INTRODUCTION
Arterial vasodilation and vascular dysregulation represent the initiating as well as perpetuating mechanism in the pathophysiology of the hyperdynamic circulatory syndrome in portal hypertension[1]. This arterial vasodilation occurs early and is most pronounced in the splanchnic circulation which therefore has been suggested to be the main culprit for reduced peripheral vascular resistance occurring with progression of portal hypertension[2,3]. A strong line of evidence suggests that vascular overproduction of nitric oxide (NO) plays a pivotal role for the development of splanchnic arterial vasodilation in this condition[4-6]. In fact, non-specific inhibition of NO synthesis has been shown to restore mesenteric vascular responsiveness[7] and to almost normalize splanchnic vascular resistance[8] thereby markedly ameliorating portal-systemic shunting and the hyperdynamic circulation[9,10].
Three isoforms of NO-synthases (NOS) have been cloned. Vascular overproduction in the splanchnic circulation in portal hypertension is derived from endothelial (eNOS) and neuronal (nNOS) but not inducible NOS (iNOS)[4,11,12]. eNOS as well as nNOS-derived NO production are regulated through distinct posttranslational modifications, one being the interaction of NOS with regulatory proteins[13]. The molecular chaperone heat shock protein-90 (HSP-90) facilitates folding and stabilization of cellular proteins thereby promoting specific signalling pathways[14]. In support of this concept, interaction of eNOS and nNOS with HSP-90 has recently been shown to facilitate NO synthesis[15,16]. In fact, in portal hypertension we have observed increased eNOS activity and an associated enhanced eNOS-dependent vasorelaxation in mesenteric arteries, which was markedly inhibited by geldanamycin, an ansamycin antibiotic and specific HSP-90 antagonist[17]. Whether HSP-90 likewise plays a crucial role in nNOS-mediated vasodilation in the splanchnic circulation in healthy as well as portal hypertensive conditions is currently unknown.
Chronic specific nNOS-inhibition markedly attenuates the hyperdynamic circulatory syndrome in experimental cirrhosis[18] underlining the relative importance of nNOS-derived NO in this scenario. Moreover, we recently reported an increased nNOS expression in the whole mesenteric vascular bed of portal vein-ligated (PVL) rats[12], and nNOS has been demonstrated to be up-regulated and to be highly expressed in vascular smooth muscle cells of the mesenteric arteries during portal hypertension[19]. nNOS expression and function in mesenteric nervous tissue independent of the arterial smooth muscle layer, however, has not been investigated so far.
Thus, the aim of our study was to analyse (1) nNOS expression in mesenteric nerves during portal hypertension; (2) whether HSP-90 co-localizes with and regulates nNOS-mediated vasorelaxation in the mesenteric arterial vasculature; and (3) if this is the case, whether alterations in HSP-90 function occur during portal hypertension.
MATERIALS AND METHODS
Animals
The investigation was performed in male Sprague-Dawley rats (Harlan Sprague Dawley Laboratories, Indianapolis, IN), weighing 300-399 g. Rats were caged at a constant room temperature of 21°C, exposed to a 12:12 hour light:dark cycle, and allowed free access to water and standard rat chow ad libitum. All experimental procedures in this study were conducted according to the German Physiological Society principles for the care and use of laboratory animals (Granted permission number 621-2531.1-23/00, Government of Oberpfalz, Bavaria).
Drugs
Acetylcholine, Sodium-Nitroprusside, Norepinephrine, Guanethidine, Atropine, Timolol, L-NAME and geldanamycin were purchased from Sigma (Deisenhofen, Germany).
Induction of portal hypertension
A prehepatic portal hypertensive animal model extensively studied in our laboratory was used[20]. Portal hypertension was induced surgically in aseptic conditions. Briefly, the rats were anaesthetized with ketamine hydrochloride (Ketalar, 100 mg/kg bw; Parke, Davis, Avon, CT). After a midline abdominal incision, the portal vein was freed from surrounding tissue. A ligature (silk gut 3-0) was placed around a 20-gauge blunt-tipped needle lying alongside the portal vein. Subsequent removal of the needle yielded a calibrated stenosis of the portal vein. In sham-operated rats, the same operation was performed with the exception that after isolating the portal vein no ligature was placed. All studies were performed in 12-18 h fasted animals 10-14 d after surgery.
In vitro perfusion
The in vitro perfusion system used was a partial modification of that originally described by McGregor and used extensively in previous studies from our laboratory and others[5,21,22]. Briefly, the superior mesenteric artery (SMA) was cannulated with a PE-60 catheter and gently perfused with 15 mL warm Krebs solution to eliminate blood. After isolating the SMA with its mesentery, the gut was cut off close to its mesenteric border. The arterial vasculature was then transferred to a 37°C waterjacketed container and perfused with oxygenated 37°C Krebs’ solution (95% O2, 5% CO2) using a roller pump (Ismatec, IPC 8-channel, Zürich, Switzerland). The Krebs solution had the following composition (mmol/L): NaCl, 118; KCl, 4.7; KH2PO4, 1.2; MgSO4, 1.2; CaCl2, 2.5; NaHCO3, 25; disodium EDTA, 0.026; and glucose, 11.0; pH 7.4. The effluent of the perfused tissue was continuously removed from the perfusing chamber. The perfusion pressure was measured with a P-23-Db strain gauge transducer (Statham, Oxnard, CA) on a side arm just before the perfusing cannula and continuously recorded (Powerlab Quadbridge and Powerlab 4/20, from AD Instruments, Spechbach, Germany).
