similar to the original scoring system of Morris and colleagues[20]; briefly, 0 = no evidence of inflammation, 1 = erythema only, 2 = erythema with slight oedema and small erosions, 3 = two or more bleeding ulcers and/or inflammation and/or moderate adhesions, and 4 = severe ulceration and/or stenosis with prestenotic dilations with or without severe adhesions.
Determination of myeloperoxidase activity: Colonic myeloperoxidase activity was measured as previously described by Grisham et al[21] and Suzuki et al[22]. Briefly, tissue was homogenised in approximately 10 mL 50 mmol/L KPO4 at pH 7.4 and centrifuged for 20 min at 20 000 g. The pellet was homogenised in 10 mL 50 mmol/L KPO4, and 10 mmol/L EDTA at pH 6.0 with 0.5% hexadecyltrimethylammonium bromide (wt/vol). After one freeze-thaw cycle, the homogenate was sonicated, and myeloperoxidase activity was determined by measuring H2O2-dependent oxidation of 3,3’,5,5’-tetramethylbenzidine and expressed as units per gram of tissue.
Microscopic scoring: Specimens were fixed in PBS containing 4% formaldehyde and embedded in paraffin. Multiple sections (5 μm thick) were stained with haematoxylin and eosin using standard techniques. The degree of inflammation was assessed by two blinded investigators using the 0-4 scoring system described by Neurath et al[23] (0 = no signs of inflammation, 1 = low levels of leucocyte infiltration, 2 = moderate levels of leucocyte infiltration, 3 = high levels of leucocyte infiltration, high vascular density, thickening of bowel wall, and 4 = transmural infiltrations, loss of goblet cells, high vascular density, significant bowel wall thickening).
Isolation of mRNA and analysis of mRNA expression by real-time RT-PCR
A total of 80 rats (normal and IBD model) were used for real-time RT-PCR analysis. The proximal part of the colon (the area with macroscopically visible mucosal folds) and the brain were stored in RNAlater (Ambion, Austin, TX) at -80°C for use in RNA extraction. RNA was extracted using the Trizol reagent (Invitrogen) according to the manufacturer’s protocol. The integrity and quality of the isolated RNA was examined by quantifying the A260/280 ratio (BioPhotometer, Eppendorf, Hamburg, Germany) and resolving the RNA on a 1% agarose gel. The cDNA was prepared from 1.0 μg RNA (OD = 1.9-2.0) in the presence of 2.5 μmol/L oligo (dT) primer and 200 U Moloney murine leukemia virus reverse transcriptase (M-MLV; Promega, Madison, WI) in a total volume of 20 μL. The reaction mixture was incubated for 1 h at 42°C and stopped by heating at 90°C for 5 min. The primers and FAM-labelled probes were designed with Primer Express v1.5 software (Applied Biosystems). For IL-6, the forward primer was 5'-GCCCTTCAGGAACAGCTATGA-3', the reverse was 5'-TGTCAACAACATCAGTCCCAAGA-3', and the probe was 5'-CTCTCCGCAAGAGACTTCCAGCCAGTT-3'. For the housekeeping gene beta-actin, the forward primer was 5'-GTCAGGTCATCACTATCGGCAAT-3', the reverse was 5'- AGAGGTCTTTACGGATGTCAACGT-3', and the probe was 5'-CCTTCCTTCCTGGGTATGGAATCCTGTG-3'. All the primers and probes were obtained from Applied Biosystems. PCR was performed in the ABI PRISM 7000 Sequence Detection operated by Sequence Detector v1.3 software (Applied Biosystems), using TaqMan Real-time PCR Master Mix (Toyobo, Japan). A threshold was set at the linear part of the amplification curve, and the number of cycles needed to reach it were calculated. Relative mRNA levels were determined by using a standard curve and by further normalisation to the housekeeping gene beta-actin. The annealing temperatures were both set to 60°C.
