Published online Apr 14, 2010. doi: 10.3748/wjg.v16.i14.1776
Revised: January 25, 2010
Accepted: February 1, 2010
Published online: April 14, 2010
AIM: To investigate the effectiveness of early infliximab use for induction and maintenance therapy in pediatric Crohn’s disease.
METHODS: We performed a retrospective chart review of 36 patients with Crohn’s disease. Ten patients (group A) were treated with mesalamine after induction therapy with oral prednisolone, and 13 patients (group B) were treated with azathioprine after induction therapy with oral prednisolone. Thirteen patients (group C) received infliximab and azathioprine for induction and maintenance therapy for the first year, and were treated with azathioprine after 1 year. All patients were followed for at least 24 mo. Efficacy was determined by the relapse rate using the pediatric Crohn’s disease activity index score in each group at 12 and 24 mo.
RESULTS: At the 1 year follow-up, the relapse rate (23.1%, 3 of 13 patients) in group C was lower than that (61.5%, 8 of 13 patients) in group B (P = 0.047). At the 2 years follow-up, the relapse rate (38.5%, 5 of 13 patients) in group C was lower than that (76.9%, 10 of 13 patients) in group B (P = 0.047). Adverse events in group C were fewer than in groups A and B.
CONCLUSION: Early induction with infliximab at diagnosis, known as “top-down” therapy, was effective for reducing the relapse rate compared to conventional therapies for at least 2 years.
- Citation: Lee JS, Lee JH, Lee JH, Lee HJ, Kim MJ, Lee HJ, Choe YH. Efficacy of early treatment with infliximab in pediatric Crohn’s disease. World J Gastroenterol 2010; 16(14): 1776-1781
- URL: https://www.wjgnet.com/1007-9327/full/v16/i14/1776.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i14.1776
Although the etiology of Crohn’s disease remains incompletely understood, environmental factors, infectious microbes, ethnic origin, genetic susceptibility, and immune system dysfunction have been implicated in the associated chronic mucosal inflammation[1]. There is no known cure for Crohn’s disease. Therefore, the goal of treatment is to mitigate inflammation and the associated clinical symptoms. The current treatment guidelines are designed to maintain remission after induction therapy.
Conventional therapy for active disease includes treatment with corticosteroids. Initially, corticosteroids are very effective and fast acting, but long term exposure to corticosteroids becomes problematic with issues of dependency and/or resistance[2]. Infliximab is an anti tumor necrosis factor (TNF)-α monoclonal antibody that was introduced for the treatment of moderate to severe Crohn’s disease[3,4]. The efficacy of infliximab suggests a new concept; that early induction with intensive therapy may reduce complications associated with conventional treatment and may improve the quality of life. Therefore, intensive therapy with early use of this biological agent has been proposed, and is known as ‘top-down’ therapy. The results of some studies suggest that infliximab is more effective in children than in adults[5,6].
Ethnic differences in the clinical characteristics of Crohn’s disease have been noted. Crohn’s disease is relatively common in developed countries, while it is not as common in Asian countries[7]. In addition, recent studies from Far East Asian countries reveal that the prevalence of NOD2 mutations, associated with increased susceptibility to Crohn’s disease in Western populations, is lower than that found in other populations[8-10]. Therefore, the clinical response to treatment for Crohn’s disease in Asian countries might differ from patients in Western countries.
The purpose of this study was to evaluate the efficacy of the early use of infliximab, compared to conventional treatments, at 1 and 2 years of follow-up, in Korean pediatric patients with Crohn’s disease.
We included newly diagnosed pediatric patients with Crohn’s disease. All included patients had not been treated with agents such as corticosteroids, mesalamine, or infliximab, and were followed for at least 24 mo at our clinic. Forty-three patients confirmed to have Crohn’s disease at Samsung Medical Center, Korea, between March 2001 and February 2007 were enrolled. Seven patients were excluded: four patients were lost to follow-up and three patients did not respond to the induction regimen with prednisolone or infliximab. Among three patients who did not achieve induction, 2 patients did not stop corticosteroids and one patient did not respond to three infusions of infliximab for induction. Therefore, 36 patients were eligible for inclusion in the study. A retrospective chart analysis was conducted of physician notes, laboratory studies, radiology reports, endoscopy records, and histology reports. Biopsies were obtained by endoscopy in all eligible patients. The diagnosis of Crohn’s disease was made in accordance with the ESPGHAN - Porto criteria[11]. Infectious diseases such as tuberculosis were ruled out by taking a detailed family history, imaging studies and confirmation of a negative purified protein derivative tuberculin (PPD) test result and a negative PCR-hybridization of Mycobacterium tuberculosis on biopsy tissue. Our study was approved by the institutional review board of our institution.
