Published online Apr 14, 2010. doi: 10.3748/wjg.v16.i14.1688
Revised: January 26, 2010
Accepted: February 2, 2010
Published online: April 14, 2010
Endoscopic submucosal dissection (ESD) is efficient for en bloc resection of large colorectal tumors. However, it has several technical difficulties, because the wall of the colon is thin and due to the winding nature of the colon. The main complications of ESD comprise postoperative perforation and hemorrhage, similar to endoscopic mucosal resection (EMR). In particular, the rate of perforation in ESD is higher than that in EMR. Perforation of the colon can cause fatal peritonitis. Endoscopic clipping is reported to be an efficient therapy for perforation. Most cases with perforation are treated conservatively without urgent surgical intervention. However, the rate of postoperative hemorrhage in ESD is similar to that in EMR. Endoscopic therapy including endoscopic clipping is performed and most of the cases are treated conservatively without blood transfusion. In blood examination, some degree of inflammation is detected after ESD. For the standardization of ESD, it is most important to decrease the rate of perforation. Adopting a safe strategy for ESD and a suitable choice of knife are both important ways of preventing perforation. Moreover, appropriate training and increasing experience can improve the endoscopic technique and can decrease the rate of perforation. In this review, we describe safe procedures in ESD to prevent complications, the complications of ESD and their management.
- Citation: Yoshida N, Yagi N, Naito Y, Yoshikawa T. Safe procedure in endoscopic submucosal dissection for colorectal tumors focused on preventing complications. World J Gastroenterol 2010; 16(14): 1688-1695
- URL: https://www.wjgnet.com/1007-9327/full/v16/i14/1688.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i14.1688
Endoscopic mucosal resection (EMR) has been generally performed for colorectal tumors worldwide, including Japan. It is difficult to perform en bloc resection by EMR for a colorectal tumor whose size is larger than 20 mm[1-3]. The rate of en bloc resection by EMR for tumors with a diameter of more than 20 mm was reported to be approximately 30%[2,3]. Piecemeal EMR enables us to remove large colorectal tumors. However, colorectal cancer has a high rate of local recurrence[2-5]. Moreover, precise histopathological diagnosis is difficult when using separate resected specimens of piecemeal EMR[6]. Thus, laparoscopic-assisted colectomy (LAC) has been regarded as a standard therapy for large colorectal tumors throughout the world[7]. However, LAC is more invasive than endoscopic treatment. Endoscopic submucosal dissection (ESD) has a high rate of en bloc resection for large colorectal tumors, and it is less invasive than LAC. The rate of en bloc resection for large colorectal tumors has been reported to be 84.0%-98.9%[8-17]. However, the procedure has not been standardized because of its associated technical difficulties. The colon is winding in nature, and the colonic wall is thinner than the gastric wall. Moreover, there are many folds in the colorectum. Therefore, the rate of perforation in ESD is reported to be higher than that in EMR. A safe strategy, suitable knife, and adoption of other equipment are necessary while performing ESD in order to prevent its associated complications, including perforation.
In Japan, a special working group consisting of experts in ESD suggested specific indications for ESD[6]. Briefly, ESD is suitable for a tumor when it is difficult to use a snare EMR for en bloc resection. ESD should be performed for tumors that are diagnosed as carcinomas with intramucosal to shallow submucosal invasion. Moreover, ESD is performed for lesions with submucosal fibrosis that cannot be removed by conventional EMR even if the size of the lesion is less than 20 mm. However, tumors in a location where the endoscope will not be able to be operated smoothly should not be removed by ESD.
ESD is performed using a general lower gastrointestinal endoscope with a single channel. In our institution, EC 590 MP (Fuji Film Medical, Tokyo, Japan) or PCF Q260AI (Olympus Medical Systems Co., Tokyo, Japan) are used. ESD requires a high-frequency generator with an automatically controlled system. In our institution, VIO300D or ICC200 (Erbe Elektromedizin Ltd., Tubingen, Germany) are used. An upper gastrointestinal endoscope is adopted in some institutions because it is slim and can be used in the retroflexed position[10]. A transparent short hood (Olympus Medical Systems Co., Tokyo, Japan) is fitted at the tip of the endoscope. A mixture of 1% hyaluronic acid solution (Mucoup; Johnson & Johnson K.K., Tokyo, Japan) and 10% glycerin solution (Glyceol; Chugai Pharmaceutical Co., Tokyo, Japan) is used as the injection liquid to induce a higher elevation of the submucosa and to lengthen the duration of the continuous elevation of the submucosa[18,19].
Before ESD, residual feces and liquid are removed from the entire colon even if the tumor is located at the rectum. The ESD procedure should be abandoned if the residual feces can not be removed enough. Residual feces prevent smooth submucosal dissection. Moreover, it is essential to remove residual feces in order to prevent the outflow of feces into the abdomen in the case of perforation.