Immunohistochemistry
IHC for detection of IL-6 was performed on paraffin sections of rat brain and colon samples; female rats were perfused under chloral hydrate anaesthesia via the abdominal aorta. After a 30 s rinse with physiological saline, a fixative containing 4% freshly prepared paraformaldehyde in 0.01 mol/L PBS buffer (pH 6.0) was perfused for 5 min. The brains and colons were cut into 1-2 mm frontal or sagittal slices and were embedded in paraffin using an automated vacuum tissue processor (Leica ASP300, Germany). For experiments, 4 μm sections were cut and mounted on specific slides. For antigen retrieval, the protocol recently described by Pizarro et al[24] and Strober et al[25] was used. Briefly, the deparaffinised and rehydrated sections were subjected to a 5 min digestion with 0.01% trypsin (Sigma) in PBS and an additional microwave treatment in 10 mmol/L citrate buffer (pH 6.0) for 15 min at 720 W, followed by a 20 min cooling period in the same buffer. After pretreatment, sections were treated for 15 min with 3% hydrogen peroxidase to block endogenous peroxidase activity and were blocked with 0.5% blocking reagent for 30 min to reduce non-specific reactions. The sections were incubated for 36 h at 4°C with rabbit anti-rat-IL-6 IgG (Biozol, Eching, Germany; diluted 1:400) in blocking buffer. Sections were washed in PBS, and biotinylated linking antibody solution (Biozol, Eching, Germany) was applied (100 μL/section) for 20 min at 37°C. Sections were then washed in PBS before application of HRP conjugated streptavidin (100 μL/section, 15 min) at 37°C. Slides were stained with DAB (IBL, Germany) after being washed in PBS for 5 min, washed in PBS and DAB (BIOS) for 5 min and counterstained with hematoxylin. For negative controls, the primary antibody was replaced with the corresponding affinity-purified preimmune IgG.
Statistical analysis
Data are expressed as mean ± SE. Statistical analysis was performed using SPSS 17.0. Differences were considered to be statistically significant when P < 0.05 by analysis of variance (ANOVA).
RESULTS
Effects of TNBS-induced colitis on clinical and macroscopic appearance
After administration of TNBS, we found that Wistar rats regularly developed colitis with severe diarrhoea, losing 15% of their body weight and suffering rectal prolapse and extensive wasting. Gross blood adhesion to the anus was noted in some rats. Macroscopically, colitis was characterised by bowel wall thickening, adhesions and necrotic foci (Figure 1). As these severe inflammatory changes covered 2-4 cm of the distal colonic mucosa, this resulted in a higher median macroscopic damage score in TNBS-treated rats compared to controls (Figure 2A, P < 0.01), especially by day 7.
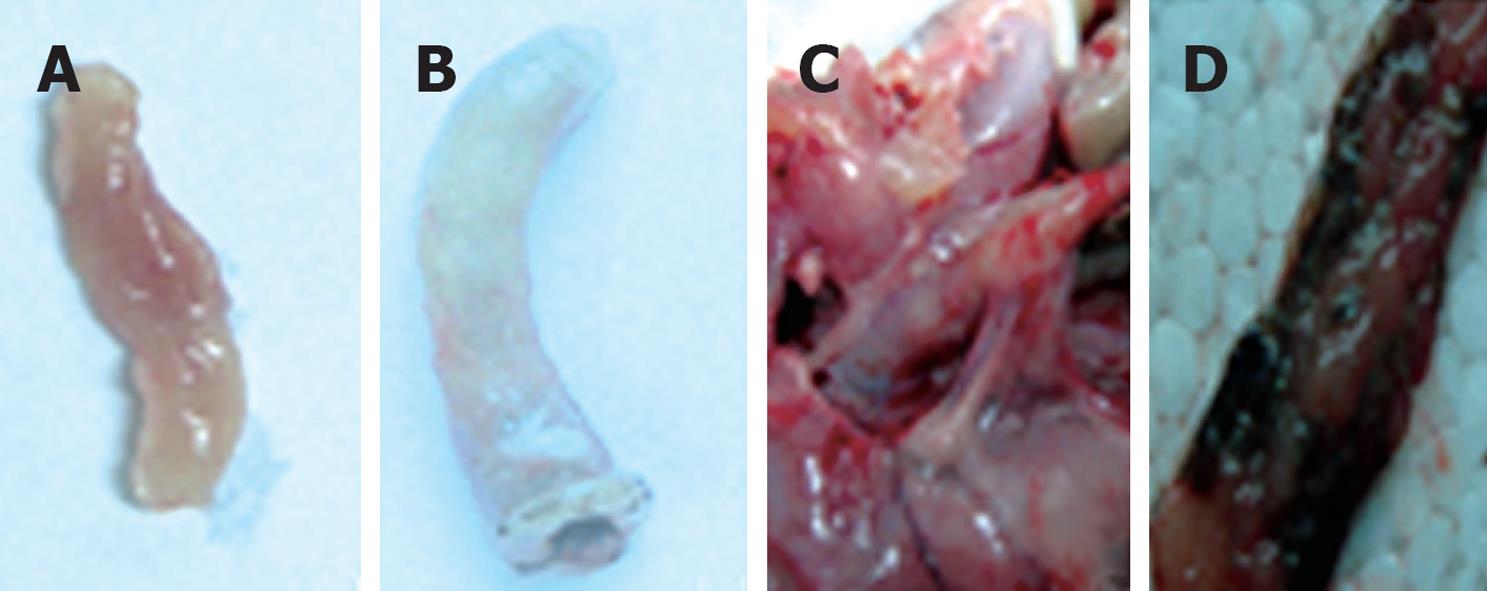
Figure 1 The macroscopic appearance of colon tissue in trinitrobenzene sulfonic acid (TNBS)-treated vs control rats.