The patients (n = 36) were divided into three subgroups according to the treatment regimen. Ten patients (group A) were treated with mesalamine after induction therapy with oral prednisolone and 13 patients (group B) were treated with azathioprine after induction therapy with oral prednisolone. In the third subgroup, 13 patients (group C) received infliximab and azathioprine for induction and maintenance therapy for the first year, and were treated with azathioprine after 1 year. The patients in group A were treated mainly from 2001 to 2003. Treatment in groups B and C occurred mainly from 2003 to 2005 and from 2005 to 2007, respectively. The oral corticosteroid, prednisolone (1-2 mg/kg per day), was used for induction therapy. Mesalamine (Pentasa, 50-80 mg/kg per day) or azathioprine (Imuran, 2-3 mg/kg per day) was provided for maintenance therapy as the conventional treatment. Infliximab (Remicade, 5 mg/kg) was administered by intravenous infusion at weeks 0, 2 and 6, in combination with daily azathioprine and subsequently every 8 wk for 10 mo. After treatment with infliximab and azathioprine for the first 12 mo, only azathioprine therapy was continued.
The group treated with early infliximab was not previously treated with other medications such as corticosteroids or immunomodulators. All patients were followed for at least 24 mo.
Adverse events and laboratory results for side effects were investigated. Patients treated with mesalamine underwent evaluations for hypersensitivity, rash, alopecia, anorexia, headache, elevated liver enzyme, pancreatitis, serum creatinine and urine analysis. For azathioprine, patients were evaluated for pancytopenia, pancreatitis, hepatotoxicity, rash, alopecia, anorexia and arthralgia. Patients receiving infliximab were assessed for anaphylaxis, dyspnea, rash, headache, nausea, elevated liver enzyme, pancreatitis, pancytopenia and serious infections. Evaluation of the well-known adverse effects of prednisolone was not included in the analysis.
We defined remission of disease as a pediatric Crohn’s disease activity index (PCDAI) score of less than 10 points and a relapse was defined as greater than 10 points[12,13]. The relapse rate was defined as the rate of the presence of relapse, more than once, after a remission was achieved with treatment. Moderate to severe disease was defined by a score greater than 30 points by the PCDAI. The relapse rate was obtained using the PCDAI score. The efficacy of the early use of infliximab was evaluated by the relapse rate in each group at 12 and 24 mo.
A comparison of the study groups was performed using the χ2 test for variables, and Fisher’s exact test was used to compare each of the measurements. All statistical analyses utilized a 0.05 level of significance.
The study population at diagnosis included 27 males (75.0%) and nine females (25.0%), with a mean age of 11.9 ± 3.5 years (Table 1). The mean age of the patients in group A was 12.9 ± 1.4 years, for group B it was 11.5 ± 2.7 years and for group C, 12.9 ± 4.0 years; there were no statistical differences between these groups (P = 0.520). The mean overall follow-up period was 51.9 mo (range: 24-101 mo) and the minimal duration of follow-up was 24 mo. The location of disease was the terminal ileum in three patients (8.3%), the colon in 10 patients (27.8%) and the ileocolon in 23 patients (63.9%). The mean PCDAI score at baseline was 32.9 ± 8.6 points. There was no significant difference in the PCDAI scores between the groups. The mean PCDAI score was 30.3 ± 6.5 in group A, 33.1 ± 9.9 in group B and 34.8 ± 8.8 in group C (P = 0.464). Baseline characteristics were not significantly different between the treatment groups (Table 2).