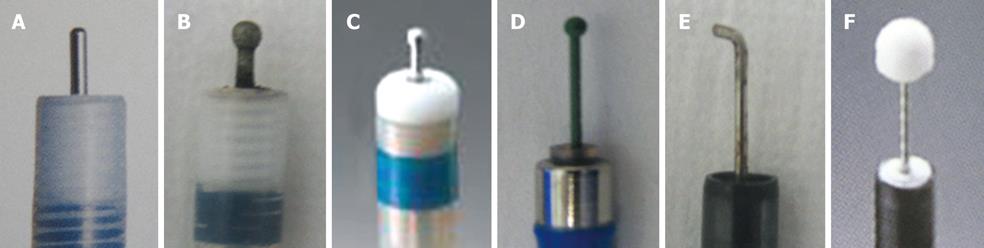
Various knives are used in ESD for colorectal tumors (Figure 1A-F). Among the obtuse short-tipped types are the Flush knife (Fujifilm Medical, Tokyo, Japan), Dual knife (Olympus Medical Systems Co., Tokyo, Japan), B-knife (Zeon Medical, Tokyo, Japan), and Splash needle (Pentax Co., Tokyo, Japan)[9,15,20]. The Flush knife and Splash needle are capable of injecting substances into the submucosa. They enable us to omit switching between the knife and the injection needle[13,15]. A Dual knife has a ball disk at the tip of the knife, enabling us to hook the submucosa. The B-knife and Flush knife both have a new type of ball tip. The insulated tipped (IT) knife (Olympus Medical Systems Co., Tokyo, Japan), whose efficacy has been reported to be satisfactory in ESD for gastric tumors, is used in certain institutions[21]. Speedy dissection can be performed with the IT knife, but it may cause large perforations due to its long blade. A Hook knife (Olympus Medical Systems Co., Tokyo, Japan) is used particularly when the dissection of the submucosa is difficult due to poor elevation of the submucosa[12]. The B-knife is the only bipolar knife; burning of the muscularis propria layer is considered to be less with this knife than with other monopolar knives. A grasping-type scissor forceps has been reported as an original knife[22]. In our institution, the Flush knife is mainly used because it can be effectively used to administer local injections, while the Hook knife is used when the risk of perforation is high due to the poor elevation of the submucosa[12,23].
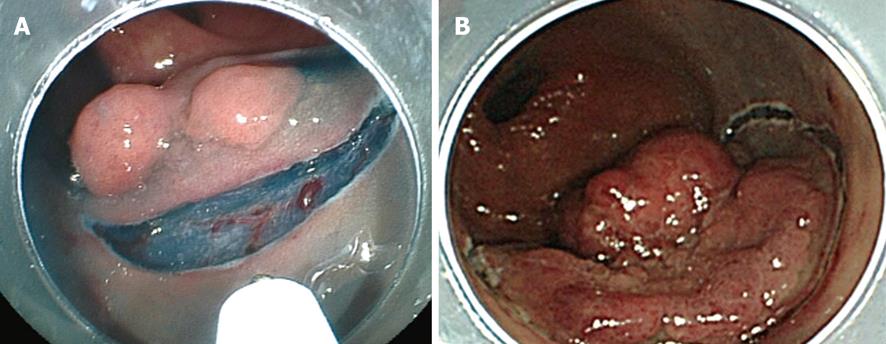
The border of the tumor is observed carefully by applying indigo carmine dye. It is generally unnecessary to make placement of borders by coagulation because in the majority of cases, the borders of the tumor are clearly visible. Injection into the submucosa for its elevation is performed with a 23-25 gauge needle (TOP Co., Tokyo, Japan) after observation of the border of the tumor. Then, a mucosal incision is taken. An initial complete circumferential incision or a partial circumferential incision is made according to the institution’s procedure and the lesion’s characteristics, as reported previously (Figure 2A and B)[8,12]. In initial complete circumferential incision, injection of hyaluronic acid solution into the submucosa is performed from the oral edge of the tumor. A mucosal incision is made after adequate elevation of the submucosa is obtained. Simultaneously, an incision up to the deep submucosa is made. Then the solution is injected into the anal edge of the tumor and the mucosal incision is made. Thus, a mucosal incision is made all around the tumor. On the other hand, in partial circumferential incision, the anal side of the tumor is the first to be incised after the injection of hyaluronic acid solution for submucosal elevation. Both types of mucosal incisions are performed with the endocut mode (e.g. Output 40W, effect 2 in ICC200; or endocut I, effect 2, duration 2, interval 1 in VIO300D). However, each incision has its own merits and demerits.