Where indicated endothelial denudation of the mesenteric vasculature was performed by a combined treatment of cholic acid (sodium salt) and distilled water as has been used before[21]. In brief, after cannulation of the SMA and gentle flushing with 10 mL of warmed Krebs’ solution to eliminate blood, perfusion with cholic acid (0.5%/1.5 mL for 10 s) followed by 15 mL of Krebs solution (to eliminate cholic acid) was performed. The preparation was then transferred to the 37°C waterjacketed container and perfused with oxygenated 37°C Krebs solution (4 mL/min) for 10 min. After the mesenteric vasculature was relaxed, 37°C warmed distilled water was perfused for 10 min before starting Krebs perfusion again. After an equilibration period of 45 min, experimental perfusion protocols were performed (Figure 1). At the end of each experiment we assessed whether the vessel was completely de-endothelialized and whether the smooth muscle function was maintained. The mesenteric preparation was kept preconstricted with methoxamine (MT; α-1-agonist: 100 μmol/L) and dose-dependent vasorelaxation to the endothelium-dependent vasodilator acetylcholine (ACh: 10-8 to 10-6 g, bolus of 0.1 mL) and the endothelium-independent vasodilator sodium nitroprusside (SNP: 10-6 to 10-5 g, bolus of 0.1 mL) was tested. Only experiments with ACh-/ and SNP-induced relaxation being less/more than 15%/60% were accounted as valid.
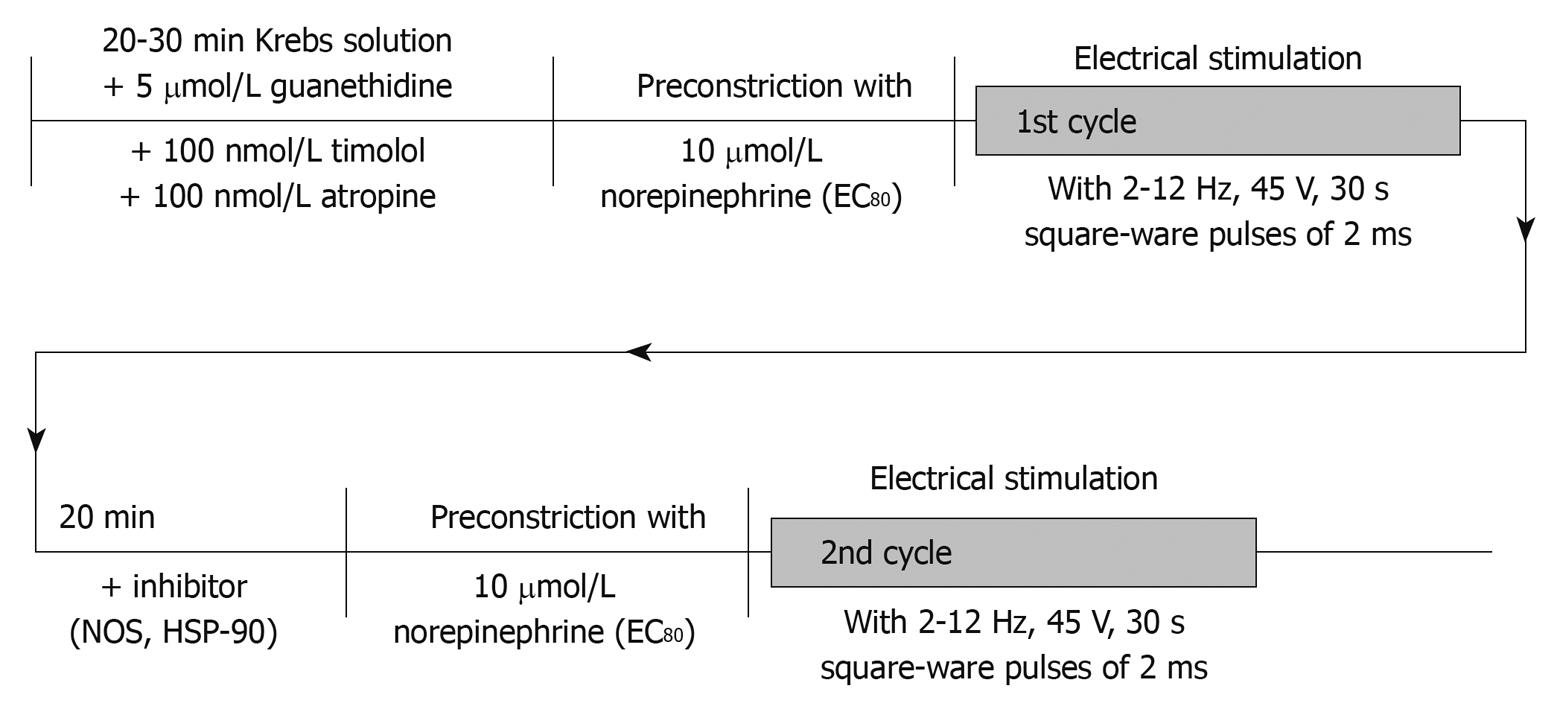
Figure 1 Experimental study protocol.
In this illustration, the chronological order of applications of drugs and performance of peri-arterial nerve stimulation (PNS) is shown. EC80: Concentration achieving 80% maximal vasoconstriction; NOS: Nitric oxide synthase; HSP-90: Heat shock protein-90.
Periarterial nerve stimulation
Two platinum electrodes, one placed around the SMA and the other shaped as a wire grid the tissue was resting on, were used for transmural electrical field stimulation. The nerves of the preparation were stimulated by means of an electronic stimulator (I-ZQ4v, Hugo Sachs Electronics, Hugstetten, Germany) , delivering single square-wave pulses (2 ms) at 45 V with a train duration of 30 s and frequencies of 2-12 Hz. In order to evaluate vasodilatory responsiveness vessels were preconstricted submaximally (EC80) using norepinephrine (NE 10-5 mol/L) before applying periarterial nerve stimulation (PNS) (Figure 1). nNOS-mediated vasorelaxation is known to be non-adrenergic and non-cholinergic in origin. Therefore, guanethidine (5 × 10-5 mol/L), atropine (10-9 mol/L) and timolol (10-9 mol/L) were added from the beginning in order to deplete endogenous noradrenaline stores and to prevent its uptake as well as to avoid cholinergic stimulation. When a stable preconstriction level was achieved, PNS was applied in a non-cumulative fashion. Sufficient time was allowed between each stimulation train for the perfusion pressure to return to a stable level, usually 5-10 min. PNS responses are expressed as percentage change of the pre-constriction level being present before PNS. Nitrergic vasodilation is known to be independent of prostaglandin synthesis[23] and it is not subject to pre-junctional inhibition by alpha2-adrenoreceptors[23]. Moreover, in previous experiments tetrodotoxin has been used to evidence the neural origin of the hemodynamic response[12].
