CASE REPORT
Case 1
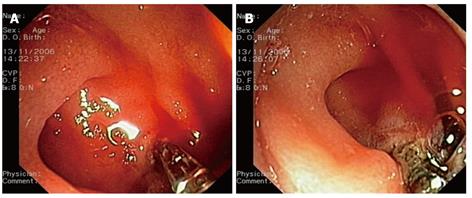
An 86-year-old woman was admitted for melena. She had a medical history of ischemic heart disease and chronic intake of low-dose aspirin. She was intravenously administered omeprazole (8 mg/h). Her endoscopic findings were suggestive of a Dieulafoy’s lesion that was located in the third duodenal portion at the level of the genu inferius. An initial attempt at bleeding control with injection of epinephrine solution at a dose of 25 mL proved to be unsuccessful. At that point, the decision was made to use a hemostatic forceps (Coagrasper, FD-410LR; Olympus, Tokyo, Japan). The blood was washed out using a water-jet-equipped, single-channel gastroscope (GIF1T 140; Olympus), with a large working channel (diameter: 3.8 mm), and the hemostatic forceps was advanced through it. The bleeding point was gently grasped and retracted with the hemostatic forceps (Figure 1A). At that point, monopolar electrocoagulation was delivered using an electrosurgical current generator (ICC 200; ERBE, Tubingen, Germany) with forced mode at a setting of 60 W (Figure 1B). The coagulation effect was evaluated by washing out the blood again. The whole hemostatic procedure was carried out with success within 5 min. The bleeding point had to be grasped twice. The total duration of complete coagulation with this setting was about 1 min. The patient tolerated the procedure well. She had no perforation or rebleeding.
Figure 1 Endoscopic images.
A: The bleeding point is pinched with a hemostatic forceps; B: Coagulation delivery at the retracted bleeding point.
Case 2
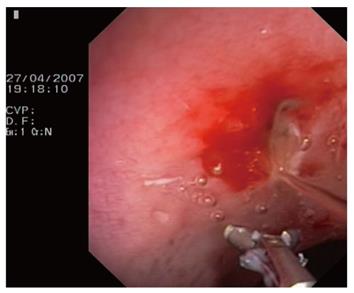
A 60-year-old man presented with ulcer bleeding in the duodenal bulb. He was managed with injections of epinephrine solution in combination with the placement of two hemoclips (QuickClip II, standard size; Olympus) and intravenous administration of omeprazole (8 mg/h). However, during the next 10 d, he developed recurrent bleeding. A repeat endoscopy demonstrated two simultaneously oozing, bleeding, minimal lesions in the ulcer area. The endoclips remained attached to the site of application. Although high doses of epinephrine solution (60 mL) were injected again, they failed to achieve hemostasis. After that, a VIO 200 ERBE generator was set to soft coagulation mode (Effect 5, 80 W) to coagulate the bleeding lesions with hemostatic forceps. The same endoscope and technique were used (Figure 2). As it was difficult to keep the endoscope stable in the retropyloric bulb, coagulation was also delivered by applying the tip of the unopened hemostatic forceps to the bleeding points. Prompt and effective hemostasis was achieved without any further episodes of bleeding. Following an uneventful recovery, the patient was discharged home a few days later.
Figure 2 An opened hemostatic forceps while washing out the blood after coagulation.
Case 3
A 66-year-old woman was referred to our Endoscopy Unit for chronic GI bleeding of unexplained origin. She was receiving combined antithrombotic treatment with low-dose aspirin and clopidogrel for advanced cardiovascular disease. She was also receiving omeprazole for ulcer prevention. On upper endoscopy, she had signs of active diverticular bleeding of the second duodenal portion. She underwent endoscopic hemostasis by using an ERBE VIO 200 generator with either soft coagulation mode (Effect 5, 80 W) or forced mode (60 W), as well as hemostatic forceps, which grasped and retracted the bleeding point. The procedure was well tolerated and resulted in bleeding control. No late-onset complications were observed.
Case 4
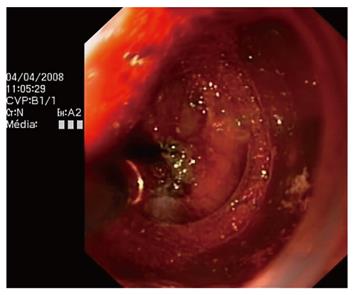
A 61-year-old woman was diagnosed with depressed-type IIc superficial adenocarcinoma in the stomach, with a diameter of approximately 1.5 cm. Cap-assisted endoscopic mucosal resection (EMR) of her neoplastic lesion was complicated with immediate bleeding. Coagulation of the spurting bleeding vessels using an ERBE VIO 200 generator (soft mode, Effect 5, 50 W) and hemostatic forceps allowed us to complete successfully the EMR procedure (Figure 3). The patient was treated with omeprazole 20 mg bid. She did not experience any further complications. She was discharged home within 48 h.
Figure 3 Coagulating the bleeding vessels during cap-assisted endoscopic mucosal resection (EMR).