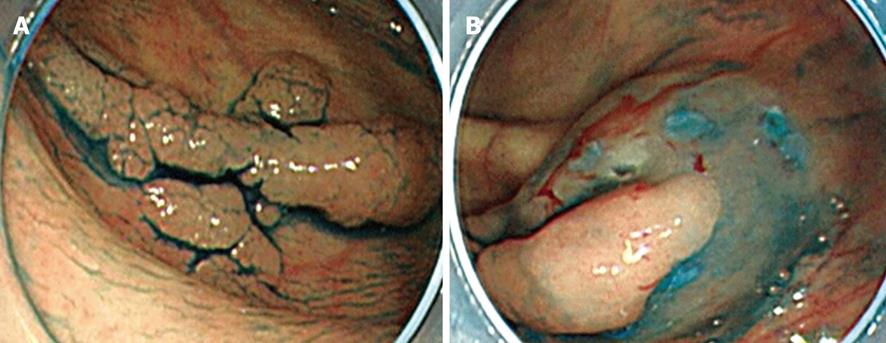
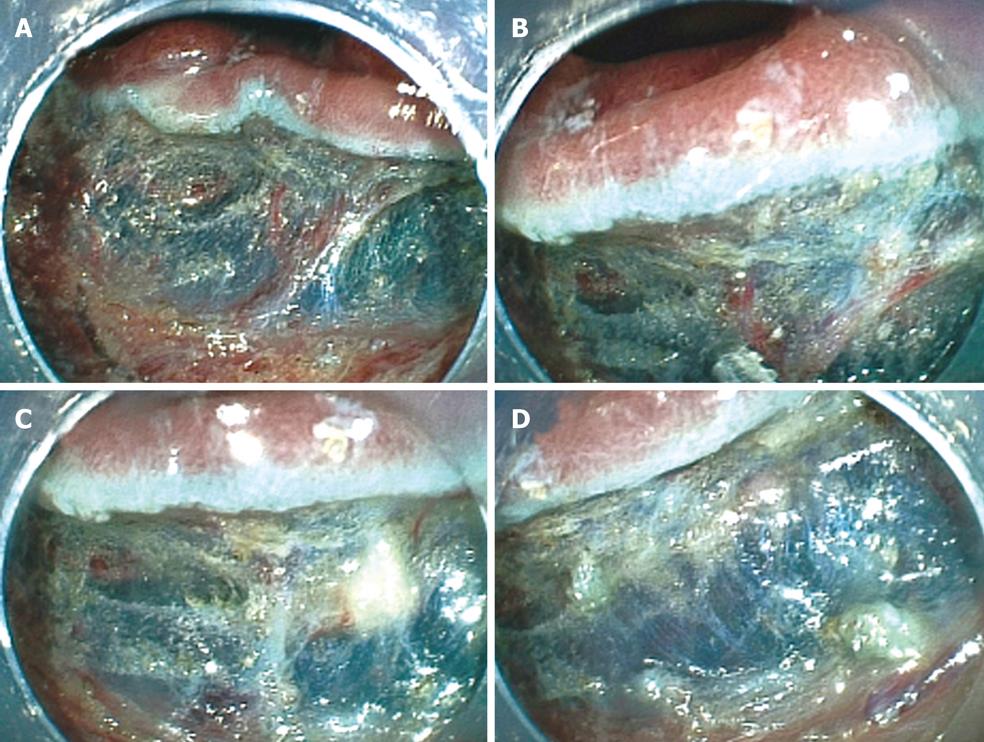
In initial complete circumferential incision, leakage of injection fluid can easily occur following which submucosal elevation cannot be obtained. Moreover, injection of the fluid into the oral side of the tumor causes the position of the tumor to be perpendicular to the endoscope (Figure 3A and B). This makes submucosal dissection difficult. In addition, the uncut residual mucosa on the oral side pulls the tumor upward (Figure 4A); however, this substance caused by the residual mucosa is lost in initial complete circumferential incision, and the tumor becomes perpendicular to the endoscope (Figure 4B). These factors are experienced frequently in tumors whose size is less than 50 mm. In partial circumferential incision, higher elevation of the submucosa can be maintained because the uncut residual mucosa on the oral side of the tumor prevents the leakage of injected fluid. However, in partial circumferential mucosal incision, it is sometimes difficult to resect the residual mucosa on the oral side owing to the presence of the partially resected tumor. Thus, each type of mucosal incision has its own merits and demerits; the type of incision to be used should be decided according to the tumor size, location of the tumor, and types of knives being used. In our institution, partial circumferential incision is performed for tumors measuring less than 50 mm or for which fluid injection into the tumor’s oral side would likely negatively influence the position of the tumor.