A: The colon of control rats, with macroscopic damage score = 0; B-D depict colonic tissue of TNBS-treated rats: B: Significant swelling and distension, with macroscopic damage score = 5; C: Extensive adhesions around the colon, with macroscopic damage score = 7; D: Necrotic foci in colon, with macroscopic damage score = 8.5.
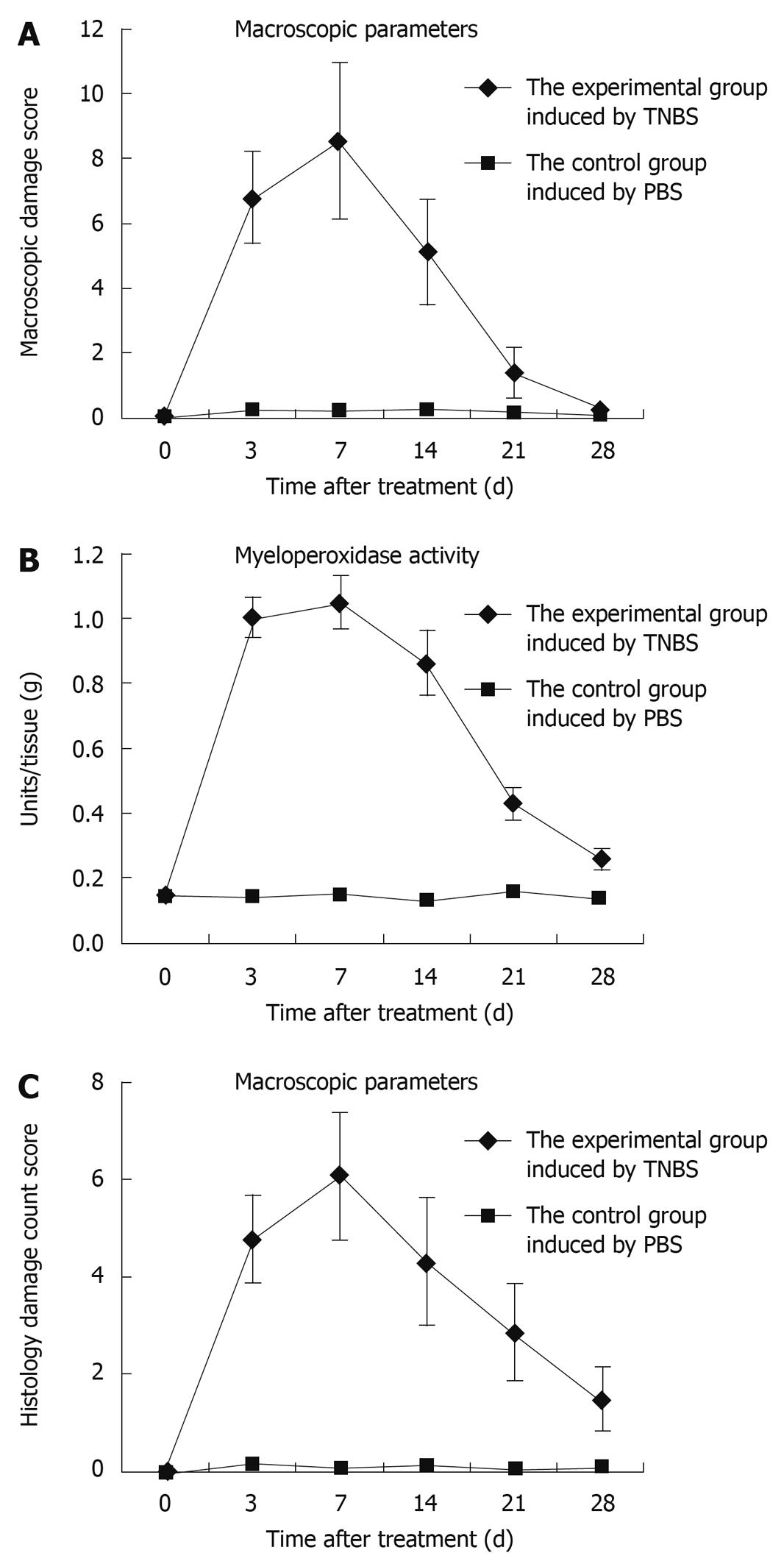
Figure 2 Assessment of TNBS-induced colitis rats by time points.
