Published online Apr 7, 2010. doi: 10.3748/wjg.v16.i13.1587
Revised: February 25, 2010
Accepted: March 4, 2010
Published online: April 7, 2010
Accurate evaluation of iron overload is necessary to establish the diagnosis of hemochromatosis and guide chelation treatment in transfusion-dependent anemia. The liver is the primary site for iron storage in patients with hemochromatosis or transfusion-dependent anemia, therefore, liver iron concentration (LIC) accurately reflects total body iron stores. In the past 20 years, magnetic resonance imaging (MRI) has emerged as a promising method for measuring LIC in a variety of diseases. We review the potential role of MRI in LIC determination in the most important disorders that are characterized by iron overload, that is, thalassemia major, other hemoglobinopathies, acquired anemia, and hemochromatosis. Most studies have been performed in thalassemia major and MRI is currently a widely accepted method for guiding chelation treatment in these patients. However, the lack of correlation between liver and cardiac iron stores suggests that both organs should be evaluated with MRI, since cardiac disease is the leading cause of death in this population. It is also unclear which MRI method is the most accurate since there are no large studies that have directly compared the different available techniques. The role of MRI in the era of genetic diagnosis of hemochromatosis is also debated, whereas data on the accuracy of the method in other hematological and liver diseases are rather limited. However, MRI is a fast, non-invasive and relatively accurate diagnostic tool for assessing LIC, and its use is expected to increase as the role of iron in the pathogenesis of liver disease becomes clearer.
- Citation: Tziomalos K, Perifanis V. Liver iron content determination by magnetic resonance imaging. World J Gastroenterol 2010; 16(13): 1587-1597
- URL: https://www.wjgnet.com/1007-9327/full/v16/i13/1587.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i13.1587
Iron homeostasis in humans depends exclusively on the modulation of iron absorption since iron excretion is passive (by shedding of intestinal and skin cells, and additionally, in women by menstruation), and cannot be actively upregulated[1]. Therefore, patients with excessive iron absorption (hemochromatosis) or with transfusion-dependent congenital or acquired anemia are at increased risk for developing iron overload[1]. Excessive iron is toxic because it generates free radicals and induces oxidative stress[2]. Iron overload can result in the development of diabetes and other endocrinopathies, liver cirrhosis and hepatocellular carcinoma (HCC), cardiomyopathy and premature death in patients with thalassemia major[3-6] and hemochromatosis[7-10]. In turn, the management of iron overload with chelation therapy in thalassemia major[11-14] and with phlebotomy in hemochromatosis reduces the risk of diabetes, liver and cardiac disease and death[7-9].
The accurate evaluation of iron overload is necessary to establish the diagnosis of hemochromatosis and guide chelation treatment in transfusion-dependent anemia[3,15-17]. Serum ferritin levels are not an accurate measure of total body iron stores, particularly in patients with high iron burden, because concurrent conditions, particularly inflammation and liver disease, increase these levels independently of iron burden[16,18,19]. Serum iron, transferrin, transferrin saturation and transferrin receptor levels are also imprecise markers of body iron stores[18]. The liver is the primary site for iron storage in patients with hemochromatosis or transfusion-dependent anemia, therefore, liver iron concentration (LIC) accurately reflects total body iron stores[1,19,20]. Liver biopsy with measurement of iron concentration by atomic absorption spectroscopy is considered the gold standard for LIC assessment[16]. However, hepatic iron distribution appears to be uneven, particularly when cirrhosis is present, but also in the absence of cirrhosis[21-25]. In addition, liver biopsy is an invasive procedure and complications requiring hospitalization are observed in approximately 0.5% of patients with thalassemia major who undergo liver biopsy, even in experienced centers[26]. The risks of liver biopsy also preclude repeated biopsies, which are necessary in patients with thalassemia major in order to adjust chelation treatment and avoid iron overload and chelation-associated toxicity[3,16,17,19].
It is apparent that there is a pressing need for non-invasive methods that can provide accurate LIC measurements. Superconducting quantum interference devices (SQUIDs) has been used for this purpose, but appear to underestimate LIC and are available in only a few centers[16]. In the past 20 years, magnetic resonance imaging (MRI) has emerged as a promising method for measuring LIC in a variety of diseases. We review the potential role of MRI in LIC determination in the most important disorders characterized by iron overload, namely, thalassemia major, other hemoglobinopathies, acquired anemia, and hemochromatosis.
A literature search (using PubMed) was performed using the following key words: “thalassemia major”, “iron overload”, “MRI”, “liver”, “hemochromatosis”, “desferrioxamine”, “deferiprone”, “deferasirox”, “thalassemia intermedia”, “sickle cell disease”, “myelodysplastic syndromes”, “bone marrow transplantation”, “hepatitis C”, “alcoholic liver disease” and “non-alcoholic liver disease” up to 13 January 2010. The authors also manually reviewed the references of retrieved articles for any pertinent material.
The measurement of iron overload with MRI is based on the shortening effect of the interaction of iron-containing molecules (particularly ferritin and hemosidirin) with hydrogen nuclei (mainly in water molecules) on T2 relaxation time[19,27,28]. Hepatic iron deposition with MRI can be quantified by measuring the ratio of the signal intensity of the liver and of a reference tissue (mainly paraspinous muscle, which does not develop siderosis)[27,28]. These signal intensity ratios (SIRs) can be derived from either spin-echo (SE) T2-weighted or from gradient recalled-echo (GRE) T2*-weighted sequences[27,28]. Liver siderosis can also be determined by the direct measurement of relaxation time (relaxometry), either T2 [or 1/T2 (R2)] from SE sequences or T2* [or 1/T2* (R2*)] from GRE sequences; it is also possible to measure both R2 and R2* relaxation times (hybrid method)[27,28]. SIR-measuring methods are faster than relaxometry but less sensitive, particularly in patients with severe iron overload[27,28]. In addition, SIR-measuring methods appear to have smaller interscanner reproducibility than do relaxometry methods[29,30]. Among relaxometry methods, R2 acquisition time is longer than R2* acquisition time[30].
Some early studies have assessed the accuracy of LIC quantification by liver/muscle SIR derived from either GRE or SE sequences[21,31,32]. The correlation between LIC measured in liver biopsy and SIR was stronger when GRE sequences were used[21,31,32]. However, both methods were inaccurate in patients with severe iron overload or liver fibrosis, who represent a sizable percentage of the thalassemia population[21,31,33]. In contrast, the presence of viral hepatitis did not affect the correlation between MRI- and liver-biopsy-based LIC measurements[31]. More recent studies also have reported moderate correlations between SIR and LIC (r = 0.65-0.89)[34-36].
Regarding relaxometry methods, two large studies (n = 80 and n = 106) have reported moderate correlations between liver T2 and T2* measured in 1.5 T scanners with LIC (r = -0.82 and r = -0.81, respectively)[37,38]. The correlation coefficient between liver T2* and LIC was stronger in patients without hepatic fibrosis (-0.93 vs -0.68 in patients with liver fibrosis)[38]. Two smaller studies (n = 46 and n = 52, respectively) reported relatively stronger correlations between LIC and liver R2 determined in a 1.5 T and 0.5 T imager, respectively (r = 0.874 and r = 0.94, respectively)[39,40]. Interestingly, the presence of liver fibrosis reduced the accuracy of the method only in the 1.5 T scanner[39,40]. Liver inflammation and chronic hepatitis C virus (HCV) infection also had no effect on the correlation between R2 and LIC in the 0.5 T unit[39,40]. A recent study also has suggested that measuring liver R2* in higher field strength imagers (i.e. 3 T vs 1.5 T) yields less accurate measurements, particularly in patients with more severe iron overload[41]. However, the latter studies evaluated patients with different characteristics and their results are not directly comparable[39-41].