After mucosal and submucosal incisions are made around the tumor, the submucosa below the tumor is dissected from the anal side of the tumor. Dissection of the submucosa is performed using the Endocut (e.g. Output 40W, effect 2 in ICC200; endocut I, effect 2, duration 2, interval 1 in VIO300D) or the coagulation mode (e.g. Forced coagulation, Output 40W in ICC200 or Forced coagulation, Output 40W, effect 3 in VIO300D). To achieve submucosal elevation, the glycerin solution or the mixture of hyaluronic acid solution and glycerin solution is injected with the injection needle or knife with the function of injection, as appropriate. Then continuing to dissect with prevention of perforation and hemorrhage, en bloc resection of the tumor is performed.
Perforation following ESD for colorectal tumors can be fatal because peritonitis caused by colorectal bacteria and feces is known to be more severe than peritonitis occurring after gastric perforation.
The rate of perforation has been reported to be 1.4%-10.4% (Table 1). In our experience with perforations, there were no statistical differences regarding the location of the tumor, i.e. in the colon or in the rectum[23]. Another report has revealed that perforation is associated with large tumor size (> 30 mm) and the presence of fibrosis[17]. The rate of perforation of ESD is dramatically high when compared with that observed for EMR[1-3]. One of the reasons for the high rate of perforations is the thinness of the colorectal wall as compared to the gastric wall. Knife coagulation is the most common cause of perforation[23]. The paradoxical movement of the endoscope during ESD due to the winding nature of the colorectum causes coagulation in the muscularis propria. A longer operation time increases the amount of air in the abdomen, causing greater paradoxical movement of the endoscope. This situation is experienced specifically in tumors located in the cecum up to the descending colon. Obtuse short-tipped knives such as the Dual knife and the Flush knife can easily cause this type of perforation. In contrast, it is difficult to cause perforations while using the Hook knife because it enables us to hook and separate the submucosa from the muscularis propria and thereby cut safely. Rare reasons for perforation include resection by using a snare, coagulation by special hemostat forceps with soft coagulation, and endoscopic clipping onto coagulated submucosa[23]. Further, endoscopic clipping is also performed when perforation is detected. Multiple endoscopic clipping is performed to close the perforation depending upon its size. Small perforations can be closed by endoscopic clipping[24,25]. If abdominal distention due to air leakage is severe, decompression of the pneumoperitonium must be performed using a 20-gauge puncture needle[14]. The majority of cases with perforation are treated conservatively without emergency surgery. Recently, it has been shown that large perforations can be closed using a new closure device consisting of a clip with loop[26]. On the other hand, there are cases in which perforation is not detected by endoscopy, but free air is detected by computed tomography (CT). The possible reasons for this are that small perforations cannot be detected during ESD or that very small perforations may occur during deep injection by the injection needle. However, these cases are generally not clinically serious because they can be successfully treated by withholding oral intake; no abdominal pain is typically detected after ESD.
| Author | Country | n | Perforation rate (%) | Postoperative hemorrhage rate (%) |
| Fujishiro et al[10] | Japan | 200 | 10.4 | 1.0 |
| Hurlstone et al[16] | UK | 42 | 2.3 | 2.3 |
| Tanaka et al[8] | Japan | 70 | 10.0 | 1.4 |
| Tamegai et al[11] | Japan | 71 | 1.4 | 0.0 |
| Toyonaga et al[15] | Japan | 468 | 1.5 | 1.5 |
| Yoshida et al[12] | Japan | 119 | 7.5 | 1.6 |
| Zhou et al[14] | China | 74 | 8.1 | 1.3 |
| Takeuchi et al[13] | Japan | 50 | 2.0 | 12.01 |
| Isomoto et al[17] | Japan | 292 | 8.2 | 0.7 |
Severe abdominal tympanic fullness, emphysema, and severe abdominal pain are possible symptoms of perforation. A high index of suspicion should be maintained when these symptoms are observed; moreover, the patient, nurse and physician should all watch out for these symptoms.
On the other hand, delayed perforation has been reported as a serious complication after ESD[10]. The rate of delayed perforation is reported to be 0.3%-0.7%[10,17,27]. The reasons for delayed perforation are unknown, but it is reported to be related to excessive coagulation in the muscularis propria. It has been reported that delayed perforations are typically large in size and require treatment by emergency surgery[10,17,27].
The rate of perforation is reported to be decreased with the increased experience of the endoscopist[8,9]. It is important to actively prevent perforations when the endoscopist does not have much experience with ESD. The indication of ESD according to endoscopist’s skill, appropriate strategy of ESD and the choice of a suitable knife in each case is important in preventing perforations[8].