DISCUSSION
Several techniques or devices have been used for the endoscopic control of active GI bleeding. The widely available endoscopic hemostatic options include injection techniques such as epinephrine or sclerosant, ablative ones such as heater probe or argon plasma coagulation (APC), and mechanical methods such as endoclips or endoscopic banding[1]. In several ulcer bleeding cases, combination endoscopic treatment with epinephrine injection and thermal coagulation or endoclipping has been recommended to achieve better outcomes[3]. Bleeding control of a Dieulafoy’s lesion may require hemostasis with multiple endoscopic treatment methods, whereas clinical experience with endotherapy of duodenal intradiverticular bleeding is limited to a small number of cases[4,5].
GI bleeding is a major complication of endoscopic surgery. Minor bleeding during ESD can be managed with coagulation using an electrosurgical knife. When that fails, conventional endoscopic means such as hemoclips or APC can be applied[6]. However, endoclipping may pose several difficulties in the completion of the dissection procedure[7].
A hemostatic forceps is a valuable tool for performing safely ESD procedures, which are usually demanding[8]. Its intraoperative use is extended from the coagulation of actively bleeding or oozing vessels to the pre-coagulation of visible, exposed vessels on the artificial ulcer. It does not only aim at facilitating an ESD procedure, so as to continue the endoscopic intervention safely, but also to prevent delayed bleeding as a late-onset complication[9].
In our small study, a hemostatic forceps was used in the endoscopic management of GI bleeding that was unrelated to ESD. To the best of our knowledge, this is the first report of its application beyond ESD in the English-language literature. Our initial experience demonstrated that monopolar coagulation using a hemostatic forceps as: (1) a second-line therapeutic approach during the same hemostatic procedure; (2) second-line retreatment of two simultaneously bleeding sources after recent combined endotherapy with other modalities; and (3) first-line treatment of a non-iatrogenic bleeding lesion or an EMR-induced lesion, was effective as well as devoid of any post-procedural complications.
Although omeprazole was introduced prior to endotherapy in all our cases, the clinical efficacy of proton pump inhibitors (PPIs) in acute bleeding is a matter of debate. They can reduce the severity of endoscopic signs of recent bleeding and the need for endotherapy, but they have not provided a clear benefit in terms of rebleeding, mortality or the need for surgery[10]. In addition, the administration of PPIs prior to ESD does not seem necessary for the prevention of postoperative bleeding[11]. However, they can prevent delayed bleeding more effectively than H2-receptor antagonists[12]. Moreover, epinephrine was initially injected in two cases. Epinephrine alone can provide initial hemostasis of 80%-100% in bleeding ulcers, but recurrent bleeding rates range from 6% to 36%. Similar to ulcers, it may only provide temporary hemostasis of bleeding of Dieulafoy’s lesions[13].
Although that hemostatic tools appears to be a reasonable alternative option for the endoscopic treatment of various bleeding lesions, a few practical and technical issues should be taken into consideration before encouraging endoscopists to use it for non-variceal, actively bleeding cases, to achieve definitive hemostasis. To date, the clinical experience of its hemostatic applications has not extended further than those interventionists that perform ESD procedures. However, it is conceivable that excessive coagulation with it might result in delayed perforation. Therefore, one should be aware of the mode of use of a hemostatic forceps, to avoid any complications. The bleeding point should be precisely pinched, retracted and coagulated with a minimal contact area for a minimum time[7]. In difficult cases, it is also possible to achieve prompt tissue coagulation with the simple contact of an unopened hemostatic forceps, as shown in our second case. However, by pinching an artery, its use seems more advantageous than other endoscopic techniques, such as APC, for avoiding unnecessary maneuvers and providing accurate hemostasis. To facilitate capture, a hemostatic forceps can be also rotated.
Several experts in ESD procedures usually coagulate the blood vessels using a hemostatic forceps in soft mode[7,9,14-20]. Others use the coagulation mode of the knife for hemostasis[21,22]. In our endoscopy unit, we generally use an ERBE VIO 200 generator in soft mode (Effect 5, 50, 80 or 100 W) for a hemostatic forceps. However, an ICC 200 generator, which can be set to soft or forced mode, is the only available option for emergency cases. This is the reason why that was used for the coagulation of the Dieulafoy’s lesion and the duodenal intradiverticular bleeding. In the case of the Dieulafoy’s lesion, the field was blood-filled and large amounts of water were required to clear it. It took us a long time to grasp the spurting lesion twice and coagulate it with the forced mode to complete the hemostatic procedure. Despite the prolonged duration of coagulation, the bleeding was controlled with no early or delayed-onset complications. It seems doubtful if the use of soft coagulation would be effective in achieving hemostasis. In the case of the large bleeding diverticulum in the second duodenal portion, which was not easily accessible, we continued the coagulation using the forced mode (60 W), because the hemostatic effect of the soft mode with 80 W current was not rapid enough. Although there might be a higher risk of excess tissue injury from thermocoagulation in thin-walled duodenal diverticula, an endoclip-induced perforation has been reported as the only complication of endotherapy of diverticular bleeding in a recent case series[5]. Possible explanations include the sharp tip of the hemoclip and excessive air inflation during therapeutic endoscopy[23].
In conclusion, our case series supports the potential value of the application of a hemostatic forceps in active GI bleeding of various origins. It suggests that it seems to be a reliable and safe therapeutic tool even for those cases of bleeding that are unrelated to ESD, which are difficult to treat with other endoscopic means, although more cases need to be studied before any firm conclusions can be made. Proper techniques and settings should be followed to avoid any procedure-related complications.