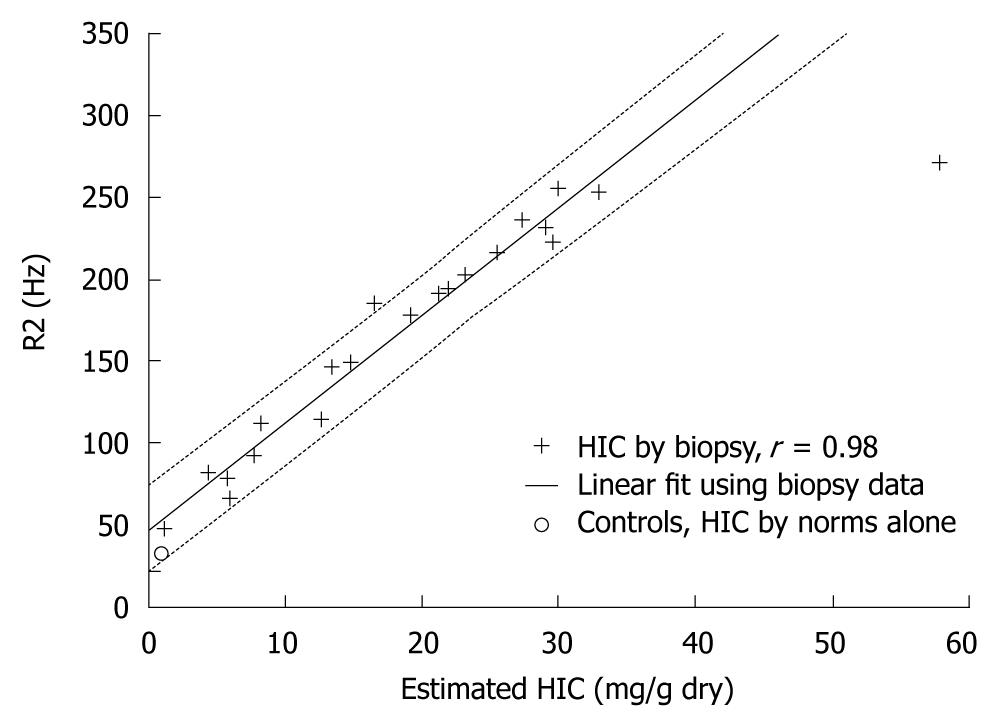
Several studies have shown that relaxometry methods are more accurate than SIR-measuring methods for LIC determination[30,36,42,43]. In an early comparative study, liver R2 correlated more strongly with biopsy-determined LIC than liver/paraspinous muscle SIR measured in SE sequences (r = 0.97 and 0.71, respectively)[42]. Moreover, the presence of liver fibrosis or inflammation did not affect the correlation between liver R2 and LIC[42]. Liver/subcutaneous fat SIR did not correlate significantly with LIC[42]. In another early small study (n = 10), liver R2 relaxation time measured with a 0.5 T MRI unit was better correlated with LIC than was R2* relaxation time[43]. In a large study in patients with thalassemia major (n = 57), sickle cell disease (SCD) (n = 34), thalassemia intermedia (n = 6) and other causes of iron overload (aplastic anemia, hemochromatosis and heme-metabolism defects; n = 5), liver R2 and R2* measured with a 1.5 T scanner showed a strong correlation with LIC (r = 0.98 and 0.97, respectively; Figure 1)[30]. R2 showed less variability between imaging slices and better reproducibility between examinations compared with R2*[30]. Combined measurement of R2 and R2* did not improve diagnostic accuracy[30]. We also recently showed in 94 patients with thalassemia major a strong correlation between liver R2, R2* and GRE-derived liver/muscle SIR in a 1.5 T unit[36]. Liver R2 was more accurate than the other methods in patients with more severe iron overload[36]. According to current guidelines for the management of patients with thalassemia major, MRI is a feasible alternative to liver biopsy for determining LIC[16]. The use of R2 sequences and local individual calibration is recommended[16].
Myocardial iron-overload-induced heart failure is the cause of death in approximately 60% of patients with thalassemia major[4]. Measurement of cardiac T2* is the currently recommended method for assessing cardiac iron overload[16]. However, several large cross-sectional studies (total, n = 429) have not identified a significant correlation between cardiac and liver T2*[38,44-47]. Only one large study (n = 180) has reported a weak, albeit significant, correlation between liver T2* and cardiac T2* (r = 0.18, P < 0.05)[48]. In smaller studies (n = 46 and 38, respectively), liver R2 and R2* did not correlate significantly with cardiac R2 and R2*[39,49,50]. We also found no correlation between myocardial R2* and liver R2, and R2* and GRE-derived liver/muscle SIR in 94 patients[36]. Only a few studies have reported a significant correlation between liver T2 and heart T2[37], or between liver R2* and cardiac R2*[51]; however, the correlation was weak (r = 0.34 and 0.23, respectively)[37,51]. Cardiac iron overload has also been reported in patients with minimal iron deposition in the liver[50-52]. Finally, in a recent large study in 652 patients with thalassemia major, liver T2* was very weakly correlated with cardiac T2* (R2 = 0.003, P = 0.04)[53]. More importantly, liver T2* was not associated with the development of heart failure or arrhythmia, whereas cardiac T2* predicted heart failure and arrhythmia[53].
The lack of correlation between liver and cardiac iron load might be explained by different mechanisms of iron uptake in the two organs[28]. In addition, small studies have suggested that iron deposition and chelation-induced clearance of iron from the heart lag behind liver iron changes[50,54]. Moreover, patients receiving treatment with different chelators might experience iron clearance from the liver and heart at different rates[46,55-58]. It appears that desferrioxamine is more or similarly effective than deferiprone in removing iron from the liver, but less effective than the latter in removing iron from the heart[46,55-58]. Deferasirox, another oral chelator, appears to be similarly effective to desferrioxamine in reducing liver iron (assessed with liver biopsy or SQUID) when appropriately dosed[59,60]. In uncontrolled studies, deferasirox also has reduced cardiac iron deposition (assessed with cardiac T2*)[61,62]. These findings suggest that myocardial iron overload should also be monitored closely in patients with thalassemia major[16]. According to recent guidelines for the management of these patients, myocardial iron deposition should be monitored with T2* MRI every year in patients with a poor chelation history, or in those with LIC, who show a non-optimal response to chelation therapy[16].
Endocrine disorders, particularly hypogonadism and diabetes, are frequently observed in patients with thalassemia major[63,64]. However, liver iron load does not appear to predict the extent of iron deposition in the pancreas or the pituitary gland, as assessed by SIR[65-67], T2[68], R2[49] or T2*[69]. However, in the largest study that has assessed the relationship between hepatic and pancreas/pituitary gland iron stores (n = 180), liver T2* correlated weakly, albeit significantly, with pancreas and pituitary gland T2* (r = 0.35 and 0.17, respectively)[48]. Liver T2* also correlated significantly with pituitary gland T2 and pituitary gland/muscle SIR (r = 0.21 and 0.63, respectively)[48]. However, liver T2* was not associated with the presence of endocrine disorders (diabetes, hypothyroidism, hypogonadism and hypoparathyroidism)[48]. No differences in liver iron overload between diabetic and non-diabetic patients were reported in other studies using T2*[69,70] or SIR obtained from SE or GRE sequences[34]. There was also no correlation between liver T2* and insulin resistance or pancreatic β-cell reserve[69]. Only one small study (n = 31) has reported more severe liver iron overload (assessed with liver/paraspinous muscle SIR in GRE sequences) in diabetic patients[66]. Regarding hypogonadotropic hypogonadism, one study of 36 patients has reported no difference in liver/subcutaneous fat SIR between patients with hypogonadotropic hypogonadism and those without pituitary gland dysfunction[67], whereas another larger study (n = 50) has identified low liver T2* as a predictor of the presence of hypogonadism (but not of diabetes)[70]. The apparent lack of association between the iron burden in liver, pancreas and pituitary gland might be partly due to different mechanisms of iron accumulation in these organs[49,65-69]. In addition, pancreatic T2 and R2 are also reduced by the development of fatty degeneration, which might also confound the relationship between liver and pancreatic relaxation times in MRI[49,66,68].