Role of HSP-90 for nNOS-mediated vasorelaxation
Most importantly, all PNS experiments were performed in de-endothelialized mesenteric vasculature in order to exclude any hemodynamic effects of endothelium-derived vasodilators. In order to evaluate the magnitude of nNOS-mediated vasorelaxation, PNS experiments were performed in the absence (1st perfusion cycle) and presence of L-NAME (10 μmol/L) (2nd perfusion cycle after incubation with L-NAME for 20 min). The differences in PNS-triggered vasorelaxation induced by L-NAME correspond to nNOS-mediated vasodilator effects. As for the role of HSP-90 in mediating this nNOS-vasodilation, vessel preparations were correspondingly pre-incubated with the HSP-90 inhibitor geldanamycin (GA 3 μg/mL) for 20 min before performing the 2nd perfusion cycle (Figure 1). GA is a benzoquinone ansamycin antibiotic that binds to the nucleotide-binding site of HSP-90 and specifically blocks HSP-90 function[24,25]. GA was first reported to inhibit iNOS activity in rat smooth muscle[26], however, as reported earlier no iNOS protein was detected in the vasculature studied, ruling out a hemodynamic role of HSP-90 related to iNOS-derived NO. As shown previously, incubation with GA does not directly affect soluble guanylate cyclase or other smooth muscle cell machinery required for NO-dependent vasodilation since vasodilation is not altered in response to SNP[17].
Immunofluorescence analysis
Mesenteric tissues were harvested by dissecting and removing the highly vascular tissue situated between the mesenteric lymph nodes and small intestine. The tissue was freed, washed in PBS, stored in 3.7% formaldehyde at 4°C over night, and then transferred into 20% sucrose solution for more than 12 h at 4°C. After this procedure the SMA were embedded in tissue tek (Herstetller, Stadt, Land) and stored at -80°C. Frozen sections (Frigocut 2800 E, Leica, Wetzlar, Germany) were incubated overnight with monoclonal antibodies directed against nNOS or HSP-90, respectively (both BA, Transduction Laboratory, San Diego, CA, USA). Immunofluorescence staining was performed by utilizing either red fluorescent or green fluorescent anti-mouse IgG (Alexa 46, MoBiTec, Göttingen, Germany) as secondary antibodies. Sections treated with secondary antibody only or with control IgG (as first antibody) did not reveal an immunosignal (data not shown).
Immunoprecipitation and Western blotting
Mesenteric nerves located in near proximity to the SMA were dissected microscopically and washed in PBS and homogenized in a lysis buffer. Protein quantification of tissue samples was performed using the Lowry assay. In experiments examining the level of nNOS and HSP-90 protein in mesenteric tissue, 100 μg of protein were used for electrophoresis. For nNOS immunoprecipitation, about 1500 μg of detergent-soluble protein was incubated with excess nNOS mAb overnight after samples were precleared with Pansorbin (Calbiochem, CA, USA). Immune complexes were precipitated by the addition of protein A-Sepharose. Protein samples were boiled in Laemmli loading buffer and separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) on a 7.5% acrylamide gel, and proteins were electroblotted onto nitrocellulose membranes. Subsequently, the membranes were washed with Tris-buffer saline with 1% Tween, blocked with 5% milk, and incubated with either HSP-90 mAb or nNOS mAb. Densitometry was performed with a scanner and analyzed with the Software Image Quant (Personal Densitometer Si, Amersham, Bioscience, UK).
Statistical analysis
Results were expressed as mean ± SE. Statistical analysis was performed using ANOVA (two-way, with repeated measurements) for comparison of study groups for PNS-induced vasorelaxation. Unpaired non-parametric tests were used for comparison of individual values between study groups. The level of statistical significance was set at P < 0.05.
RESULTS
Animals
There were no differences in body weight in the experimental groups (349 ± 36 g for PVL and 363 ± 42 g for sham rats, respectively). PVL rats showed elevated spleen weights, expressed in relation to body weight (PVL: 3.25 ± 0.30 g/kg bw vs sham: 2.48 ± 0.50 g/kg bw, P < 0.0001).