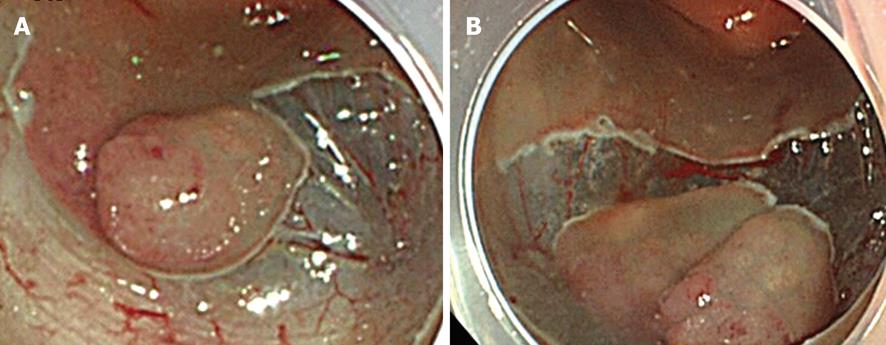
To prevent hemorrhage during ESD, when a vessel less than 2 mm in diameter is detected in the submucosa it is cut with a knife in the coagulation mode (e.g. Forced coagulation, Output 40W in ICC or Forced coagulation Output 40W, effect 3 in VIO300D). When a vessel more than 2 mm in diameter is detected, special hemostat forceps (e.g. Coagrasper; FD-410LR, Olympus Optical Co, Tokyo, Japan) are used in the soft coagulation mode (e.g. Output 50W in ICC; Output 60W, effect 5 in VIO300D) to prevent hemorrhage during ESD[12]. These forceps can be rotated and they are used to gently catch and lift the vessels upward from the muscularis propria. In our institution, a unique use of the hemostat forceps has been adopted for resecting vessels. In brief, a vessel is coagulated using hemostatic forceps in the soft coagulation mode and then resected with the forceps in the endocut mode. Moreover, the coagulated submucosa surrounding the vessel is also resected with the forceps. Removing the coagulated vessel and the surrounding submucosa ensure that the subsequent submucosal dissection is safer and easier than otherwise (Figure 5A-D). When massive bleeding that cannot be stopped by the knife occurs during ESD, special hemostat forceps are used in the soft coagulation mode as described above. Endoscopic clipping is performed when bleeding cannot be controlled with the special forceps.
The rate of postoperative hemorrhage in ESD is reported to be 0%-12.0% (Table 1)[8-17]. This rate is comparable to that reported for EMR[1-3]. A study has reported the rate of postoperative hemorrhage to be 12.0%, including mild cases[13]. Most cases of postoperative hemorrhage are treated only by endoscopic clipping and withholding oral intake without emergency surgery or blood transfusion.
Severe restlessness of the patient owing to abdominal fullness and pain have rendered submucosal dissection impossible in some cases. Conscious sedation is effective for some patients for the prevention of restlessness. Carbon dioxide insufflations have been reported to be effective for the prevention of abdominal fullness[28]. In our institution, conscious sedation is performed with midazolam (Dormicum; Astellas Pharma Inc., Tokyo, Japan) and pentazocine (Pentajin; Daiichi Sankyo Co., Tokyo, Japan) with monitoring using an automatic blood pressure monitor. In our ESD study that included 105 cases, there were 22 patients for whom the operation time exceeded 2.5 h, and patient restlessness occurred in 15 out of these 22 cases (68.1%) despite conscious sedation. In contrast, in cases with an operation time less than 2.5 h, patient restlessness occurred in only 10 out of 83 cases (12.0%). Thus, restlessness due to abdominal fullness and pain occurs frequently in cases with an operation time exceeding 2.5 h. Therefore, according to our experience, ESD is indicated when the operation time is expected to be less than 2.5 h. On the other hand, the use of propofol for conscious sedation extends the possibility of longer operation times than 2.5 h without causing restlessness and discomfort[14]. However, this drug requires further examination before its standardized use in ESD procedures.
Inflammation has been reported to a certain degree in some cases. In our previous report, the mean amount of C-reactive protein 2 d after ESD was 5.82 ± 12.10 mg/L in cases with perforation and 1.27 ± 2.00 mg/L in cases without perforation[29]. Fever and abdominal pain were also reported without perforation. A rare complication was acute colon obstruction after ESD of a colonic tumor located at the cecal base[30].