Allogeneic bone marrow transplantation (BMT) from an HLA-identical donor is a potentially curative treatment for thalassemia major, particularly when pre-transplantation chelation treatment is adequate and there is no portal fibrosis or hepatomegaly[71-74]. In these patients, liver iron overload progressively decreases without phlebotomy but may persist for 4-6 years after BMT in older patients[75,76]. In patients who have undergone BMT for thalassemia major, higher LIC independently predicts progression of liver fibrosis[77]. In these patients, phlebotomy reduces liver iron overload and improves liver and myocardial function[78-80]. Liver MRI has also been used in these patients to assess iron overload[81]. Measurement of liver T2 and T2* with a 1.5 T imager revealed iron overload in four out of eight patients, even though a mean time of 11.3 years had elapsed since BMT, and seven patients had received iron chelation treatment with phlebotomy, desferrioxamine or their combination for a mean 39 mo[81]. In contrast, heart T2 and T2* were normal in all patients[81].
Thalassemia intermedia is a diverse group of hemoglobinopathies that are characterized by less severe transfusion dependency and iron overload than in thalassemia major[82,83]. Liver iron overload assessed with measurement of the T2* relaxation time is less pronounced in patients with thalassemia intermedia than in those with thalassemia major[84]. Even though patients with thalassemia intermedia have liver iron overload compared with healthy controls, cardiac T2* does not differ between the former and the latter[84]. In a study of 26 patients with thalassemia intermedia, liver T2 strongly correlated with LIC (r = -0.82, P = 0.0003) but not with heart T2 (r = -0.17, P = 0.41)[37]. Iron overload can develop in patients with thalassemia intermedia who have not been regularly transfused[82,83]. In patients with thalassemia intermedia who received < 10 red blood cell (RBC) units, liver R2 revealed iron overload in two-thirds of the patients, whereas cardiac T2* was normal in all patients[85]. In a very recent study in 49 patients with thalassemia intermedia, liver T2* revealed the presence of hepatic iron overload in 77.5% of patients, even though 44.9% had never been transfused and only 8.2% were being regularly transfused[86]. In contrast, no patient had cardiac iron overload and liver T2* did not correlate with cardiac T2*[86].
Beta thalassemia/hemoglobin E, a frequent hemoglobinopathy in Asia, manifests as thalassemia intermedia in half of the patients and as thalassemia major in the other half[87,88]. Iron overload can develop even in patients who do not require regular transfusions and is due to increased intestinal absorption of iron[87,88]. In a pivotal study in patients with beta thalassemia/hemoglobin E (n = 41), thalassemia major (n = 9) or hereditary hemochromatosis (n = 23), liver R2 strongly correlated with LIC measured in biopsy (r = 0.98, P < 0.0001)[89]. However, R2 variability increased with increasing LIC[89].
Hemoglobin H (Hb H) disease is another frequent hemoglobinopathy in Asia, which results from the deletion of three of the four α-globin genes (deletional Hb H disease) or from deletion of two α-globin genes and a non-deletional mutation of a third α-globin gene (non-deletional Hb H disease). In all cases, there is an excess of β-globin chains that form β4 tetramers (Hb H)[88,90,91]. The clinical phenotype of Hb H disease is variable and often resembles thalassemia intermedia[88,90,91]. Regular transfusions are infrequently required but iron overload can develop even in never-transfused patients[88,91]. Increased intestinal iron absorption due to hemolysis and ineffective erythropoiesis appears to explain the development of iron overload in these patients[91]. Non-deletional Hb H disease has more severe presentation and more frequently leads to iron overload than does deletional Hb H disease[91,92]. In an early study in 36 non-transfusion-dependent patients with Hb H disease, liver/paraspinous muscle SIR obtained from GRE sequences was more sensitive in detecting iron overload than SIR determined from SE sequences[93]. Liver iron overload was observed in 33/36 patients, whereas iron overload in the heart and pancreas were present in only one and six patients, respectively[93]. In a large study in 114 Chinese patients with Hb H disease, liver/paraspinous muscle SIR measured from GRE sequences revealed liver iron overload in 85% of the patients[92]. Iron overload was present even though only one patient was transfusion-dependent and only eight had received more than five transfusions (median number of transfusions: 5.5; range: 5-20)[92]. Patients with deletional Hb H disease had more severe iron overload than those with non-deletional Hb H disease[92]. In a smaller recent study in 37 patients with Hb H disease or thalassemia intermedia and serum ferritin levels > 1000 pmol/L, liver T2* was abnormal in most patients (84%)[94]. Log-liver T2* correlated with serum ferritin levels but not with heart, pancreas or pituitary siderosis, as assessed by MRI, or with abnormalities in pancreatic function or the growth hormone axis[94].
Chronic RBC transfusion therapy is increasingly being used in patients with SCD, particularly for the primary or secondary prevention of stroke[95]. However, iron overload frequently develops in patients with SCD who are receiving chronic RBC transfusion therapy[96]. The development of iron overload in these patients increases the risk for hospitalization[97] and death[98], whereas chelation treatment reduces mortality[97]. In a recent study in patients with SCD, thalassemia major or bone marrow failure, liver R2* was strongly correlated with LIC assessed with liver biopsy (r = 0.96-0.98, P < 0.001), regardless of the presence of fibrosis[99]. In another study in 35 patients with SCD, liver T2 was moderately correlated with LIC (r = -0.80, P = 0.00001) but not with heart T2 (r = 0.10, P = 0.56)[37].
Iron overload frequently develops in patients with myelodysplastic syndromes (MDSs), which can result in abnormal liver function, diabetes and heart failure and increase the risk of death[100-104]. In these patients, treatment with desferrioxamine improves liver, pancreas and pituitary gland function[100,102,105]. Several small studies (n = 10 or 11) have assessed liver iron deposition by measuring liver T2* and have revealed liver iron overload in almost all patients[106-109]. Log liver T2* correlates with RBC units transfused[106,107]. In contrast, myocardial siderosis was present in only 10%-15% of patients and cardiac T2* did not correlate with liver T2*[106,108,109]. Only one study has assessed pancreas and pituitary gland siderosis with MRI in patients with MDS, and has reported no association between liver T2* and pancreas or pituitary gland siderosis, but a correlation between log liver T2* and indices of insulin resistance and reduced beta cell reserve[106]. According to recent guidelines, iron overload should be monitored in patients with MDS, using serum ferritin and transferrin levels, whereas liver MRI is considered useful but not essential[92].
Iron overload is frequently observed in patients who have undergone allogeneic BMT for hematological diseases other than thalassemia major, and results primarily from RBC transfusions[110-112]. Iron overload in these patients appears to be associated with increased risk for infections, veno-occlusive disease, hepatic dysfunction[110,113,114] and death[111,114-118]. Phlebotomy improves liver function in this population[118]. It is recommended that patients who have undergone BMT and have iron overload should be treated with phlebotomy and/or chelation therapy[112]. Liver MRI is a useful tool for quantifying body iron stores in this population[110]. In an early study in 13 children who had undergone autologous BMT, mostly for non-hematological disorders (neuroblastoma in 8 patients), liver iron overload assessed with liver/paraspinous muscle SIR measured in SE sequences was present in 10 patients (77%)[119]. Liver iron overload is correlated with RBC units transfused[119]. Three small studies (n = 32, 19 and 20) have revealed liver iron overload in the majority of patients (97%, 95% and 85%, respectively) who had undergone allogeneic BMT for leukemia, MDS, multiple myeloma, lymphoma, aplastic anemia or myelofibrosis, who had serum ferritin levels greater than the upper limit of the normal range, > 1000 ng/mL or > 1600 pmol/L, respectively[120-122]. Liver iron deposition was assessed by liver/paraspinous muscle SIR measured from GRE sequences[122], R2[121] or T2*[120]. Liver siderosis correlated with RBC units transfused[122]. Patients with more advanced liver siderosis more frequently exhibited elevated transaminases, which normalized in most of them after phlebotomy[122]. However, liver iron deposition did not correlate with heart, pancreas or pituitary siderosis as assessed by MRI, or with abnormalities in pancreatic function or the growth hormone axis[120]. In addition, the duration of post-BMT follow-up, type of graft or conditioning, and the presence of chronic graft-versus-host-disease had no effect on the presence of liver iron overload[121].