Western blotting and co-association of nNOS and HSP-90 in mesenteric nerves
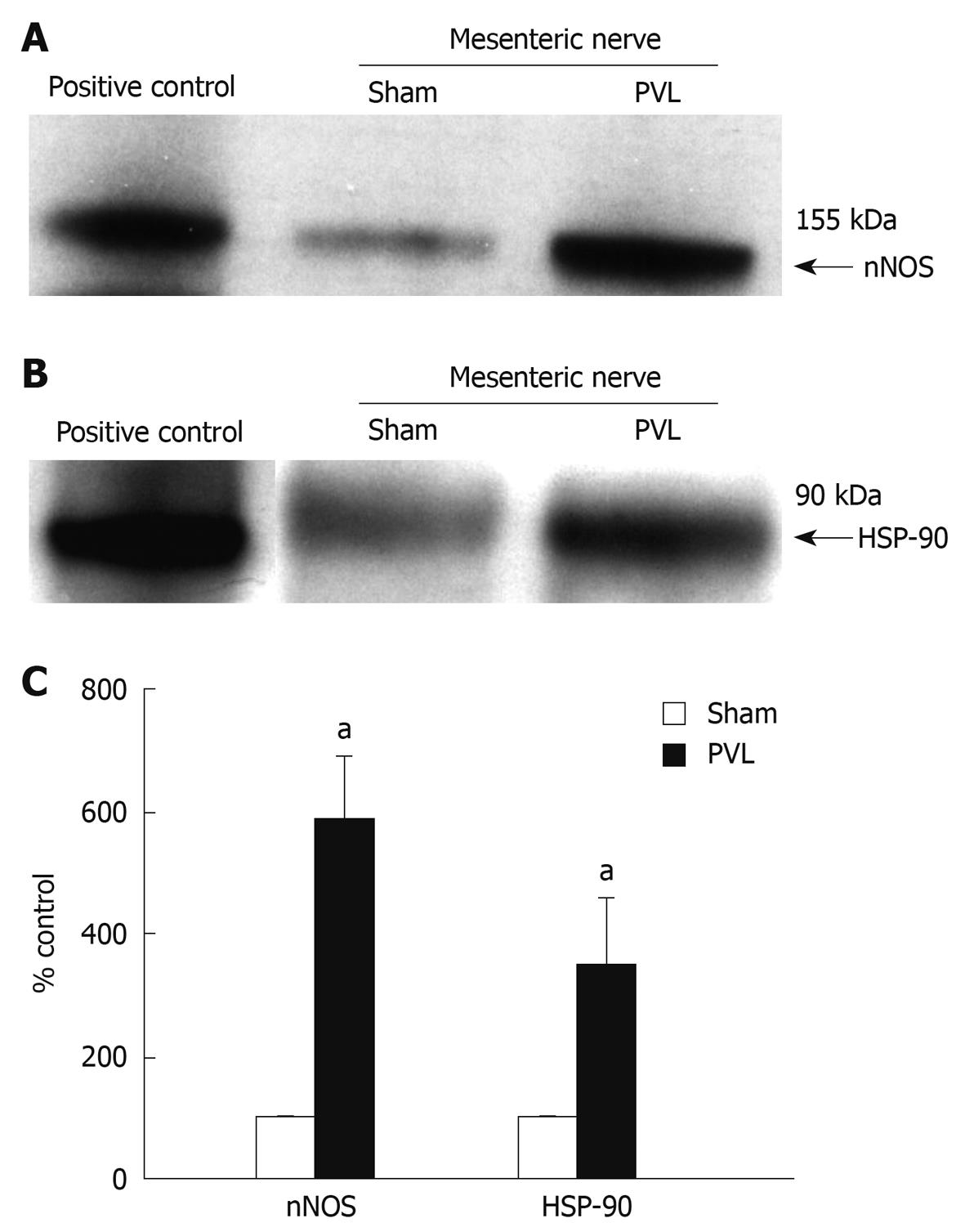
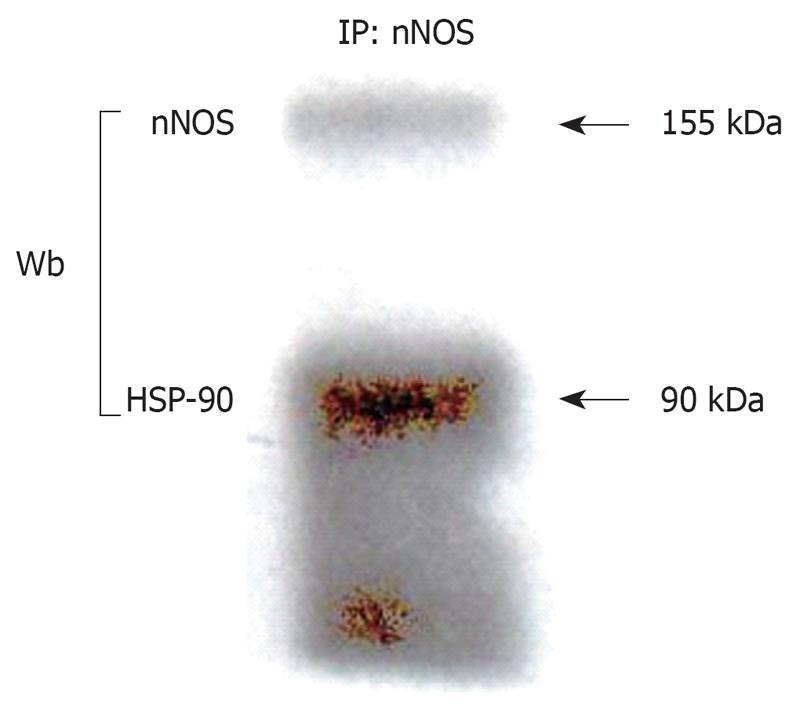
To determine the importance of HSP-90 for neuronal vascular control in mesenteric arteries, we first examined expression, association and localization of nNOS and HSP-90 in mesenteric nerves. nNOS expression was found to be increased in PVL rats as compared to sham rats (Figure 2A and C). In addition, HSP-90 expression was enhanced in mesenteric nerves in PVL rats as compared to sham rats (Figure 2B and C) and nNOS co-precipitated with HSP-90 from mesenteric nerves (Figure 3) evidencing direct protein-protein interaction.
Figure 2 n-nitric oxide synthase (nNOS) and HSP-90 expression in mesenteric nerves.
nNOS (A) and HSP-90 (B) in mesenteric nerves of portal-vein-ligated rats (PVL) and sham rats by Western blotting analysis. Protein lysate from rat brain tissue was used as positive control; C: Densitometric analysis of Western blottings revealed a more than 5-fold increase of nNOS and approximately 3-fold increase of HSP-90 protein expression in mesenteric nerves of PVL rats as compared to sham rats. aP < 0.05, n = 3 per group.
Figure 3 Co-immunoprecipitation.
nNOS was immunoprecipitated from detergent-soluble protein lysates prepared from de-endothelialized mesenteric tissue harvested as described in materials and methods and prepared for gel electrophoresis or, alternatively, protein samples were directly prepared for gel electrophoresis. nNOS and HSP-90 are both abundantly expressed in mesenteric tissue and immunoprecipitation (IP) of nNOS coprecipitates HSP-90 under basal conditions. Wb: Western blotting.