As these severe inflammatory changes covered 2-4 cm of the distal colonic mucosa, this resulted in a higher median macroscopic damage score in TNBS-treated rats compared to controls (A, P < 0.01), especially by day 7; compared with the control rats, MPO activity increased gradually, peaked by day 7, and decreased to nearly the baseline value (B); The score reached a peak at day 7, and decreased gradually to nearly the normal value (C).
Effects of TNBS-induced colitis on colonic myeloperoxidase activity
Myeloperoxidase (MPO) activity was determined by using accumulation of polymorphonuclear leucocytes as an indicator. Compared with the control rats, MPO activity increased gradually, peaked by day 7, and decreased to nearly the baseline value (Figure 2B).
Effects of TNBS-induced colitis on colonic histology
The degree of inflammation was assessed using the scoring system at 3, 7, 14, 21, and 28 d after TNBS induction. The score reached a peak at day 7, and then decreased gradually to nearly the normal value (Figure 2C). Microscopic evaluation of colons from control rats showed normal colonic histological features (Figure 3A). Histological evaluation of the colon at each period after TNBS induction is shown in Figure 3B-F. TNBS caused the inflammatory features typical of colitis in the rats’ colonic architecture: severe oedema and haemorrhage at the muscular layer of the mucosa, ulceration, goblet cell depletion, and a mixed cell infiltration that was mainly composed of mononuclear cells such as macrophages, lymphocytes and plasmocytes.
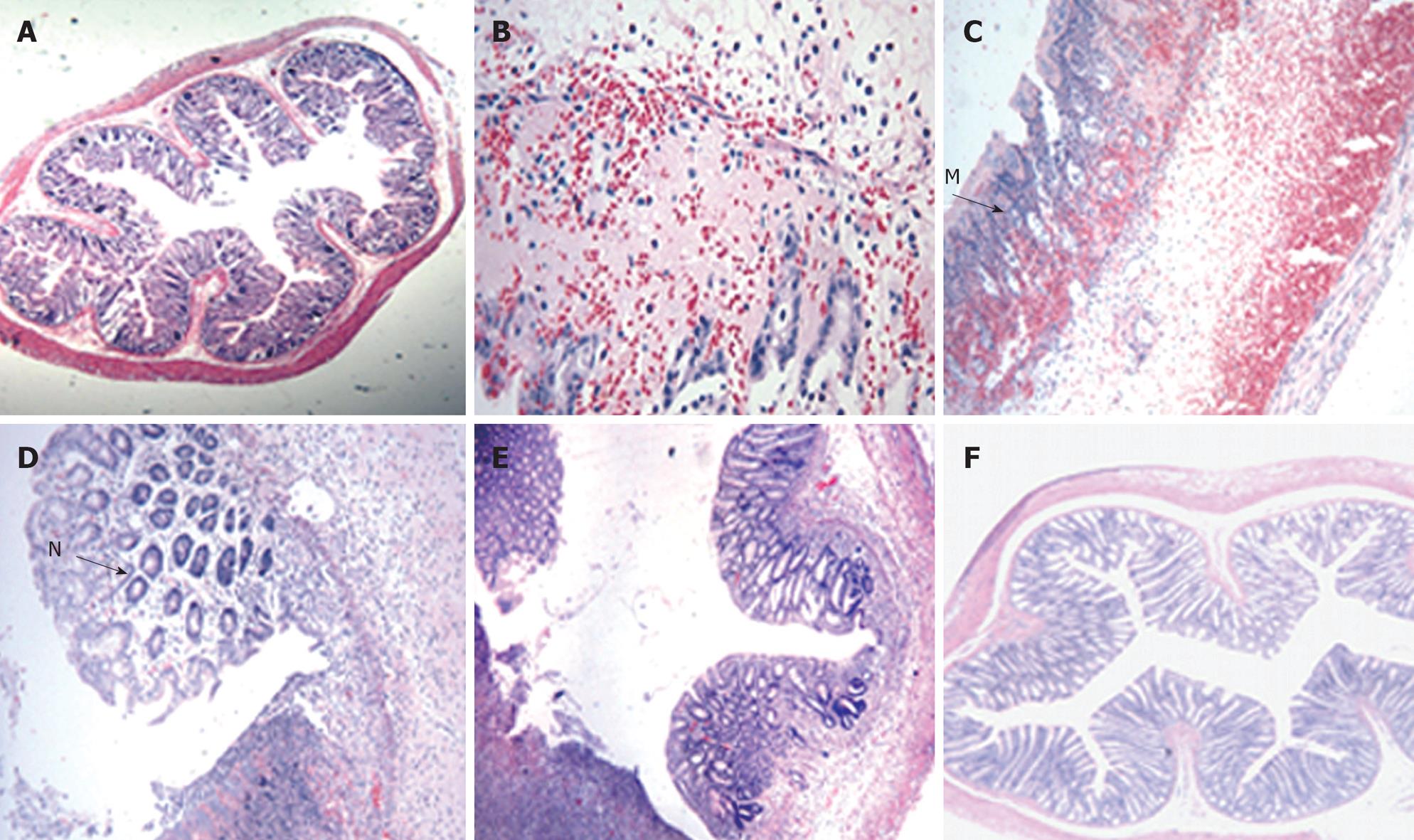
Figure 3 Light photomicrograph of colon tissues from TNBS-induced colitis rats.