Two small case-series (n = 11 and 3, respectively) have evaluated iron overload with MRI in patients with acute leukemia or solid tumors who received multiple RBC transfusions due to chemotherapy-induced anemia[123,124]. Liver/muscle SIR identified liver iron overload in all patients, whereas cardiac T2* was marginally reduced and pancreas/skeletal muscle SIR was normal in all patients[123,124].
Hemochromatosis is an autosomal recessive disease with variable penetrance. In most patients, it results from a mutation of the HFE gene, which leads to iron overload by increasing intestinal iron absorption and by enhancing iron release from macrophages after phagocytosis of erythrocytes[7,8,15]. In an early study in 20 patients with hemochromatosis and 18 with hematological diseases (mainly MDS), liver/muscle SIR obtained from SE sequences correlated strongly with LIC (r2 = 0.98)[125]. However, in subsequent studies in patients with suspected hemochromatosis, SIR obtained from GRE sequences has provided a more accurate determination of LIC than from SE sequences[126,127]. In a more recent and larger study in 174 patients with suspected hemochromatosis or with chronic HCV infection, Gandon et al[128] have shown that liver/muscle SIR obtained from GRE sequences identifies liver iron overload with high sensitivity and specificity. The severity of hepatic siderosis and the presence of cirrhosis does not affect the accuracy of MRI measurements. The diagnostic accuracy of the technique proposed by Gandon et al[128] has been replicated in other studies in patients with suspected hemochromatosis, chronic HCV infection, or persistent elevation of transaminase levels[129,130]. However, it should be mentioned that this method does not appear to be accurate in assessing LIC in patients with thalassemia major or MDS, who have higher LIC than patients with hemochromatosis[35]. Two smaller studies (n = 11 and 23, respectively) have reported a strong correlation between liver R2 and biopsy-determined LIC in hemochromatosis[89,131]. Some experts consider liver MRI to be the method of choice for documenting iron overload in patients with suspected hemochromatosis[15]. Nevertheless, others argue that MRI has limited sensitivity to detect mild iron overload in patients with suspected hemochromatosis[7].
Besides hemochromatosis, mild to moderate iron overload is also present in some patients with other liver diseases, including chronic hepatitis B or C, alcoholic liver disease and non-alcoholic fatty liver disease[1,132-136]. In chronic hepatitis B or C and alcoholic liver disease, low levels of hepcidin, a protein that regulates iron absorption, may play a role in the development of iron overload[1,132-134,136]. Increased iron uptake from hepatocytes due to upregulation of transferrin receptors might also contribute[136]. In non-alcoholic fatty liver disease, insulin-induced redistribution of intracellular transferrin receptors to the hepatocyte membrane, and downregulation of the iron exporter protein due to the pro-inflammatory state present in these patients, may contribute to the development of liver iron overload[132]. MRI is a sensitive method for evaluating liver iron deposition in patients with chronic HCV infection[128-130]. However, liver biopsy is required in most of these patients to assess the presence of inflammation, fibrosis and cirrhosis.
Increased LIC is frequently observed in patients with non-biliary cirrhosis (not due to hemochromatosis) and can occasionally be severe; in contrast, liver siderosis is rare in biliary cirrhosis[1,23,134]. Liver/paraspinous muscle SIR obtained from SE or GRE sequences has revealed liver iron overload in 40% of cirrhosis patients[137]. However, in studies in patients with cirrhosis of various etiologies in whom liver biopsy had been performed, there was only a moderate correlation between liver/muscle SIR in GRE sequences and histological grading of liver siderosis (r = 0.515, P < 0.001)[138]. Moreover, others did not find a difference in liver iron load (assessed with SIR obtained from GRE sequences) between patients with viral hepatitis and cirrhosis[139]. In addition, liver siderosis in either SE or GRE sequences did not increase with the progression of viral hepatitis-induced cirrhosis[140]. Despite these discrepant findings, MRI might be particularly useful when cirrhosis is present because of the risk of bleeding in these patients during liver biopsy and because of the uneven iron distribution in the cirrhotic liver[21-25].
Finally, MRI might be useful for the evaluation of iron stores in porphyria cutanea tarda, a familial or sporadic disease that is characterized by decreased activity of hepatic uroporphyrinogen decarboxylase (UROD), which is frequently associated with iron overload[141]. In these patients, iron inhibits UROD activity, and phlebotomy is the treatment of choice[136,141]. A recent study in 20 patients with porphyria cutanea tarda measured liver/paraspinous muscle SIR from GRE sequences and identified liver iron overload in 11 (55%)[142].
MRI is a potentially useful non-invasive method for evaluating liver iron stores in a wide spectrum of hematological and liver diseases. Most studies have been performed in thalassemia major and MRI is currently a widely accepted method for guiding chelation treatment in these patients. However, the lack of correlation between liver and cardiac iron stores suggests that both organs should be evaluated with MRI, since cardiac disease is the leading cause of death in this population. It is also unclear which MRI method is the most accurate because there are no large studies that have directly compared the different techniques. Another issue is the reproducibility of the various methods in different centers. Liver/muscle SIR obtained from GRE sequences[128-130] and liver and heart T2* have been shown to be reproducible in different scanners[143-145] but more data are needed. The role of MRI in the era of genetic diagnosis of hemochromatosis is also debated, whereas data on the accuracy of the method in other hematological and liver diseases are rather limited. However, MRI is a fast non-invasive and relatively accurate diagnostic tool for assessing liver iron content, and its use is expected to increase as the role of iron in the pathogenesis of liver disease becomes clearer.