Immunofluoresence of nNOS and HSP-90 in mesenteric nerves
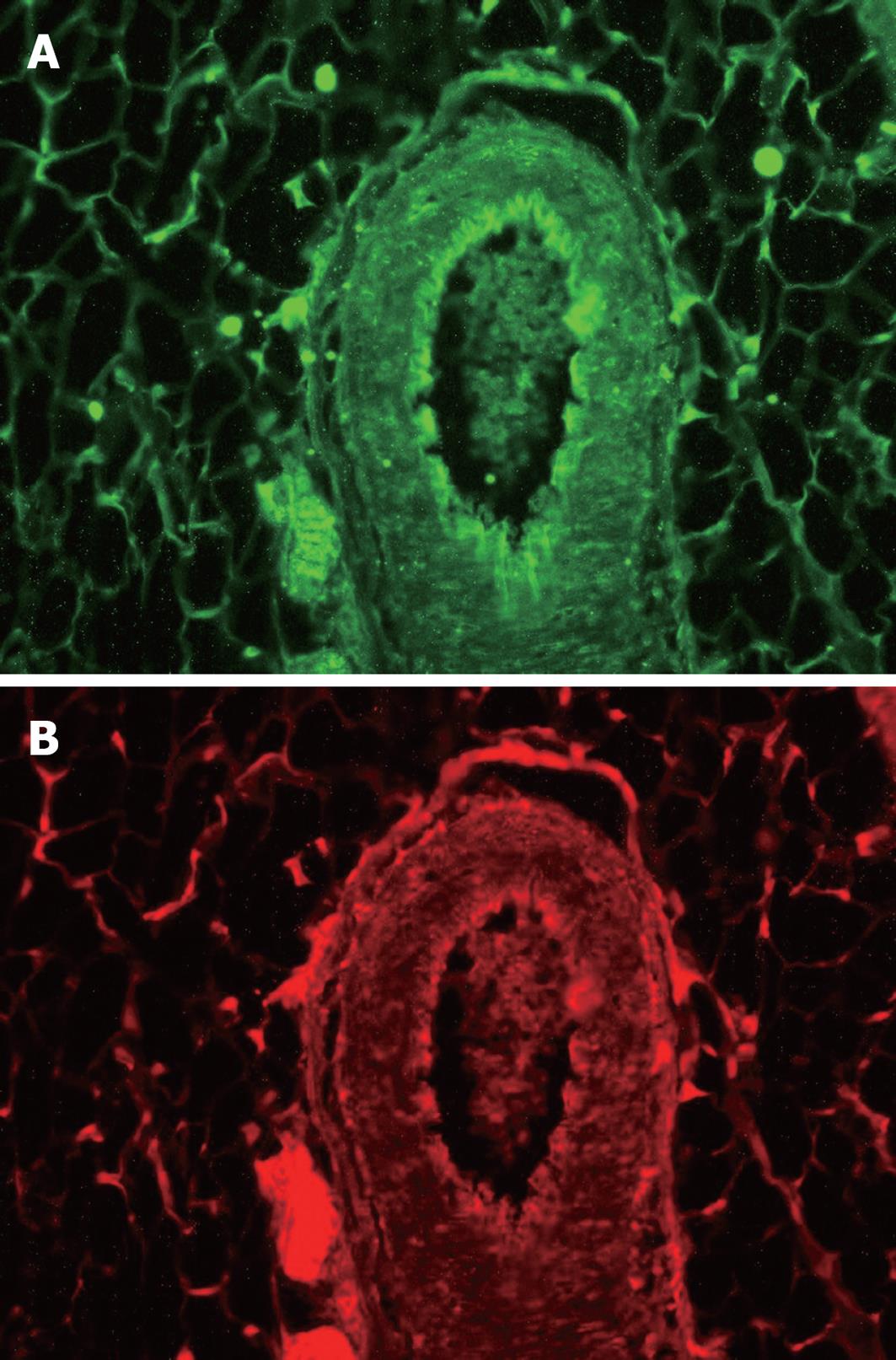
We previously reported that both nNOS and HSP-90 are abundantly expressed in mesenteric vasculature in rats[17]. Here we extend this information with a focus on the mesenteric neurons. In fact, immunofluorescence revealed co-localization, with nNOS and HSP-90 being distributed equally in perivascular neurons (Figure 4A and B). Indeed, identical localization and most obviously similar magnitude of protein expression for nNOS as well as HSP-90 were observed at the entry of the nerve supply into the mesenteric vasculature.
Figure 4 Immunofluoresence analysis of nNOS and HSP-90.
As can be seen immunofluorescence shows strong signals for nNOS (A) and HSP-90 (B) mainly at the entry of the nerve bundle along the adventitia revealing identical localization of nNOS and HSP-90 protein in mesenteric nervous tissue.