In control rats, normal histological colon morphology is evident (A, × 4). At day 3, oedema and haemorrhage at the mucosa with goblet cell depletion were seen (B, × 20). At day 7, severe transmural infiltration of the colon wall developed, with submucosal oedema and haemorrhage, lymphocytic and neutrophilic infiltration (M) and coagulative necrosis (C, × 10). At day 14, goblet cell regeneration (N), oedema and haemorrhage regressed by degrees (D, × 20). At day 21, abundant goblet cells and crypts developed (E, × 10). At day 28, oedema and haemorrhage had almost entirely disappeared, the structure of the muscular layer became compact and the intestinal glands developed highly (F, × 4).
Analysis of IL-6 mRNA expression
We used real-time RT-PCR to measure expression of IL-6 mRNA in the brain and colon at various time points after induction of colitis. Our results demonstrated a remarkable difference (P < 0.01) in colon and brain tissues in the experimental vs the control group. IL-6 mRNA expression levels increased by day 3 and returned to near baseline levels by day 28, reaching their highest point at day 7. Thus, there is similarity on the increasing trend (Figure 4A).
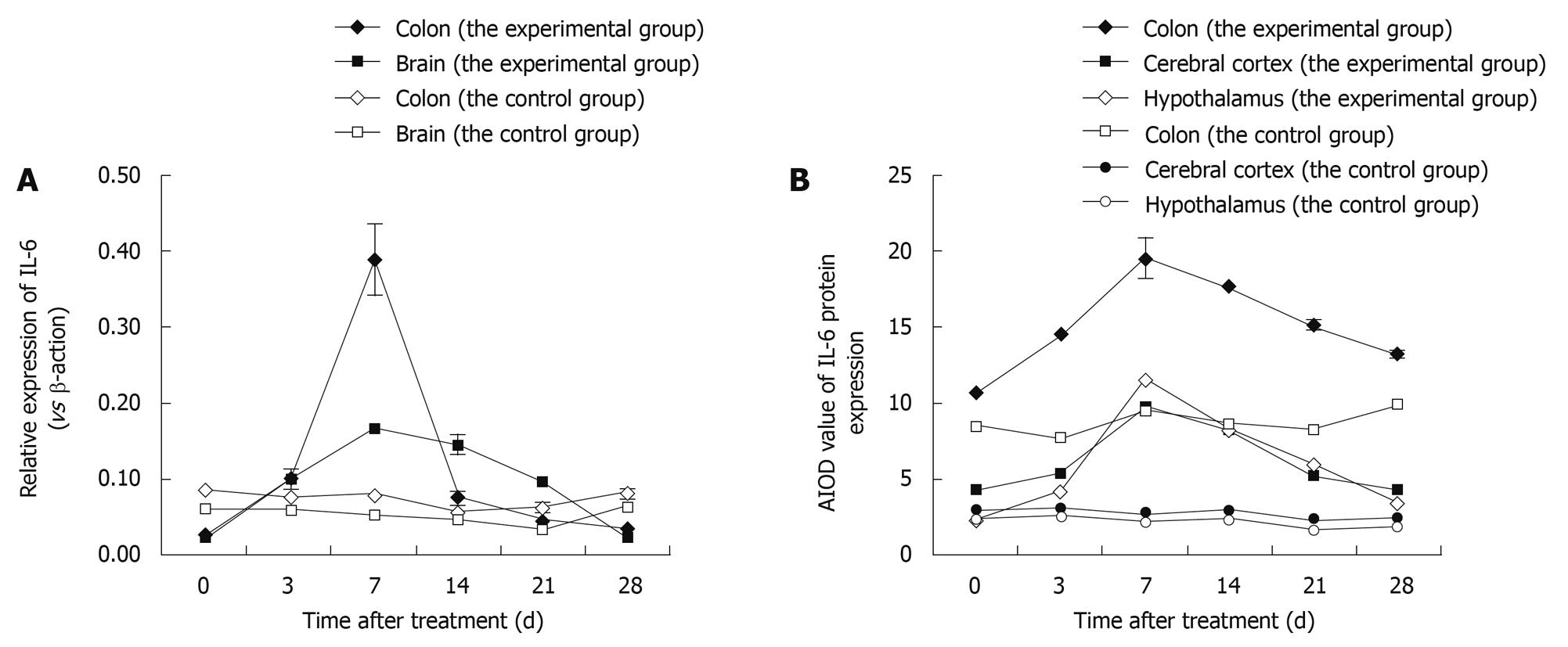
Figure 4 Variation in expression of interleukin-6 (IL-6) in the colon and brain of TNBS-induced colitis rats by time points (at days 3, 7, 14, 21 and 28) vs control rats.
A: The expression of IL-6 mRNA after real-time RT-PCR; Data shown as mean ± SD, n = 4-8; B: The average integrated optical density (AIOD) values for IL-6 protein expression after immunohistochemical staining. The results were determined by ANOVA.