Peer reviewer: Paul E Sijens, PhD, Associate Professor, Radiology, UMCG, Hanzeplein 1, 9713GZ Groningen, The Netherlands
S- Editor Tian L L- Editor Kerr C E- Editor Ma WH
| 1. | Batts KP. Iron overload syndromes and the liver. Mod Pathol. 2007;20 Suppl 1:S31-S39. |
| 2. | Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117:285-297. |
| 3. | Rund D, Rachmilewitz E. Beta-thalassemia. N Engl J Med. 2005;353:1135-1146. |
| 4. | Borgna-Pignatti C, Rugolotto S, De Stefano P, Zhao H, Cappellini MD, Del Vecchio GC, Romeo MA, Forni GL, Gamberini MR, Ghilardi R. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica. 2004;89:1187-1193. |
| 5. | Telfer PT, Prestcott E, Holden S, Walker M, Hoffbrand AV, Wonke B. Hepatic iron concentration combined with long-term monitoring of serum ferritin to predict complications of iron overload in thalassaemia major. Br J Haematol. 2000;110:971-977. |
| 6. | Olivieri NF, Nathan DG, MacMillan JH, Wayne AS, Liu PP, McGee A, Martin M, Koren G, Cohen AR. Survival in medically treated patients with homozygous beta-thalassemia. N Engl J Med. 1994;331:574-578. |
| 8. | Pietrangelo A. Hereditary hemochromatosis--a new look at an old disease. N Engl J Med. 2004;350:2383-2397. |
| 9. | Niederau C, Fischer R, Pürschel A, Stremmel W, Häussinger D, Strohmeyer G. Long-term survival in patients with hereditary hemochromatosis. Gastroenterology. 1996;110:1107-1119. |
| 10. | Loréal O, Deugnier Y, Moirand R, Lauvin L, Guyader D, Jouanolle H, Turlin B, Lescoat G, Brissot P. Liver fibrosis in genetic hemochromatosis. Respective roles of iron and non-iron-related factors in 127 homozygous patients. J Hepatol. 1992;16:122-127. |
| 11. | Brittenham GM, Griffith PM, Nienhuis AW, McLaren CE, Young NS, Tucker EE, Allen CJ, Farrell DE, Harris JW. Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. N Engl J Med. 1994;331:567-573. |
| 12. | Ehlers KH, Giardina PJ, Lesser ML, Engle MA, Hilgartner MW. Prolonged survival in patients with beta-thalassemia major treated with deferoxamine. J Pediatr. 1991;118:540-545. |
| 13. | Aldouri MA, Wonke B, Hoffbrand AV, Flynn DM, Ward SE, Agnew JE, Hilson AJ. High incidence of cardiomyopathy in beta-thalassaemia patients receiving regular transfusion and iron chelation: reversal by intensified chelation. Acta Haematol. 1990;84:113-117. |
| 14. | Wolfe L, Olivieri N, Sallan D, Colan S, Rose V, Propper R, Freedman MH, Nathan DG. Prevention of cardiac disease by subcutaneous deferoxamine in patients with thalassemia major. N Engl J Med. 1985;312:1600-1603. |
| 15. | Brissot P, Troadec MB, Bardou-Jacquet E, Le Lan C, Jouanolle AM, Deugnier Y, Loréal O. Current approach to hemochromatosis. Blood Rev. 2008;22:195-210. |
| 16. | Angelucci E, Barosi G, Camaschella C, Cappellini MD, Cazzola M, Galanello R, Marchetti M, Piga A, Tura S. Italian Society of Hematology practice guidelines for the management of iron overload in thalassemia major and related disorders. Haematologica. 2008;93:741-752. |
| 17. | Fischer R, Harmatz PR. Non-invasive assessment of tissue iron overload. Hematology Am Soc Hematol Educ Program. 2009;215-221. |
| 18. | Kushner JP, Porter JP, Olivieri NF. Secondary iron overload. Hematology. Am Soc Hematol Educ Program. 2001;47-61. |
| 19. | Brittenham GM, Badman DG. Noninvasive measurement of iron: report of an NIDDK workshop. Blood. 2003;101:15-19. |
| 20. | Angelucci E, Brittenham GM, McLaren CE, Ripalti M, Baronciani D, Giardini C, Galimberti M, Polchi P, Lucarelli G. Hepatic iron concentration and total body iron stores in thalassemia major. N Engl J Med. 2000;343:327-331. |
| 21. | Chan YL, Li CK, Lam CW, Yu SC, Chik KW, To KF, Yeung DK, Howard R, Yuen PM. Liver iron estimation in beta-thalassaemia: comparison of MRI biochemical assay and histological grading. Clin Radiol. 2001;56:911-916. |
| 22. | Emond MJ, Bronner MP, Carlson TH, Lin M, Labbe RF, Kowdley KV. Quantitative study of the variability of hepatic iron concentrations. Clin Chem. 1999;45:340-346. |
| 23. | Ludwig J, Hashimoto E, Porayko MK, Moyer TP, Baldus WP. Hemosiderosis in cirrhosis: a study of 447 native livers. Gastroenterology. 1997;112:882-888. |
| 24. | Villeneuve JP, Bilodeau M, Lepage R, Côté J, Lefebvre M. Variability in hepatic iron concentration measurement from needle-biopsy specimens. J Hepatol. 1996;25:172-177. |
| 25. | Ambu R, Crisponi G, Sciot R, Van Eyken P, Parodo G, Iannelli S, Marongiu F, Silvagni R, Nurchi V, Costa V. Uneven hepatic iron and phosphorus distribution in beta-thalassemia. J Hepatol. 1995;23:544-549. |
| 26. | Angelucci E, Baronciani D, Lucarelli G, Baldassarri M, Galimberti M, Giardini C, Martinelli F, Polchi P, Polizzi V, Ripalti M. Needle liver biopsy in thalassaemia: analyses of diagnostic accuracy and safety in 1184 consecutive biopsies. Br J Haematol. 1995;89:757-761. |
| 27. | Argyropoulou MI, Astrakas L. MRI evaluation of tissue iron burden in patients with beta-thalassaemia major. Pediatr Radiol. 2007;37:1191-1200; quiz 1308-1309. |
| 28. | Wood JC. Magnetic resonance imaging measurement of iron overload. Curr Opin Hematol. 2007;14:183-190. |
| 29. | Virtanen JM, Komu ME, Parkkola RK. Quantitative liver iron measurement by magnetic resonance imaging: in vitro and in vivo assessment of the liver to muscle signal intensity and the R2* methods. Magn Reson Imaging. 2008;26:1175-1182. |
| 30. | Wood JC, Enriquez C, Ghugre N, Tyzka JM, Carson S, Nelson MD, Coates TD. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood. 2005;106:1460-1465. |
| 31. | Angelucci E, Giovagnoni A, Valeri G, Paci E, Ripalti M, Muretto P, McLaren C, Brittenham GM, Lucarelli G. Limitations of magnetic resonance imaging in measurement of hepatic iron. Blood. 1997;90:4736-4742. |
| 32. | Bonetti MG, Castriota-Scanderbeg A, Criconia GM, Mazza P, Sacco M, Amurri B, Masi C. Hepatic iron overload in thalassemic patients: proposal and validation of an MRI method of assessment. Pediatr Radiol. 1996;26:650-656. |
| 33. | Perifanis V, Tziomalos K, Tsatra I, Karyda S, Patsiaoura K, Athanassiou-Metaxa M. Prevalence and severity of liver disease in patients with b thalassemia major. A single-institution fifteen-year experience. Haematologica. 2005;90:1136-1138. |
| 34. | Ooi GC, Khong PL, Chan GC, Chan KN, Chan KL, Lam W, Ng I, Ha SY. Magnetic resonance screening of iron status in transfusion-dependent beta-thalassaemia patients. Br J Haematol. 2004;124:385-390. |
| 35. | Rose C, Vandevenne P, Bourgeois E, Cambier N, Ernst O. Liver iron content assessment by routine and simple magnetic resonance imaging procedure in highly transfused patients. Eur J Haematol. 2006;77:145-149. |
| 36. | Christoforidis A, Perifanis V, Spanos G, Vlachaki E, Economou M, Tsatra I, Athanassiou-Metaxa M. MRI assessment of liver iron content in thalassamic patients with three different protocols: comparisons and correlations. Eur J Haematol. 2009;82:388-392. |
| 37. | Voskaridou E, Douskou M, Terpos E, Papassotiriou I, Stamoulakatou A, Ourailidis A, Loutradi A, Loukopoulos D. Magnetic resonance imaging in the evaluation of iron overload in patients with beta thalassaemia and sickle cell disease. Br J Haematol. 2004;126:736-742. |