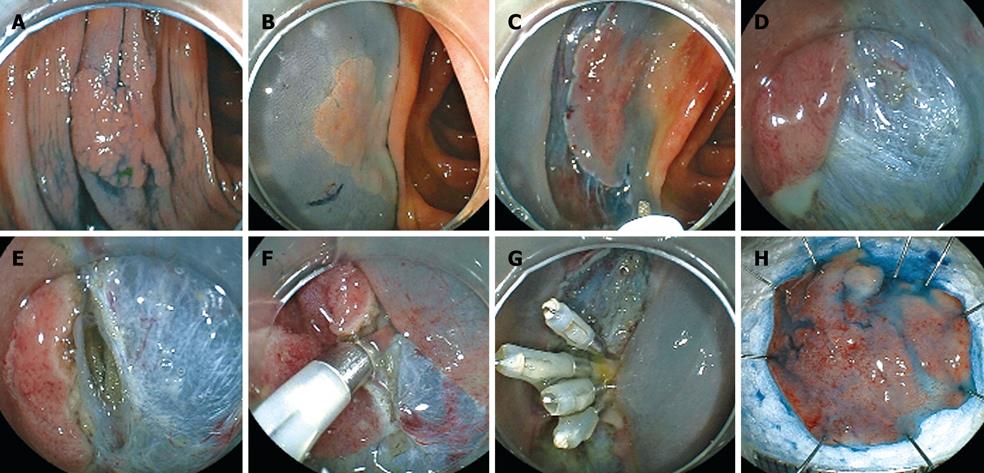
We present here the case of a 71-year-old male with a tumor graded 0-IIa, measuring 20 mm, and located in the ascending colon (Figure 6A). The surface of the tumor was slightly depressed and the deformity of the colonic wall was detected with indigo carmine dye. Magnifying endoscopy revealed a VI pit pattern[31]. The tumor was diagnosed as early colonic cancer with invasion up to the mucosa, and ESD was performed. Injection was first performed from the anal side of the tumor. However, the elevation of the tumor by injection was poor on the oral side of the tumor (Figure 6B). Therefore, either severe fibrosis or submucosal invasion was suspected. A mucosal incision was made and submucosal dissection was performed below the tumor using a Flush knife from the anal side of the tumor (Figure 6C). After that, a mucosal incision was made on the oral side of the tumor. Severe fibrosis was detected at the oral side of the tumor (Figure 6D). Then submucosal dissection was then performed with a Hook knife. However, owing to a thin submucosa, perforation was caused while hooking the submucosa (Figure 6E). Endoscopic clipping was performed minimally as the clipping did not prevent resection of the tumor, and the tumor was immediately resected using a snare. Then several endoscopic clippings were performed (Figure 6G). A small amount of free air was detected by abdominal CT after ESD. The patient had neither abdominal pain nor severe inflammation following surgery and was discharged 5 d after ESD. The resected specimen was fixed and the tumor was diagnosed by histopathological examination as early colonic cancer. The macroscopic tumor type was 0-IIa and the tumor was 20 mm in diameter. Invasion of the tumor was limited to the mucosa. Lymphatic and venous invasion was not detected. Lateral and vertical margins of the tumor were histopathologically free of the tumor (Figure 6F).
ESD is a feasible endoscopic treatment because of its high rate of en bloc resection for large colorectal tumors. Hospitalization after ESD is less than that after LAC[8,9,12]. However, ESD has disadvantages, with longer operation times and the possibility of perforation[32]. Increased experience in the procedure can, however, solve these problems[8,9].
Visits to other institutions with ESD experts and observation of such experts at work is an important component of training in performing ESD procedures. Adequate practice at performing ESD may be obtained by using animal models. Systematic training systems for use of ESD in colorectal tumors are essential for the prevention of perforation and long procedure times. Acquiring experience in gastric ESD prior to attempting colorectal ESD is reported to be a safer way to prevent perforation[8]. In our institution, EMR with circumferential mucosal incision has been used as training for ESD[15]. Moreover, better devices that are particularly suitable for ESD, such as knives, endoscopes, and other new equipment, need to be designed for shortening the operation times and to prevent perforation[33-36]. The indication of ESD should be decided according to the technique of the endoscopists in each institution. ESD for colorectal tumors is now improving, and a standardized method is expected to be developed in the near future. However, it is extremely important to diagnose the colorectal tumor correctly with suitable modalities, such as magnifying endoscopy; further, based on the diagnosis, the most appropriate methods of therapy should be considered, such as ESD, piecemeal EMR, and LAC.
In this review, we have assessed technical aspects and complications of ESD for colorectal tumors. For the standardization of ESD, it is most important to decrease the rate of perforation. Adopting a safe strategy of ESD and a suitable choice of knife are both efficient in the prevention of perforation. Moreover, appropriate training and increasing experience can improve the endoscopic technique and decrease the rate of perforation. We hope that standardization of ESD will be established in the near future, and that ESD will be performed in US and Europe where ESD is not widely adopted.