IHC
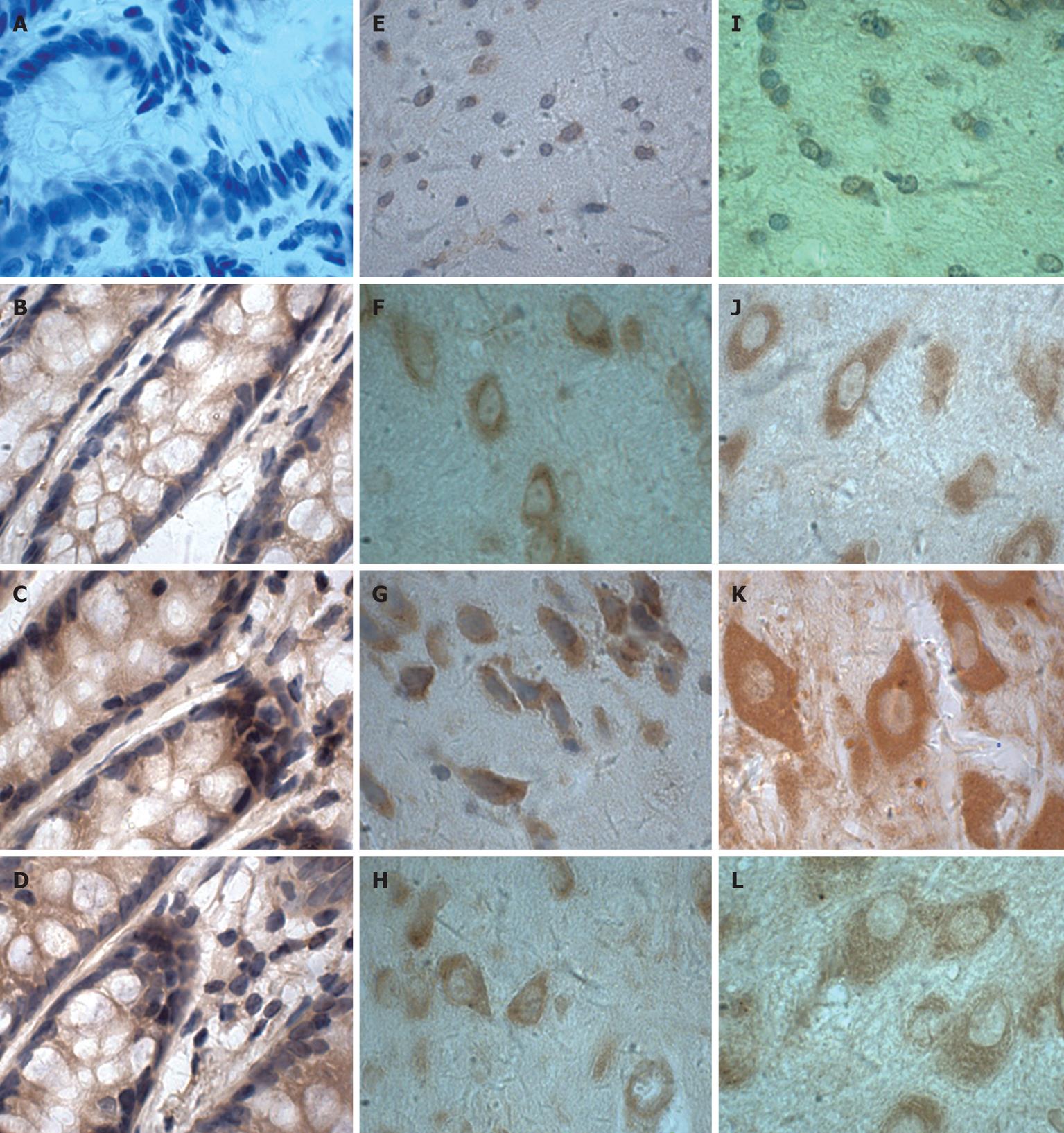
IHC was performed to determine IL-6 localisation in the colon, cerebral cortex and hypothalamus. The distribution, cellular localisation, and intensity of staining were assessed using light microscopy. The distribution and relative abundance of IL-6 in the parts of the colon, cerebral cortex and hypothalamus examined in this study are presented in Figure 4B; representative sections are shown in Figure 5. Control sections immunolabeled with the preimmune serum were devoid of immunolabeling for IL-6. We found that immunostaining was clearly increased in the colon, cerebral cortex and hypothalamus after induction of colitis, reaching a peak at day 7 and decreasing thereafter. Morphologically, analyses showed that the number of IL-6 immunopositive cells was significantly increased (P < 0.01) in the colon, cerebral cortex and hypothalamus after induction compared to control animals.
Figure 5 Immunohistochemical localisation of IL-6 proteins in colon (A-D), cerebral cortex (E-H) and hypothalamus (I-L) from TNBS-induced colitis (× 100).
C, G, K: Day 7; D, H, L: Day 28; B, F, J: Day 0; A, E, I: Negative control (the primary antibody was replaced with the corresponding affinity-purified preimmune IgG).
DISCUSSION
Over the past several years, a variety of IBD animal models have been developed and investigated, with the ultimate goal of understanding the causes of CD and UC[24,25]. To study the inflammatory pathways and pathogenesis of IBD, we have established models of colitis after chemical induction (TNBS) of hapten-induced gut inflammation; with this model, we have detected mRNA expression and cellular localisation of IL-6 using RT-PCR and IHC in brain and colonic tissues.
Our IHC and RT-PCR analysis of the colon demonstrate an important role for IL-6 in the development of IBD. The induction-related changes in IL-6 described here are not limited to our animal models and are seen in human IBD as well[26,27]. Although the pathogenesis of IBD is unclear, many earlier experiments have proved that IL-6 plays an important role in the course of colitis[28,29]. IL-6 is one of several inflammatory mediators that induce lesions in the intestinal mucosa; however, if a small amount is generated, the organism’s immune function may be enhanced (particularly anti-viral, anti-infectious and anti-tumour activities), while excessive production of IL-6 may induce septic shock and pyosepticaemia. IL-6 expression in CD and UC has been found to be increased[30]. Using RT-PCR, Ishiguro et al[31] found enhanced mRNA expression of IL-1β, IL-6, IL-8 and TNF-α in CD and UC, as well as a positive correlation of IL-6 expression levels with severity of inflammation. These results showed that when pathological changes in intestinal canal were increased, IL-6 expression in colonic tissues was gradually enhanced; with the alleviation of pathological changes in the intestinal canal, colonic IL-6 expression gradually declined and tended towards normal. It should be noted that inflammatory cells secreting IL-6 were present in intestinal inflammation sites, and their number was closely related to the severity of inflammation.