| 38. | Anderson LJ, Holden S, Davis B, Prescott E, Charrier CC, Bunce NH, Firmin DN, Wonke B, Porter J, Walker JM. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22:2171-2179. |
| 39. | Alexopoulou E, Stripeli F, Baras P, Seimenis I, Kattamis A, Ladis V, Efstathopoulos E, Brountzos EN, Kelekis AD, Kelekis NL. R2 relaxometry with MRI for the quantification of tissue iron overload in beta-thalassemic patients. J Magn Reson Imaging. 2006;23:163-170. |
| 40. | Papakonstantinou O, Kostaridou S, Maris T, Gouliamos A, Premetis E, Kouloulias V, Nakopoulou L, Kattamis C. Quantification of liver iron overload by T2 quantitative magnetic resonance imaging in thalassemia: impact of chronic hepatitis C on measurements. J Pediatr Hematol Oncol. 1999;21:142-148. |
| 41. | Storey P, Thompson AA, Carqueville CL, Wood JC, de Freitas RA, Rigsby CK. R2* imaging of transfusional iron burden at 3T and comparison with 1.5T. J Magn Reson Imaging. 2007;25:540-547. |
| 42. | Papakonstantinou OG, Maris TG, Kostaridou V, Gouliamos AD, Koutoulas GK, Kalovidouris AE, Papavassiliou GB, Kordas G, Kattamis C, Vlahos LJ. Assessment of liver iron overload by T2-quantitative magnetic resonance imaging: correlation of T2-QMRI measurements with serum ferritin concentration and histologic grading of siderosis. Magn Reson Imaging. 1995;13:967-977. |
| 43. | Gomori JM, Horev G, Tamary H, Zandback J, Kornreich L, Zaizov R, Freud E, Krief O, Ben-Meir J, Rotem H. Hepatic iron overload: quantitative MR imaging. Radiology. 1991;179:367-369. |
| 44. | Leung AW, Chu WC, Lam WW, Lee V, Li CK. Magnetic resonance imaging assessment of cardiac and liver iron load in transfusion dependent patients. Pediatr Blood Cancer. 2009;53:1054-1059. |
| 45. | Maris TG, Papakonstantinou O, Chatzimanoli V, Papadakis A, Pagonidis K, Papanikolaou N, Karantanas A, Gourtsoyiannis N. Myocardial and liver iron status using a fast T*2 quantitative MRI (T*2qMRI) technique. Magn Reson Med. 2007;57:742-753. |
| 46. | Perifanis V, Christoforidis A, Vlachaki E, Tsatra I, Spanos G, Athanassiou-Metaxa M. comparison of effects of different long-term iron-chelation regimens on myocardial and hepatic iron concentrations assessed with T2* magnetic resonance imaging in patients with beta-thalassemia major. Int J Hematol. 2007;86:385-389. |
| 47. | Tanner MA, Galanello R, Dessi C, Westwood MA, Smith GC, Nair SV, Anderson LJ, Walker JM, Pennell DJ. Myocardial iron loading in patients with thalassemia major on deferoxamine chelation. J Cardiovasc Magn Reson. 2006;8:543-547. |
| 48. | Au WY, Lam WW, Chu WW, Yuen HL, Ling AS, Li RC, Chan HM, Lee HK, Law MF, Liu HS. A cross-sectional magnetic resonance imaging assessment of organ specific hemosiderosis in 180 thalassemia major patients in Hong Kong. Haematologica. 2008;93:784-786. |
| 49. | Papakonstantinou O, Alexopoulou E, Economopoulos N, Benekos O, Kattamis A, Kostaridou S, Ladis V, Efstathopoulos E, Gouliamos A, Kelekis NL. Assessment of iron distribution between liver, spleen, pancreas, bone marrow, and myocardium by means of R2 relaxometry with MRI in patients with beta-thalassemia major. J Magn Reson Imaging. 2009;29:853-859. |
| 50. | Noetzli LJ, Carson SM, Nord AS, Coates TD, Wood JC. Longitudinal analysis of heart and liver iron in thalassemia major. Blood. 2008;112:2973-2978. |
| 51. | Aessopos A, Fragodimitri C, Karabatsos F, Hatziliami A, Yousef J, Giakoumis A, Dokou A, Gotsis ED, Berdoukas V, Karagiorga M. Cardiac magnetic resonance imaging R2* assessments and analysis of historical parameters in patients with transfusion-dependent thalassemia. Haematologica. 2007;92:131-132. |
| 52. | Anderson LJ, Westwood MA, Prescott E, Walker JM, Pennell DJ, Wonke B. Development of thalassaemic iron overload cardiomyopathy despite low liver iron levels and meticulous compliance to desferrioxamine. Acta Haematol. 2006;115:106-108. |
| 53. | Kirk P, Roughton M, Porter JB, Walker JM, Tanner MA, Patel J, Wu D, Taylor J, Westwood MA, Anderson LJ. Cardiac T2* magnetic resonance for prediction of cardiac complications in thalassemia major. Circulation. 2009;120:1961-1968. |
| 54. | Anderson LJ, Westwood MA, Holden S, Davis B, Prescott E, Wonke B, Porter JB, Walker JM, Pennell DJ. Myocardial iron clearance during reversal of siderotic cardiomyopathy with intravenous desferrioxamine: a prospective study using T2* cardiovascular magnetic resonance. Br J Haematol. 2004;127:348-355. |
| 55. | Anderson LJ, Wonke B, Prescott E, Holden S, Walker JM, Pennell DJ. Comparison of effects of oral deferiprone and subcutaneous desferrioxamine on myocardial iron concentrations and ventricular function in beta-thalassaemia. Lancet. 2002;360:516-520. |
| 56. | Pepe A, Lombardi M, Positano V, Cracolici E, Capra M, Malizia R, Prossomariti L, De Marchi D, Midiri M, Maggio A. Evaluation of the efficacy of oral deferiprone in beta-thalassemia major by multislice multiecho T2*. Eur J Haematol. 2006;76:183-192. |
| 57. | Pennell DJ, Berdoukas V, Karagiorga M, Ladis V, Piga A, Aessopos A, Gotsis ED, Tanner MA, Smith GC, Westwood MA. Randomized controlled trial of deferiprone or deferoxamine in beta-thalassemia major patients with asymptomatic myocardial siderosis. Blood. 2006;107:3738-3744. |
| 58. | Maggio A, D’Amico G, Morabito A, Capra M, Ciaccio C, Cianciulli P, Di Gregorio F, Garozzo G, Malizia R, Magnano C. Deferiprone versus deferoxamine in patients with thalassemia major: a randomized clinical trial. Blood Cells Mol Dis. 2002;28:196-208. |
| 59. | Piga A, Galanello R, Forni GL, Cappellini MD, Origa R, Zappu A, Donato G, Bordone E, Lavagetto A, Zanaboni L. Randomized phase II trial of deferasirox (Exjade, ICL670), a once-daily, orally-administered iron chelator, in comparison to deferoxamine in thalassemia patients with transfusional iron overload. Haematologica. 2006;91:873-880. |
| 60. | Cappellini MD, Cohen A, Piga A, Bejaoui M, Perrotta S, Agaoglu L, Aydinok Y, Kattamis A, Kilinc Y, Porter J. A phase 3 study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with beta-thalassemia. Blood. 2006;107:3455-3462. |
| 61. | Pathare A, Taher A, Daar S. Deferasirox (Exjade) significantly improves cardiac T2* in heavily iron-overloaded patients with beta-thalassemia major. Ann Hematol. 2010;89:405-409. |
| 62. | Pennell DJ, Porter JB, Cappellini MD, El-Beshlawy A, Chan LL, Aydinok Y, Elalfy MS, Sutcharitchan P, Li CK, Ibrahim H. Efficacy of deferasirox in reducing and preventing cardiac iron overload in {beta}-thalassemia. Blood. 2009;Epub ahead of print. |
| 63. | Christoforidis A, Perifanis V, Tsatra I, Vlachaki E, Athanassiou-Metaxa M. Evolution of OGTT in patients with beta-thalassaemia major in relation to chelation therapy. Diabetes Res Clin Pract. 2007;76:6-11. |
| 64. | Cunningham MJ, Macklin EA, Neufeld EJ, Cohen AR. Complications of beta-thalassemia major in North America. Blood. 2004;104:34-39. |
| 65. | Christoforidis A, Haritandi A, Tsitouridis I, Tsatra I, Tsantali H, Karyda S, Dimitriadis AS, Athanassiou-Metaxa M. Correlative study of iron accumulation in liver, myocardium, and pituitary assessed with MRI in young thalassemic patients. J Pediatr Hematol Oncol. 2006;28:311-315. |