Peer reviewer: Javier San Martín, Chief, Gastroenterology and Endoscopy, Sanatorio Cantegril, Av. Roosevelt y P 13, Punta del Este 20100, Uruguay
S- Editor Tian L L- Editor O’Neill M E- Editor Ma WH
| 1. | Tanaka S, Haruma K, Oka S, Takahashi R, Kunihiro M, Kitadai Y, Yoshihara M, Shimamoto F, Chayama K. Clinicopathologic features and endoscopic treatment of superficially spreading colorectal neoplasms larger than 20 mm. Gastrointest Endosc. 2001;54:62-66. |
| 2. | Saito Y, Fukuzawa M, Matsuda T, Fukunaga S, Sakamoto T, Uraoka T, Nakajima T, Ikehara H, Fu KI, Itoi T. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc. 2010;24:343-352. |
| 3. | Iishi H, Tatsuta M, Iseki K, Narahara H, Uedo N, Sakai N, Ishikawa H, Otani T, Ishiguro S. Endoscopic piecemeal resection with submucosal saline injection of large sessile colorectal polyps. Gastrointest Endosc. 2000;51:697-700. |
| 4. | Higaki S, Hashimoto S, Harada K, Nohara H, Saito Y, Gondo T, Okita K. Long-term follow-up of large flat colorectal tumors resected endoscopically. Endoscopy. 2003;35:845-849. |
| 5. | Hurlstone DP, Sanders DS, Cross SS, Adam I, Shorthouse AJ, Brown S, Drew K, Lobo AJ. Colonoscopic resection of lateral spreading tumours: a prospective analysis of endoscopic mucosal resection. Gut. 2004;53:1334-1339. |
| 6. | Tanaka S, Oka S, Chayama K. Colorectal endoscopic submucosal dissection: present status and future perspective, including its differentiation from endoscopic mucosal resection. J Gastroenterol. 2008;43:641-651. |
| 7. | Schwenk W, Haase O, Neudecker J, Müller JM. Short term benefits for laparoscopic colorectal resection. Cochrane Database Syst Rev. 2005;CD003145. |
| 8. | Tanaka S, Oka S, Kaneko I, Hirata M, Mouri R, Kanao H, Yoshida S, Chayama K. Endoscopic submucosal dissection for colorectal neoplasia: possibility of standardization. Gastrointest Endosc. 2007;66:100-107. |
| 9. | Saito Y, Uraoka T, Matsuda T, Emura F, Ikehara H, Mashimo Y, Kikuchi T, Fu KI, Sano Y, Saito D. Endoscopic treatment of large superficial colorectal tumors: a case series of 200 endoscopic submucosal dissections (with video). Gastrointest Endosc. 2007;66:966-973. |
| 10. | Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, Yamamichi N, Tateishi A, Oka M, Ogura K. Outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms in 200 consecutive cases. Clin Gastroenterol Hepatol. 2007;5:678-683; quiz 645. |
| 11. | Tamegai Y, Saito Y, Masaki N, Hinohara C, Oshima T, Kogure E, Liu Y, Uemura N, Saito K. Endoscopic submucosal dissection: a safe technique for colorectal tumors. Endoscopy. 2007;39:418-422. |
| 12. | Yoshida N, Naito Y, Sakai K, Sumida Y, Kanemasa K, Inoue K, Morimoto Y, Konishi H, Wakabayashi N, Kokura S. Outcome of endoscopic submucosal dissection for colorectal tumors in elderly people. Int J Colorectal Dis. 2010;25:455-461. |
| 13. | Takeuchi Y, Uedo N, Ishihara R, Iishi H, Kizu T, Inoue T, Chatani R, Hanaoka N, Taniguchi T, Kawada N. Efficacy of an endo-knife with a water-jet function (Flushknife) for endoscopic submucosal dissection of superficial colorectal neoplasms. Am J Gastroenterol. 2010;105:314-322. |
| 14. | Zhou PH, Yao LQ, Qin XY. Endoscopic submucosal dissection for colorectal epithelial neoplasm. Surg Endosc. 2009;23:1546-1551. |
| 15. | Toyonaga T, Man-I M, Morita Y, Sanuki T, Yoshida M, Kutsumi H, Inokuchi H, Azuma T. The new resources of treatment for early stage colorectal tumors: EMR with small incision and simplified endoscopic submucosal dissection. Dig Endosc. 2009;21 Suppl 1:S31-S37. |
| 16. | Hurlstone DP, Atkinson R, Sanders DS, Thomson M, Cross SS, Brown S. Achieving R0 resection in the colorectum using endoscopic submucosal dissection. Br J Surg. 2007;94:1536-1542. |
| 17. | Isomoto H, Nishiyama H, Yamaguchi N, Fukuda E, Ishii H, Ikeda K, Ohnita K, Nakao K, Kohno S, Shikuwa S. Clinicopathological factors associated with clinical outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms. Endoscopy. 2009;41:679-683. |
| 18. | Yamamoto H, Kawata H, Sunada K, Sasaki A, Nakazawa K, Miyata T, Sekine Y, Yano T, Satoh K, Ido K. Successful en-bloc resection of large superficial tumors in the stomach and colon using sodium hyaluronate and small-caliber-tip transparent hood. Endoscopy. 2003;35:690-694. |