| Gender (male/female) | 27/9 |
| Median age (range, yr) | 13 (1-16) |
| Duration of follow up [mean (range), mo] | 51.9 (24-101) |
| Disease location | |
| Small intestine | 3 |
| Colon | 10 |
| Small intestine & colon | 23 |
| PCDAI at diagnosis (mean ± SD) | 32.9 ± 8.6 |
| Group A | Group B | Group C | P | |
| Median age (range, yr) | 13 (10-14) | 12.5 (4-14) | 14 (1-16) | 0.520 |
| Duration of follow up (mo) (mean ± SD) | 82.7 ± 29.1 | 50.3 ± 15.5 | 29.6 ± 7.4 | 0.234 |
| PCDAI (mean ± SD) | 30.3 ± 6.5 | 33.1 ± 9.9 | 34.8 ± 8.8 | 0.464 |
The cumulative relapse rate, including infliximab use, was 52.8% (19 patients) at 12 mo and 66.7% (24 patients) at 24 mo; there was no significant difference in the relapse rate between the different age groups (P = 0.553); 69.2% (9 out of 13 patients) for patients less than 13 years of age and 65.2% (15 out of 23 patients) for those more than 13 years of age (Table 3). The relapse rate in patients who had colon only involvement was 50.0% (5 out of 10 patients). The relapse rate in patients with terminal ileum only involvement was 66.7% (2 out of 3 patients), and for patients with ileocolonic involvement the relapse rate was 73.9% (17 out of 23 patients). There was no difference in the relapse rate between patients with mild disease, with a PCDAI score less than 30 points at diagnosis (7 out of 11 patients, 63.6%), and moderate to severe disease, with a PCDAI score more than 30 points (17 out of 25 patients, 68.0%).
| n (%) | P | |
| Age at diagnosis | ||
| < 13 yr (n = 13) | 9 (69.2) | 0.553 |
| ≥ 13 yr (n = 23) | 15 (65.2) | |
| Involvement | ||
| Colon (n = 10) | 5 (50.0) | 0.416 |
| Terminal ileum (n = 3) | 2 (66.7) | |
| Small and large bowel (n = 23) | 17 (73.1) | |
| PCDAI at diagnosis | ||
| < 30 (n = 11) | 7 (63.6) | 0.544 |
| ≥ 30 (n = 25) | 17 (68.0) |
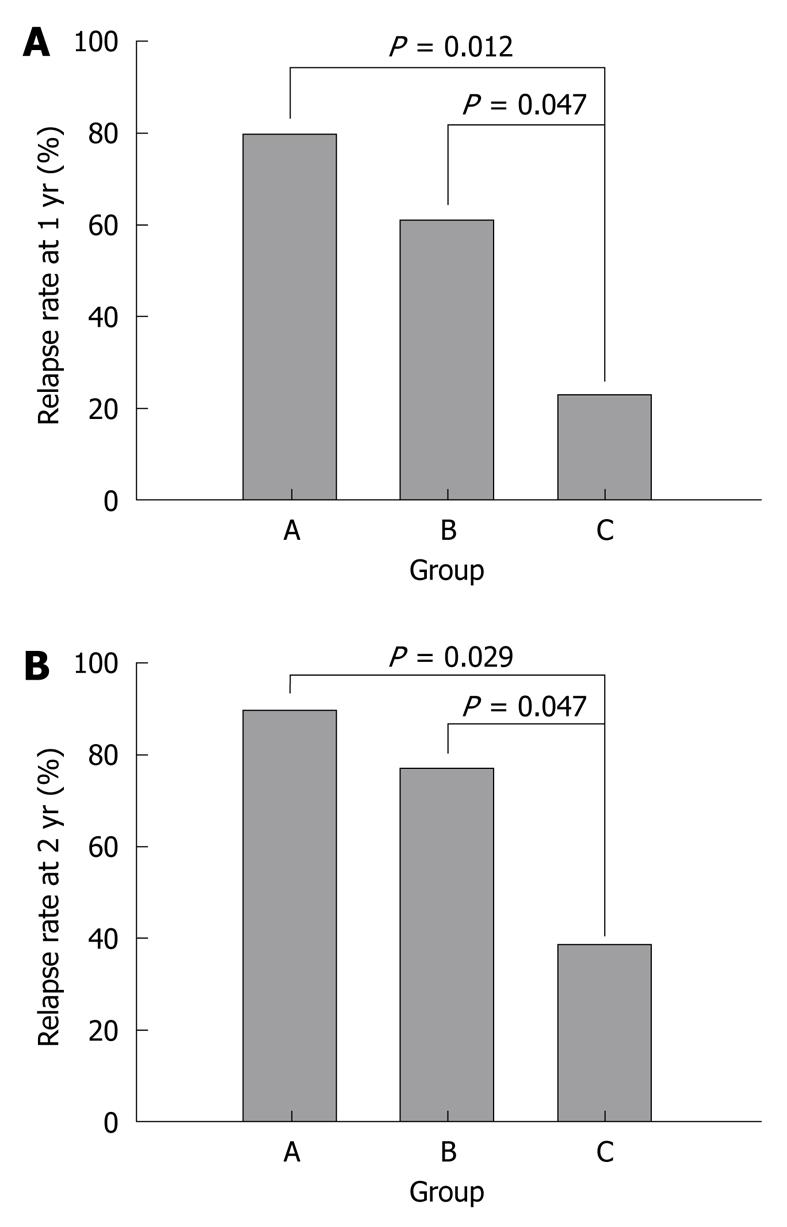
The relapse rate at 1 year was 23.1% [95% confidence interval (CI): 0.2%-46.0%] in group C (3 out of 13 patients), 61.5% (95% CI: 35.1%-88.0%) in group B (8 out of 13 patients) and 80.0% (95% CI: 55.2%-100.0%) in group A (8 out of 10 patients). At 1 year, the absolute difference between groups C and B was 38.5% (95% CI: 3.5%-73.4%) and was 56.9% (95% CI: 23.2%-90.7%) between groups C and A. There was a significant difference between each of the groups (P = 0.047, P = 0.012) (Figure 1A). At the 2 years follow up, the relapse rate in group C [38.5%, 5 out of 13 patients (95% CI: 12.0%-64.9%)] was lower than the relapse rate in groups B [76.9%, 10 out of 13 patients (95% CI: 54.0%-99.8%)] and A [90.0%, 9 out of 10 patients (95% CI: 71.4%-100.0%)]. At 2 years, the absolute difference between groups C and B was 38.5% (95% CI: 3.5%-73.4%) and was 51.5% (95% CI: 19.2%-83.9%) between groups C and A. There was a statistically significant difference between each group (P = 0.047, P = 0.029) (Figure 1B).