| 66. | Papakonstantinou O, Ladis V, Kostaridou S, Maris T, Berdousi H, Kattamis C, Gourtsoyiannis N. The pancreas in beta-thalassemia major: MR imaging features and correlation with iron stores and glucose disturbances. Eur Radiol. 2007;17:1535-1543. |
| 67. | Argyropoulou MI, Kiortsis DN, Efremidis SC. MRI of the liver and the pituitary gland in patients with beta-thalassemia major: does hepatic siderosis predict pituitary iron deposition? Eur Radiol. 2003;13:12-16. |
| 68. | Argyropoulou MI, Kiortsis DN, Astrakas L, Metafratzi Z, Chalissos N, Efremidis SC. Liver, bone marrow, pancreas and pituitary gland iron overload in young and adult thalassemic patients: a T2 relaxometry study. Eur Radiol. 2007;17:3025-3030. |
| 69. | Au WY, Lam WW, Chu W, Tam S, Wong WK, Liang R, Ha SY. A T2* magnetic resonance imaging study of pancreatic iron overload in thalassemia major. Haematologica. 2008;93:116-119. |
| 70. | Lam WW, Au WY, Chu WC, Tam S, Ha SY, Pennell DJ. One-stop measurement of iron deposition in the anterior pituitary, liver, and heart in thalassemia patients. J Magn Reson Imaging. 2008;28:29-33. |
| 71. | Di Bartolomeo P, Santarone S, Di Bartolomeo E, Olioso P, Bavaro P, Papalinetti G, Di Carlo P, Papola F, Nicolucci A, Di Nicola M. Long-term results of survival in patients with thalassemia major treated with bone marrow transplantation. Am J Hematol. 2008;83:528-530. |
| 72. | Lucarelli G, Clift RA, Galimberti M, Angelucci E, Giardini C, Baronciani D, Polchi P, Andreani M, Gaziev D, Erer B. Bone marrow transplantation in adult thalassemic patients. Blood. 1999;93:1164-1167. |
| 73. | Lucarelli G, Galimberti M, Polchi P, Angelucci E, Baronciani D, Giardini C, Andreani M, Agostinelli F, Albertini F, Clift RA. Marrow transplantation in patients with thalassemia responsive to iron chelation therapy. N Engl J Med. 1993;329:840-844. |
| 74. | Lucarelli G, Galimberti M, Polchi P, Angelucci E, Baronciani D, Giardini C, Politi P, Durazzi SM, Muretto P, Albertini F. Bone marrow transplantation in patients with thalassemia. N Engl J Med. 1990;322:417-421. |
| 75. | Muretto P, Del Fiasco S, Angelucci E, De Rosa F, Lucarelli G. Bone marrow transplantation in thalassemia: modifications of hepatic iron overload and associated lesions after long-term engrafting. Liver. 1994;14:14-24. |
| 76. | Lucarelli G, Angelucci E, Giardini C, Baronciani D, Galimberti M, Polchi P, Bartolucci M, Muretto P, Albertini F. Fate of iron stores in thalassaemia after bone-marrow transplantation. Lancet. 1993;342:1388-1391. |
| 77. | Angelucci E, Muretto P, Nicolucci A, Baronciani D, Erer B, Gaziev J, Ripalti M, Sodani P, Tomassoni S, Visani G. Effects of iron overload and hepatitis C virus positivity in determining progression of liver fibrosis in thalassemia following bone marrow transplantation. Blood. 2002;100:17-21. |
| 78. | Angelucci E, Muretto P, Lucarelli G, Ripalti M, Baronciani D, Erer B, Galimberti M, Giardini C, Gaziev D, Polchi P. Phlebotomy to reduce iron overload in patients cured of thalassemia by bone marrow transplantation. Italian Cooperative Group for Phlebotomy Treatment of Transplanted Thalassemia Patients. Blood. 1997;90:994-998. |
| 79. | Mariotti E, Angelucci E, Agostini A, Baronciani D, Sgarbi E, Lucarelli G. Evaluation of cardiac status in iron-loaded thalassaemia patients following bone marrow transplantation: improvement in cardiac function during reduction in body iron burden. Br J Haematol. 1998;103:916-921. |
| 80. | Angelucci E, Muretto P, Lucarelli G, Ripalti M, Baronciani D, Erer B, Galimberti M, Annibali M, Giardini C, Gaziev D. Treatment of iron overload in the “ex-thalassemic”. Report from the phlebotomy program. Ann N Y Acad Sci. 1998;850:288-293. |
| 81. | Mavrogeni S, Gotsis ED, Berdousi E, Ladis V, Verganelakis D, Toulas P, Cokkinos DV. Myocardial and hepatic T2* magnetic resonance evaluation in ex-thalassemic patients after bone-marrow transplantation. Int J Cardiovasc Imaging. 2007;23:739-745. |
| 82. | Taher A, Isma’eel H, Cappellini MD. Thalassemia intermedia: revisited. Blood Cells Mol Dis. 2006;37:12-20. |
| 83. | Borgna-Pignatti C. Modern treatment of thalassaemia intermedia. Br J Haematol. 2007;138:291-304. |
| 84. | Mavrogeni S, Gotsis E, Ladis V, Berdousis E, Verganelakis D, Toulas P, Cokkinos DV. Magnetic resonance evaluation of liver and myocardial iron deposition in thalassemia intermedia and b-thalassemia major. Int J Cardiovasc Imaging. 2008;24:849-854. |
| 85. | Origa R, Barella S, Argiolas GM, Bina P, Agus A, Galanello R. No evidence of cardiac iron in 20 never- or minimally-transfused patients with thalassemia intermedia. Haematologica. 2008;93:1095-1096. |
| 86. | Roghi A, Cappellini MD, Wood JC, Musallam KM, Patrizia P, Fasulo MR, Cesaretti C, Taher AT. Absence of cardiac siderosis despite hepatic iron overload in Italian patients with thalassemia intermedia: an MRI T2* study. Ann Hematol. 2009;Epub ahead of print. |
| 87. | Vichinsky E. Hemoglobin e syndromes. Hematology Am Soc Hematol Educ Program. 2007;79-83. |
| 88. | Cohen AR, Galanello R, Pennell DJ, Cunningham MJ, Vichinsky E. Thalassemia. Hematology Am Soc Hematol Educ Program. 2004;14-34. |
| 89. | St Pierre TG, Clark PR, Chua-anusorn W, Fleming AJ, Jeffrey GP, Olynyk JK, Pootrakul P, Robins E, Lindeman R. Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood. 2005;105:855-861. |
| 90. | Fucharoen S, Viprakasit V. Hb H disease: clinical course and disease modifiers. Hematology Am Soc Hematol Educ Program. 2009;26-34. |
| 91. | Chui DH, Fucharoen S, Chan V. Hemoglobin H disease: not necessarily a benign disorder. Blood. 2003;101:791-800. |
| 92. | Chen FE, Ooi C, Ha SY, Cheung BM, Todd D, Liang R, Chan TK, Chan V. Genetic and clinical features of hemoglobin H disease in Chinese patients. N Engl J Med. 2000;343:544-550. |
| 93. | Ooi GC, Chen FE, Chan KN, Tsang KW, Wong YH, Liang R, Chan V, Ngan H. Qualitative and quantitative magnetic resonance imaging in haemoglobin H disease: screening for iron overload. Clin Radiol. 1999;54:98-102. |
| 94. | Au WY, Lam WW, Chu WW, Tam S, Wong WK, Lau J, Yeung YM, Liu HS, Liang R. Organ-specific hemosiderosis and functional correlation in Chinese patients with thalassemia intermedia and hemoglobin H disease. Ann Hematol. 2009;88:947-950. |
| 96. | Harmatz P, Butensky E, Quirolo K, Williams R, Ferrell L, Moyer T, Golden D, Neumayr L, Vichinsky E. Severity of iron overload in patients with sickle cell disease receiving chronic red blood cell transfusion therapy. Blood. 2000;96:76-79. |
| 97. | Fung EB, Harmatz P, Milet M, Ballas SK, De Castro L, Hagar W, Owen W, Olivieri N, Smith-Whitley K, Darbari D. Morbidity and mortality in chronically transfused subjects with thalassemia and sickle cell disease: A report from the multi-center study of iron overload. Am J Hematol. 2007;82:255-265. |
| 98. | Ballas SK. Iron overload is a determinant of morbidity and mortality in adult patients with sickle cell disease. Semin Hematol. 2001;38:30-36. |