nNOS-dependent mesenteric vasorelaxation and HSP-90
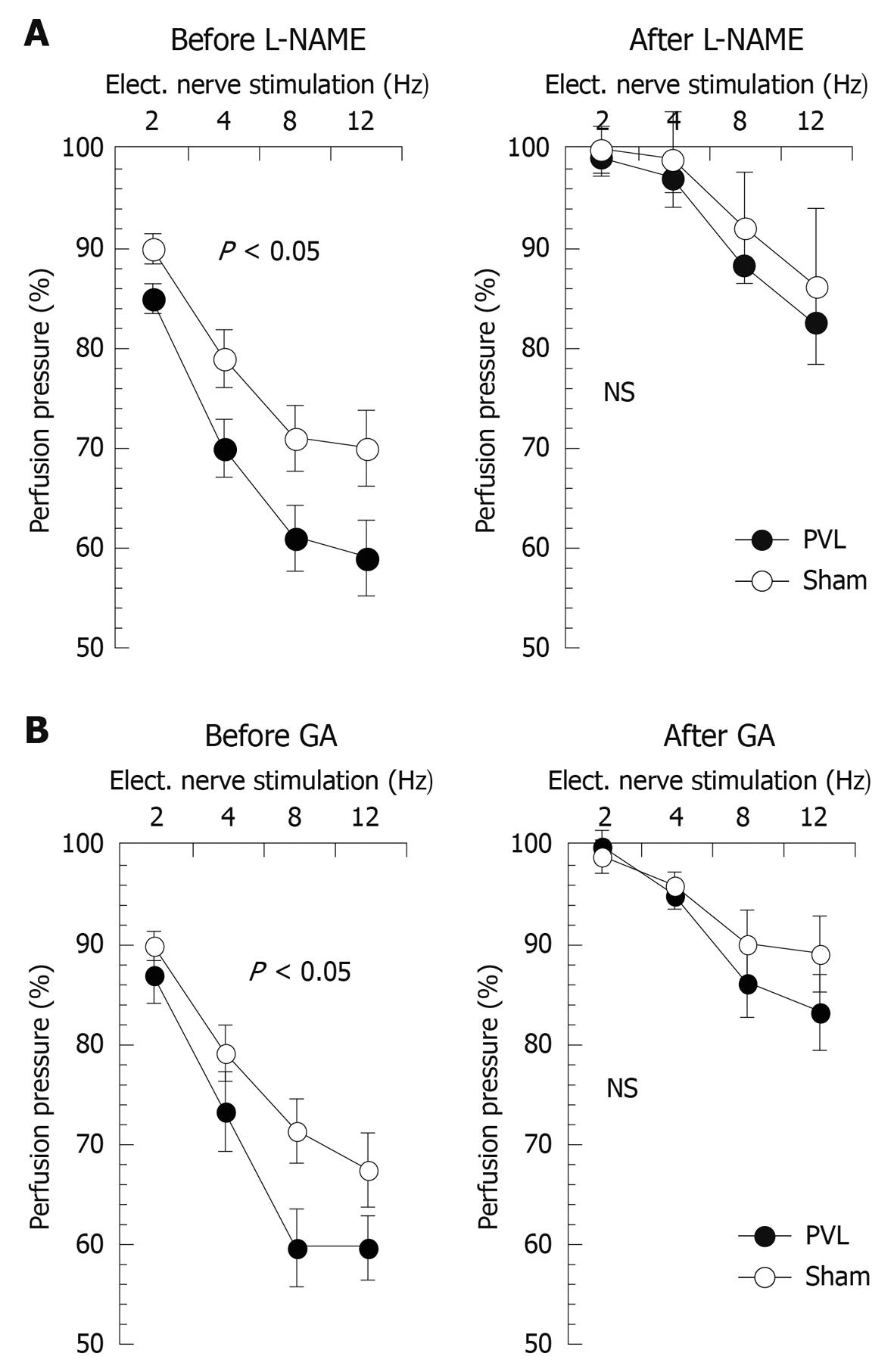
Baseline perfusion pressures before removal of the endothelium were significantly lower in PVL as compared to sham rats (16.0 ± 6.1 mmHg vs 23.4 ± 8.0 mmHg, P < 0.01). After removal of the endothelium no difference in basal perfusion pressure between study groups was noted anymore (data not shown). The vasodilator response to acetylcholine at the highest dose used was 7.5% ± 5.0% and 9.0% ± 6.0% in PVL and sham rats, respectively (NS), demonstrating a sufficient de-endothelialization. The vasodilator response to sodium nitroprusside at the highest dose applied was 65% ± 7% for PVL and 58% ± 9% for sham rats (NS) demonstrating the functional integrity of the vascular smooth muscle cells in the vascular bed studied. NE-induced pre-constriction levels were similar in both study groups (95 ± 5 mmHg vs 88 ± 6 mmHg) indicating similar vascular tone before neuronal stimulation. Incubation with either L-NAME or GA did not affect baseline or pre-constriction levels in either group. All vessels responded to PNS with a frequency-dependent decrease in perfusion pressure (Figure 5). This neurally mediated vasodilatory response was significantly more pronounced in PVL as compared to sham rats (Figure 5). Non-specific NO-inhibition by L-NAME (Figure 5A) abolished the difference in PNS-induced vasorelaxation between the study groups evidencing NO as being the vasodilator mediating this difference and thus reflecting nNOS-mediated vasorelaxation. Moreover, HSP-90 inhibition using GA likewise abolished the difference in PNS-induced vasorelaxation between PVL and sham rats (Figure 5B). This indicates that the observed increase in nNOS-mediated vasorelaxation in PVL rats is dependent on HSP-90 signalling.
Figure 5 nNOS- and HSP-90-mediated neuronal vasorelaxation in PVL and sham rats.
PNS-induced nitrergic vasorelaxation in de-endothelialized mesenteric vasculature is depicted before and after L-NAME (A) as well as before and after HSP-90-inhibition with geldanamycin (B). It can be appreciated that no more significant differences in frequency-dependent neuronal vasorelaxation were observed between the study groups after L-NAME as well as after geldanamycin. Values given as mean ± SE. PVL-group: n = 6; Sham group: n = 6. P < 0.05 for ANOVA comparing study groups. Elect. nerve stimulation: Electric nerve stimulation; NS: Not significant.