Patients treated with mesalamine had three adverse events. Five patients treated with azathioprine were found to have adverse events. Only one patient had dyspnea and tachycardia after the third infusion of infliximab (Table 4).
| n (%) | Details | |
| Group A (mesalamine) | 3 (30.0) | Anorexia (n = 2), Pancreatitis (n = 1) |
| Group B (azathioprine) | 5 (38.5) | Anorexia (n = 1), Pancytopenia (n = 3), Pancreatitis (n = 1) |
| Group C (infliximab) | 1 (8.3) | Dyspnea and tachycardia (n = 1) |
To our knowledge, this is the first study reported in Asia on the long-term efficacy of ‘top-down’ infliximab treatment for newly diagnosed pediatric Crohn’s disease. We have used infliximab as induction therapy since 2005. Our patients had a better response to infliximab treatment compared to conventional therapy. The improved response, when compared to Western data, suggests that there might be ethnic differences in the response to treatment. A review of the medical literature showed that the incidence and prevalence of Crohn’s disease in Korea is lower than in developed countries; however, it is rapidly increasing[7]. In addition, the pathogenesis associated with the common NOD2 variant in Asian populations differs from Western countries.
The results of our study showed that induction and maintenance therapy with infliximab and azathioprine was more effective than conventional treatments in children. After discontinuation of infliximab maintenance therapy for 1 year, we showed that the relapse rate in group C was lower than that in the other groups. The remission rate achieved with infliximab was higher in our study than in the study reported by Hyams et al[14]; at 1 year, 55.8% (29 out of 52) of patients in the study by Hyams receiving infliximab every 8 wk were in clinical remission; on the other hand, 76.9% (10 out of 13) of the patients in our study group C achieved remission. There was no difference in the PCDAI score between the study by Hyams and our study, 42.1 ± 9.2 and 34.8 ± 8.8, respectively. The reason why remission was maintained longer in our study compared to the findings from other developed countries remains unclear. Three hypotheses can be posited: first, it is likely that there are ethnic differences in the clinical response to infliximab, as seen with NOD2 variations; second, we used infliximab as ‘top-down’ therapy, while in the study by Hyams the patients who had received other drugs such as oral corticosteroids and aminosalicylates were also included in the infliximab treatment group; finally, the number of patients in our study was relatively small, and a selection bias cannot be ruled out. Further study is needed to confirm our findings. However, even with the differences in the number of patients and inclusion criteria, the clinical response to early use of infliximab in our report was much better than the responses reported by Hyams.