There is increased evidence to support the hypothesis of bidirectional circuits between the immune system, CNS and endocrine system[12]. Neuroimmunomodulation is one of the fastest-growing fields of study in current biomedical science. There is also abundant evidence suggesting that the CNS is not an immune-privileged area and that T lymphocytes are present in the CNS. Microglial cells and astrocytes in the CNS that are equivalent to macrophages in other tissues are the primary cells responsible for an inflammatory reaction. In normal cerebral tissues, these glial cells are quiescent, but they are highly reactive and can secrete cytokines rapidly; this is followed by changes in internal cellular environments and in cell-surface antigens. Cytokines produced by the CNS, including interleukins 1-24, TNF and TGF, can reduce the production of proinflammatory cytokines, lowering the occurrence of some pathological behaviours regulated by the CNS[32,33]. Thus, cytokines may not only play an important role in the immune system, but may also show wide-ranging central regulatory effects. Together with neuromediators and endocrine hormones, cytokines act as the intercellular signalling molecules in organisms and are involved in immune activation and information transmission using neurotransmitters and hormones; thus they are closely related to psychological reactions and mental disorders. In-depth studies on cytokines will contribute to our understanding of IBD pathogenesis and potential therapeutic options. Because neural cells produce IL-6 and have IL-6 receptors, several studies have suggested that IL-6 plays a role in regulating their response to stress. Moreover, IL-6 may be an essential neurotrophic factor in the midbrain after long-term stress[34]. Loss of IL-6 in the CNS does not lead to an improvement in memory function, but instead to memory impairment[35]. Moreover, IL-6 trans-signalling may be of paramount importance in the pathogenesis of several immune-mediated disorders, as well as diseases of the CNS[36,37]. IL-6 is an important CNS-related cytokine and is involved in disease occurrence and course, by direct or indirect regulation of neuronal excitability and neurotransmitter release after receptor binding. IL-6 also plays a significant role in glial cell differentiation and proliferation and reduces antigen presentation by glial cells in the CNS[38]. It is currently recognised that neurons and glial cells can also secrete and express cytokines, and cytokines are active during nerve growth and in the adult nervous system under normal and pathological conditions. This evidence speaks for a potential and crucial role for IL-6 in regulating the interaction of the immune system, CNS and endocrine system. We have confirmed this conclusion through the detection of IL-6 expression in the brains of IBD rats, using IHC and RT-PCR.
Over the course of IBD, we found that expression of IL-6 in cerebral cortex and hypothalamus increased when IBD was exacerbated and gradually declined with disease alleviation. Neither cytokines in cerebral tissues nor those in peripheral blood can cross the blood-brain barrier[39], and there are no reports of an association between cerebral IL-6 expression and colonic cytokines or of what their relationship might be in IBD. The CNS is a central link in the nerve-endocrine-immune regulation network and plays a leading role in the maintenance of the nerve-endocrine-immune network homeostasis. Therefore, by exploring the role of IL-6 in IBD rats from the perspective of the nerve-endocrine-immune network and homeostatic regulation, we have explored the general role of the nerve-endocrine-immune network over the course of IBD. Our results show that pathological changes were most obvious and severe at day 7 after induction of colitis, with necrosis and exfoliation of the intestinal epithelial cells and severe oedema and haemorrhage in the mucosa and muscularis layers, as well as increased expression of IL-6 in the cerebral tissues. Conversely, with the resolution of enteropathy and alleviation of symptoms, IL-6 expression in cerebral tissues began to decline and gradually trended towards levels in normal rats. Therefore, there may be a correlation between IL-6 expression in cerebral and colonic tissues. We propose that cerebral tissues may regulate and control the occurrence and development of IBD via IL-6.
This experiment provides new evidence concerning IBD pathogenesis, as well as an experimental model for further enriching and improving the nerve-endocrine-immune network theory. Future studies that explore signal transduction of IL-6, e.g. in IBD rats, may help to further elucidate its role in neuroimmunomodulation.
COMMENTS
Background
Since inflammatory bowel disease (IBD) appeared in Northern Europe and North America, the incidence has been rising in these areas. Currently the incidence of IBD is increasing rapidly in Asian countries especially in the last two decades. Although considerable progress has been made, a major gap in knowledge of the pathogenesis of IBD remains and has precluded the discovery of lasting, effective forms of therapy.
Research frontiers
It has become increasingly evident that interactions between the nervous system and the immune system play an important role in the pathophysiology of IBD. However, how neuroimmunomodulation plays its role in the occurrence and development of IBD is unknown. In this study, the authors demonstrate that interleukin-6 (IL-6) could play a potential role in the course of colitis.
Innovations and breakthroughs
Recent reports have highlighted the importance of cytokines, including pro- and anti-inflammatory cytokines, in the development of IBD. In particular, the imbalance of the cytokines can lead to improper immune responses and initiation of the inflammatory process. So the authors demonstrated similar trends of IL-6 in the brain and in the colon of the experimental IBD model.
Applications
By understanding the role of IL-6 in the development of IBD, this study may open a new strategy for research into the pathogenesis, prevention and treatment of the autoimmune colitis.
Peer review
This is simple and solid work with some new and interesting results.