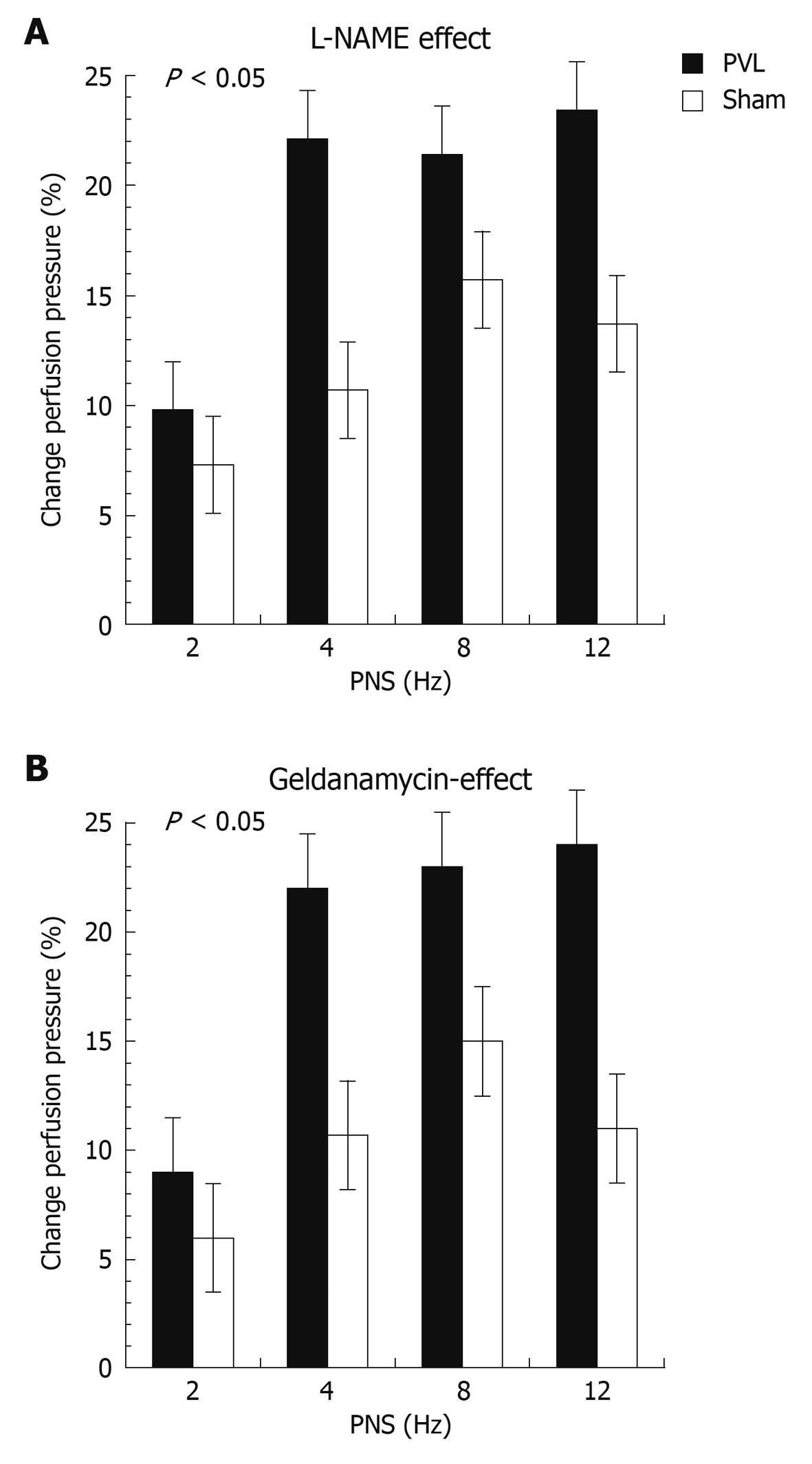
When expressing PNS-induced vasorelaxation as percent change from the pre-constriction level a markedly increased response was observed in vessel preparations from PVL rats as compared to sham rats (Figure 6). Maximal vasodilation was reached at 8-12 Hz, confirming previous investigations of SMA in rats[27]. Finally, the effect of NO inhibition (Figure 6A) and HSP-90 inhibition (Figure 6B) expressed as percent change in perfusion pressure from PNS-induced vasorelaxation observed during the corresponding 1st perfusion cycle induced by L-NAME or GA was significantly more pronounced in PVL rats as compared to sham rats (P < 0.05). In addition, the magnitude of effect induced by L-NAME and GA was almost identical indicating that the majority of nNOS-mediated vasorelaxation is dependent on HSP-90 signalling.
Figure 6 Changes in PNS-induced vasorelaxation induced by inhibition of NOS or HSP-90.
Changes in pressure response to PNS induced by L-NAME (A) and geldanamycin (B) presented as percent change in perfusion pressure as compared to values obtained during the first perfusion cycle. P < 0.05 (ANOVA comparing all conditions between study groups) vs sham, respectively.
DISCUSSION
HSP-90 acts as an intermediate in the signalling cascade leading to activation of nNOS[28,29]. In this study, we examined the role of this protein interaction in mediating neuronal regulation of vascular tone in the mesenteric vascular bed, where vascular resistance contributes to the regulation of portal pressure. The novel findings of this study include the interaction of HSP-90 and nNOS in mesenteric nerves and the participation of this interaction in regulating vasomotor function in resistance vessels based on inhibition of HSP-90 with GA. Moreover, we demonstrate that after PVL, enhanced HSP-90 signalling contributes to the well-known elevated nNOS-dependent vasorelaxation.
Numerous investigations have presented evidence that nitrergic nerves are involved in vascular homeostasis[30,31]. However, mechanisms leading to nNOS-derived NO release from nitrergic nerve endings are missing. Under physiological conditions, NO production is strictly modulated to accommodate the need of homeostasis because abnormalites of NO formation cause disease[32,33]. Since NO is highly diffusable and cannot be stored in intracellular compartments modulation of NO production in cells is primarily mediated through regulating NOS activity. Several mechanisms have been revealed in NOS regulation. These include protein-protein interactions, protein phosphorylation, and subcellular targeting[34]. For example, regulation via protein-protein interaction such as HSP-90 and NOS appears particularly important. HSP-90 is one of the most abundant proteins in cells[35] constituting 1%-2% of total intracellular proteins. As a molecular chaperone, HSP-90 has been shown to regulate more than 100 signal transduction pathways by controlling function, trafficking and turnover of a variety of signalling proteins[36]. In respect to NOS regulation, HSP-90 was first reported to associate with eNOS acting as an allosteric enhancer[15]. Subsequent studies with purified nNOS preparations indicated that HSP-90 likewise functions as an allosteric enhancer for nNOS and inhibition of HSP-90 results in the inability to form the dimeric active nNOS[28]. Coupling of nNOS with HSP-90 is crucial for normal nNOS function[37]. Here, we report co-localization of HSP-90 and nNOS in mesenteric nerves. In addition, nNOS of mesenteric nerves co-immunoprecipitates with HSP-90 indicating protein-protein interaction in this neuronal tissue. Finally, GA was used to probe the importance of HSP-90 in cellular pathways, since it directly binds to the amino-terminal ATP binding domain of HSP-90[38] and specifically labels HSP-90 in cellular extracts[25]. Since NANC-induced vasorelaxation in the mesenteric vasculature is reduced after inhibition of HSP-90 by GA, the hemodynamic functional relevance of the stated protein-protein interaction is demonstrated. To our knowledge, this is the first report to demonstrate a physiological role of HSP-90 for regulation of neuronal control of vascular tone via modulation of nNOS-derived NO release. This is in accordance with previous reports demonstrating that HSP-90 inhibition causes a decrease of NO production in nNOS-transfected cells[16,39].