Pediatric Crohn’s disease is characterized by frequent relapses, a widespread disease extent, a high prevalence of extraintestinal manifestations, and in general a severe clinical course[15,16]. In pediatric patients, both the disease itself and the treatment used to control it, most commonly corticosteroids, can seriously impair quality of life as well as cause significant side effects such as growth failure and an increased risk of infections. For these reasons clinicians tend to use corticosteroids for short durations. Although corticosteroids are effective in inducing clinical remission in patients with active Crohn’s disease, these agents have limited efficacy for maintaining remission and the healing of mucosal lesions[17].
Infliximab, is an anti TNF-α monoclonal antibody that was initially used in corticosteroid-dependent and corticosteroid-refractory Crohn’s disease[18]. The use of infliximab has been well documented as an effective treatment for moderate to severe Crohn’s disease in children, in several studies[14,19]. Infliximab, as a scheduled treatment, is effective for the induction and maintenance of remission in patients with Crohn’s disease[20,21]. Early use of infliximab with immunosuppressants, known as ‘top-down’ therapy, in patients with newly diagnosed Crohn’s disease has resulted in better outcomes in adult patients[22]. An 8-wk maintenance treatment schedule with infliximab has been shown to be a cost-effective approach in adult patients with active luminal or fistulizing Crohn’s disease[23]. Such therapy offers the potential for altering the natural history of Crohn’s disease, and is changing treatment paradigms. One possible explanation for the prolonged remissions with infliximab treatment is that this medication might help heal the mucosal lesions in patients with severe as well as those with mild disease[24]. However, the new concept of an early aggressive or ‘top-down’ treatment approach is not widely accepted yet, especially in children, even though a few studies have reported that infliximab was more effective in children than in adults[5,6].
The limitations of this study include the following: This was a single center study with a small number of patients who were reviewed retrospectively. As an open trial, the physicians and patients knew the treatment regimen, which could have biased the assessment of treatment efficacy. However, selection bias was unlikely because the distribution of treatment regimens among the three groups was made based on chronological grouping.
In conclusion, the results of this study showed that early and intensive treatment of pediatric Crohn’s disease patients with infliximab, at initial diagnosis, was more effective for maintaining remission and reducing flares. Treatment with azathioprine monotherapy, after discontinuation of infliximab, for 1 year, provided prolonged remission.
The etiology of Crohn’s disease remains incompletely understood. There is no known cure for Crohn’s disease. Therefore, the goal of treatment is to mitigate inflammation and the associated clinical symptoms. Conventional therapy for active disease includes treatment with corticosteroids, which is very effective and fast acting. However, long-term exposure to corticosteroids becomes problematic with issues of dependency and/or resistance.
Infliximab is an anti tumor necrosis factor α monoclonal antibody that was introduced for the treatment of moderate to severe Crohn’s disease. The efficacy of infliximab suggests a new concept; that early induction with intensive treatment, known as ‘top-down’ therapy, may reduce complications associated with conventional treatment and may improve quality of life.
This is the first study reported in Asia on the long-term efficacy of ‘top-down’ infliximab treatment for pediatric Crohn’s disease. The results of this study showed that early and intensive treatment using infliximab, at initial diagnosis, was more effective for maintaining remission and reducing flares for 2 years.
Early induction treatment with infliximab at diagnosis, known as “top-down” therapy, was effective in reducing relapse rate and adverse events compared to conventional therapies for at least 2 years.
This is a useful cohort that will serve as a useful reference for pediatricians considering infliximab induction therapy for Crohn's disease.