| 19. | Fujishiro M, Yahagi N, Kashimura K, Mizushima Y, Oka M, Matsuura T, Enomoto S, Kakushima N, Imagawa A, Kobayashi K. Different mixtures of sodium hyaluronate and their ability to create submucosal fluid cushions for endoscopic mucosal resection. Endoscopy. 2004;36:584-589. |
| 20. | Fujishiro M, Kodashima S, Goto O, Ono S, Muraki Y, Kakushima N, Omata M. Technical feasibility of endoscopic submucosal dissection of gastrointestinal epithelial neoplasms with a splash-needle. Surg Laparosc Endosc Percutan Tech. 2008;18:592-597. |
| 21. | Saito Y, Fukuzawa M, Matsuda T, Fukunaga S, Sakamoto T, Uraoka T, Nakajima T, Ikehara H, Fu KI, Itoi T. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc. 2010;24:343-352. |
| 22. | Akahoshi K, Motomura Y, Kubokawa M, Matsui N, Oda M, Okamoto R, Endo S, Higuchi N, Kashiwabara Y, Oya M. Endoscopic submucosal dissection of a rectal carcinoid tumor using grasping type scissors forceps. World J Gastroenterol. 2009;15:2162-2165. |
| 23. | Yoshida N, Wakabayashi N, Kanemasa K, Sumida Y, Hasegawa D, Inoue K, Morimoto Y, Kashiwa A, Konishi H, Yagi N. Endoscopic submucosal dissection for colorectal tumors: technical difficulties and rate of perforation. Endoscopy. 2009;41:758-761. |
| 24. | Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, Kobayashi K, Hashimoto T, Yamamichi N, Tateishi A. Successful nonsurgical management of perforation complicating endoscopic submucosal dissection of gastrointestinal epithelial neoplasms. Endoscopy. 2006;38:1001-1006. |
| 25. | Uraoka T, Kawahara Y, Kato J, Saito Y, Yamamoto K. Endoscopic submucosal dissection in the colorectum: present status and future prospects. Dig Endosc. 2009;21 Suppl 1:S13-S16. |
| 26. | Sakamoto N, Beppu K, Matsumoto K, Shibuya T, Osada T, Mori H, Shimada Y, Konno A, Kurosawa A, Nagahara A. “Loop Clip”, a new closure device for large mucosal defects after EMR and ESD. Endoscopy. 2008;40 Suppl 2:E97-E98. |
| 27. | Toyanaga T, Man-I M, Ivanov D, Sanuki T, Morita Y, Kutsumi H, Inokuchi H, Azuma T. The results and limitations of endoscopic submucosal dissection for colorectal tumors. Acta Chir Iugosl. 2008;55:17-23. |
| 28. | Saito Y, Uraoka T, Matsuda T, Emura F, Ikehara H, Mashimo Y, Kikuchi T, Kozu T, Saito D. A pilot study to assess the safety and efficacy of carbon dioxide insufflation during colorectal endoscopic submucosal dissection with the patient under conscious sedation. Gastrointest Endosc. 2007;65:537-542. |
| 29. | Yoshida N, Kanemasa K, Sakai K, Sumida Y, Morimoto Y, Kashiwa , Hasegawa D, Wakabayashi N, Inaba S, Yanagiswa A. Experience of endoscopic submucosal dissection (ESD) to colorectal tumor-especially about clinical course of cases with perforation (Japanese literature with English abstract). Gastroenterol Endosc. 2008;50:1472-1483. |
| 30. | Park SY, Jeon SW. Acute intestinal obstruction after endoscopic submucosal dissection: report of a case. Dis Colon Rectum. 2008;51:1295-1297. |
| 31. | Kashida H, Kudo SE. Early colorectal cancer: concept, diagnosis, and management. Int J Clin Oncol. 2006;11:1-8. |
| 32. | Cao Y, Liao C, Tan A, Gao Y, Mo Z, Gao F. Meta-analysis of endoscopic submucosal dissection versus endoscopic mucosal resection for tumors of the gastrointestinal tract. Endoscopy. 2009;41:751-757. |
| 33. | Uraoka T, Kato J, Ishikawa S, Harada K, Kuriyama M, Takemoto K, Kawahara Y, Saito Y, Okada H. Thin endoscope-assisted endoscopic submucosal dissection for large colorectal tumors (with videos). Gastrointest Endosc. 2007;66:836-839. |
| 34. | Saito Y, Emura F, Matsuda T, Uraoka T, Nakajima T, Ikematsu H, Gotoda T, Saito D, Fujii T. A new sinker-assisted endoscopic submucosal dissection for colorectal cancer. Gastrointest Endosc. 2005;62:297-301. |
| 35. | Sakamoto N, Osada T, Shibuya T, Beppu K, Matsumoto K, Mori H, Kawabe M, Nagahara A, Otaka M, Ogihara T. Endoscopic submucosal dissection of large colorectal tumors by using a novel spring-action S-O clip for traction (with video). Gastrointest Endosc. 2009;69:1370-1374. |