Portal hypertension is characterized by decreased systemic and particularly splanchnic vascular resistance which is the initiating mechanism for the development of the hyperdynamic circulatory syndrome in this entity[40]. Therefore, any measure improving splanchnic vascular resistance is of high hemodynamic and clinical relevance. Vascular overproduction of NO has been shown to be the pathophysiological hallmark leading to this splanchnic vasodilation and to be due to a marked upregulation of eNOS and nNOS-derived NO synthesis[4-6,12]. Indeed, either non-specific or nNOS-specific NOS inhibition has been demonstrated to ameliorate the development of the hyperdynamic circulatory syndrome in experimental portal hypertension[9,18]. In addition, enhanced eNOS and nNOS protein expression as well as an increased vasodilatory response to eNOS and nNOS stimulation has been reported by us and others in the mesenteric vasculature during experimental portal hypertension[5,12,41]. Beside AKT phosphorylation[42], a marked contributory role of HSP signalling for eNOS-derived NO overproduction has been reported by us previously[17]. Here, we show that HSP-90 also largely mediates the enhancement in nNOS-dependent NO release in the mesenteric vasculature of pre-hepatic portal hypertensive rats. This is based on the observation of enhanced HSP-90 expression in mesenteric nerves of portal hypertensive rats and the finding that inhibition of HSP-90 signalling preferentially blocks NANC vasorelaxation in these animals.
Enhanced HSP-90 protein expression has been reported previously in whole mesenteric tissue of portal vein ligated rats[43]. However, evaluation of HSP-90 expression in mesenteric nerves has not been reported before. Nonetheless, it has been evidenced in cell lines as well as in studies with purified nNOS that HSP-90 directly augments NO synthesis of nNOS in a dose-dependent manner[16,37,44]. This effect has been attributed mainly to an increase in binding of calmodulin to nNOS mediated by HSP-90[44]. By this mode of action, most importantly HSP-90 treatment has been shown to increase even the maximal nNOS activity and associated maximal nNOS-dependent NO synthesis achievable[44]. Therefore, we propose that increased HSP-90 protein expression observed in mesenteric nerves of portal hypertensive rats results in enhanced nNOS-mediated vasorelaxation in the mesenteric vasculature of these animals due to its well-known augmenting effect on the nNOS-machinery. However, neither inhibition of NO nor HSP-90 did completely abrogate NANC vasorelaxation. Remaining vasodilation may well be mediated by other endogenous neurogenic vasodilators such as calcitonin-gene-related peptide or vasoactive-intestinal peptide known to be released from NANC nerves, particularly in rats. Nonetheless, these mediators are not mediating any differences in NANC-vasodilatory responses between the study groups since no significant difference in NANC vasorelaxation remained after NOS inhibition.
In summary, HSP-90 co-localizes with and binds to nNOS in mesenteric nerves playing a key role in nNOS-dependent vasoregulation in the mesenteric vasculature. This interaction serves to mediate the increased nitrergic vasorelaxation observed in the splanchnic circulation during experimental portal hypertension. In conjunction with our previous report on the excessive eNOS-derived NO overproduction depending mainly on HSP-90 signalling this study may provide the rational for pharmacological inhibition of HSP-90 as a novel target for the treatment of the hyperdynamic state in portal hypertension.
COMMENTS
Background
Portal hypertension is the main cause of death in end-stage liver disease leading to multiple complications such as variceal hemorrhage, ascites and hepatorenal syndrome. Vasodilatation in the mesenteric circulation is of essential pathophysiological relevance in portal hypertension and n-nitric oxide synthase (nNOS) has gained much attraction as a vasodilation mediator in other clinical entities recently.
Research frontiers
Heat shock protein-90 (HSP-90) is a molecular chaperone required for the stability and activity of a diverse range of client proteins that have critical roles in signal transduction, cellular trafficking, cell growth, differentiation etc. but also vasoregulation. Among NOS it has been shown to be critical for full e- and nNOS activity. However, the role of HSP-90 in respect to neural nNOS-mediated vasoregulation has not been addressed in healthy or in portal hypertensive conditions. The strength of the current investigation relates to the utilization of the McGregor preparation enabling physiological and pharmacological testing of the whole mesenteric vasculature in its original anatomy and innervations. In contrast to arterial strips, this ensures testing of neural vasoregulation at close to in vivo conditions.
Innovations and breakthroughs
By this approach, pronounced nNOS-mediated vasodilation is confirmed in pre-hepatic portal hypertension and most strikingly is shown to be in large parts due to HSP-90 signalling. This is also the first time that HSP-90 has been established as a signaling mediator in neural vascular responses in healthy conditions.
Applications
Considering the well-known role of HSP-90 as an eNOS chaperone and enhanced eNOS-derived NO overproduction in portal hypertension, HSP-90 may well be an ideal target in treatment of portal hypertension.
Peer review
This is a good experimental investigation in which authors analyze the role of HSP-90 for neural vasoregulation in the mesenteric circulation in healthy and portal hypertensive conditions. The results are interesting and suggest that HSP-90 indeed is the main signaling intermediate translating into enhanced nNOS-dependent vasodilation during portal hypertension. Therefore, inhibition of HSP-90 could be used to treat portal hypertension.