Peer reviewer: John K Marshall, MD, Associate Professor of Medicine, Division of Gastroenterology (4W8), McMaster University Medical Centre, 1200 Main Street West, Hamilton, Ontario L8N 3Z5, Canada
S- Editor Tian L L- Editor Webster JR E- Editor Zheng XM
| 1. | Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427-434. |
| 2. | Markowitz J, Hyams J, Mack D, Leleiko N, Evans J, Kugathasan S, Pfefferkorn M, Mezoff A, Rosh J, Tolia V. Corticosteroid therapy in the age of infliximab: acute and 1-year outcomes in newly diagnosed children with Crohn's disease. Clin Gastroenterol Hepatol. 2006;4:1124-1129. |
| 3. | Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359:1541-1549. |
| 4. | Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, DeWoody KL, Schaible TF, Rutgeerts PJ. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med. 1997;337:1029-1035. |
| 5. | Hyams JS, Markowitz J, Wyllie R. Use of infliximab in the treatment of Crohn's disease in children and adolescents. J Pediatr. 2000;137:192-196. |
| 6. | Kugathasan S, Werlin SL, Martinez A, Rivera MT, Heikenen JB, Binion DG. Prolonged duration of response to infliximab in early but not late pediatric Crohn's disease. Am J Gastroenterol. 2000;95:3189-3194. |
| 7. | Yang SK, Yun S, Kim JH, Park JY, Kim HY, Kim YH, Chang DK, Kim JS, Song IS, Park JB. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986-2005: a KASID study. Inflamm Bowel Dis. 2008;14:542-549. |
| 8. | Inoue N, Tamura K, Kinouchi Y, Fukuda Y, Takahashi S, Ogura Y, Inohara N, Núñez G, Kishi Y, Koike Y. Lack of common NOD2 variants in Japanese patients with Crohn's disease. Gastroenterology. 2002;123:86-91. |
| 9. | Hsiao CH, Wei SC, Wong JM, Lai HS, Chang MH, Ni YH. Pediatric Crohn disease: clinical and genetic characteristics in Taiwan. J Pediatr Gastroenterol Nutr. 2007;44:342-346. |
| 10. | Wang YF, Zhang H, Ouyang Q. Clinical manifestations of inflammatory bowel disease: East and West differences. J Dig Dis. 2007;8:121-127. |
| 11. | IBD Working Group of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition. Inflammatory bowel disease in children and adolescents: recommendations for diagnosis--the Porto criteria. J Pediatr Gastroenterol Nutr. 2005;41:1-7. |
| 12. | Otley A, Loonen H, Parekh N, Corey M, Sherman PM, Griffiths AM. Assessing activity of pediatric Crohn's disease: which index to use? Gastroenterology. 1999;116:527-531. |
| 13. | Hyams J, Markowitz J, Otley A, Rosh J, Mack D, Bousvaros A, Kugathasan S, Pfefferkorn M, Tolia V, Evans J. Evaluation of the pediatric crohn disease activity index: a prospective multicenter experience. J Pediatr Gastroenterol Nutr. 2005;41:416-421. |
| 14. | Hyams J, Crandall W, Kugathasan S, Griffiths A, Olson A, Johanns J, Liu G, Travers S, Heuschkel R, Markowitz J. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn's disease in children. Gastroenterology. 2007;132:863-873; quiz 1165-1166. |
| 15. | Vernier-Massouille G, Balde M, Salleron J, Turck D, Dupas JL, Mouterde O, Merle V, Salomez JL, Branche J, Marti R. Natural history of pediatric Crohn's disease: a population-based cohort study. Gastroenterology. 2008;135:1106-1113. |
| 16. | Van Limbergen J, Russell RK, Drummond HE, Aldhous MC, Round NK, Nimmo ER, Smith L, Gillett PM, McGrogan P, Weaver LT. Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology. 2008;135:1114-1122. |
| 17. | Rutgeerts PJ. Review article: the limitations of corticosteroid therapy in Crohn's disease. Aliment Pharmacol Ther. 2001;15:1515-1525. |
| 18. | Lichtenstein GR. Approach to corticosteroid-dependent and corticosteroid-refractory Crohn's disease. Inflamm Bowel Dis. 2001;7 Suppl 1:S23-S29. |
| 19. | de Ridder L, Benninga MA, Taminiau JA, Hommes DW. Infliximab as first-line therapy in severe pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2006;43:388-390. |
| 20. | Akobeng AK, Zachos M. Tumor necrosis factor-alpha antibody for induction of remission in Crohn's disease. Cochrane Database Syst Rev. 2004;CD003574. |
| 21. | Behm BW, Bickston SJ. Tumor necrosis factor-alpha antibody for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev. 2008;CD006893. |
| 22. | D'Haens G, Baert F, van Assche G, Caenepeel P, Vergauwe P, Tuynman H, De Vos M, van Deventer S, Stitt L, Donner A. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn's disease: an open randomised trial. Lancet. 2008;371:660-667. |
| 23. | Lindsay J, Punekar YS, Morris J, Chung-Faye G. Health-economic analysis: cost-effectiveness of scheduled maintenance treatment with infliximab for Crohn's disease--modelling outcomes in active luminal and fistulizing disease in adults. Aliment Pharmacol Ther. 2008;28:76-87. |
| 24. | Geboes K, Rutgeerts P, Opdenakker G, Olson A, Patel K, Wagner CL, Marano CW. Endoscopic and histologic evidence of persistent mucosal healing and correlation with clinical improvement following sustained infliximab treatment for Crohn's disease. Curr Med Res Opin. 2005;21:1741-1754. |