| 99. | Hankins JS, McCarville MB, Loeffler RB, Smeltzer MP, Onciu M, Hoffer FA, Li CS, Wang WC, Ware RE, Hillenbrand CM. R2* magnetic resonance imaging of the liver in patients with iron overload. Blood. 2009;113:4853-4855. |
| 100. | Takatoku M, Uchiyama T, Okamoto S, Kanakura Y, Sawada K, Tomonaga M, Nakao S, Nakahata T, Harada M, Murate T. Retrospective nationwide survey of Japanese patients with transfusion-dependent MDS and aplastic anemia highlights the negative impact of iron overload on morbidity/mortality. Eur J Haematol. 2007;78:487-494. |
| 101. | Malcovati L, Porta MG, Pascutto C, Invernizzi R, Boni M, Travaglino E, Passamonti F, Arcaini L, Maffioli M, Bernasconi P. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J Clin Oncol. 2005;23:7594-7603. |
| 102. | Jensen PD, Jensen FT, Christensen T, Nielsen JL, Ellegaard J. Relationship between hepatocellular injury and transfusional iron overload prior to and during iron chelation with desferrioxamine: a study in adult patients with acquired anemias. Blood. 2003;101:91-96. |
| 103. | Schafer AI, Cheron RG, Dluhy R, Cooper B, Gleason RE, Soeldner JS, Bunn HF. Clinical consequences of acquired transfusional iron overload in adults. N Engl J Med. 1981;304:319-324. |
| 104. | Cazzola M, Barosi G, Gobbi PG, Invernizzi R, Riccardi A, Ascari E. Natural history of idiopathic refractory sideroblastic anemia. Blood. 1988;71:305-312. |
| 105. | Schafer AI, Rabinowe S, Le Boff MS, Bridges K, Cheron RG, Dluhy R. Long-term efficacy of deferoxamine iron chelation therapy in adults with acquired transfusional iron overload. Arch Intern Med. 1985;145:1217-1221. |
| 106. | Au W, Lam W, Chu W, Tam S, Wong W, Chan H, Law M, Liu H, Liang R. A pilot MRI study of organ specific hemosiderosis and functional correlation in Chinese patients with myelodysplasia and aplastic anemia with raised ferritin levels. Hematol Oncol. 2008;26:225-228. |
| 107. | Di Tucci AA, Matta G, Deplano S, Gabbas A, Depau C, Derudas D, Caocci G, Agus A, Angelucci E. Myocardial iron overload assessment by T2* magnetic resonance imaging in adult transfusion dependent patients with acquired anemias. Haematologica. 2008;93:1385-1388. |
| 108. | Konen E, Ghoti H, Goitein O, Winder A, Kushnir T, Eshet Y, Rachmilewitz E. No evidence for myocardial iron overload in multitransfused patients with myelodysplastic syndrome using cardiac magnetic resonance T2 technique. Am J Hematol. 2007;82:1013-1016. |
| 109. | Chacko J, Pennell DJ, Tanner MA, Hamblin TJ, Wonke B, Levy T, Thomas PW, Killick SB. Myocardial iron loading by magnetic resonance imaging T2* in good prognostic myelodysplastic syndrome patients on long-term blood transfusions. Br J Haematol. 2007;138:587-593. |
| 110. | Majhail NS, Lazarus HM, Burns LJ. Iron overload in hematopoietic cell transplantation. Bone Marrow Transplant. 2008;41:997-1003. |
| 111. | Altès A, Remacha AF, Sureda A, Martino R, Briones J, Canals C, Brunet S, Sierra J, Gimferrer E. Iron overload might increase transplant-related mortality in haematopoietic stem cell transplantation. Bone Marrow Transplant. 2002;29:987-989. |
| 112. | Socié G, Salooja N, Cohen A, Rovelli A, Carreras E, Locasciulli A, Korthof E, Weis J, Levy V, Tichelli A. Nonmalignant late effects after allogeneic stem cell transplantation. Blood. 2003;101:3373-3385. |
| 113. | Pullarkat V. Objectives of iron chelation therapy in myelodysplastic syndromes: more than meets the eye? Blood. 2009;114:5251-5255. |
| 114. | Pullarkat V, Blanchard S, Tegtmeier B, Dagis A, Patane K, Ito J, Forman SJ. Iron overload adversely affects outcome of allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2008;42:799-805. |
| 115. | Mahindra A, Bolwell B, Sobecks R, Rybicki L, Pohlman B, Dean R, Andresen S, Sweetenham J, Kalaycio M, Copelan E. Elevated pretransplant ferritin is associated with a lower incidence of chronic graft-versus-host disease and inferior survival after myeloablative allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2009;146:310-316. |
| 116. | Kataoka K, Nannya Y, Hangaishi A, Imai Y, Chiba S, Takahashi T, Kurokawa M. Influence of pretransplantation serum ferritin on nonrelapse mortality after myeloablative and nonmyeloablative allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15:195-204. |
| 117. | Armand P, Kim HT, Cutler CS, Ho VT, Koreth J, Alyea EP, Soiffer RJ, Antin JH. Prognostic impact of elevated pretransplantation serum ferritin in patients undergoing myeloablative stem cell transplantation. Blood. 2007;109:4586-4588. |
| 118. | McKay PJ, Murphy JA, Cameron S, Burnett AK, Campbell M, Tansey P, Franklin IM. Iron overload and liver dysfunction after allogeneic or autologous bone marrow transplantation. Bone Marrow Transplant. 1996;17:63-66. |
| 119. | Kornreich L, Horev G, Yaniv I, Stein J, Grunebaum M, Zaizov R. Iron overload following bone marrow transplantation in children: MR findings. Pediatr Radiol. 1997;27:869-872. |
| 120. | Au WY, Lam WM, Chu WC, Tam S, Wong WK, Pennell DJ, Lie AK, Liang R. A magnetic resonance imaging study of iron overload in hemopoietic stem cell transplant recipients with increased ferritin levels. Transplant Proc. 2007;39:3369-3374. |
| 121. | Majhail NS, DeFor T, Lazarus HM, Burns LJ. High prevalence of iron overload in adult allogeneic hematopoietic cell transplant survivors. Biol Blood Marrow Transplant. 2008;14:790-794. |
| 122. | Rose C, Ernst O, Hecquet B, Maboudou P, Renom P, Noel MP, Yakoub-Agha I, Bauters F, Jouet JP. Quantification by magnetic resonance imaging and liver consequences of post-transfusional iron overload alone in long term survivors after allogeneic hematopoietic stem cell transplantation (HSCT). Haematologica. 2007;92:850-853. |
| 123. | Lutz K, von Komorowski G, Dürken M, Engelhardt R, Dinter DJ. Myocardial iron overload in transfusion-dependent pediatric patients with acute leukemia. Pediatr Blood Cancer. 2008;51:691-693. |
| 124. | Emy PY, Levin TL, Sheth SS, Ruzal-Shapiro C, Garvin J, Berdon WE. Iron overload in reticuloendothelial systems of pediatric oncology patients who have undergone transfusions: MR observations. AJR Am J Roentgenol. 1997;168:1011-1015. |
| 125. | Jensen PD, Jensen FT, Christensen T, Ellegaard J. Non-invasive assessment of tissue iron overload in the liver by magnetic resonance imaging. Br J Haematol. 1994;87:171-184. |
| 126. | Bonkovsky HL, Rubin RB, Cable EE, Davidoff A, Rijcken TH, Stark DD. Hepatic iron concentration: noninvasive estimation by means of MR imaging techniques. Radiology. 1999;212:227-234. |
| 127. | Gandon Y, Guyader D, Heautot JF, Reda MI, Yaouanq J, Buhé T, Brissot P, Carsin M, Deugnier Y. Hemochromatosis: diagnosis and quantification of liver iron with gradient-echo MR imaging. Radiology. 1994;193:533-538. |
| 128. | Gandon Y, Olivié D, Guyader D, Aubé C, Oberti F, Sebille V, Deugnier Y. Non-invasive assessment of hepatic iron stores by MRI. Lancet. 2004;363:357-362. |
| 129. | Alústiza JM, Artetxe J, Castiella A, Agirre C, Emparanza JI, Otazua P, García-Bengoechea M, Barrio J, Mújica F, Recondo JA. MR quantification of hepatic iron concentration. Radiology. 2004;230:479-484. |
| 130. | Ernst O, Sergent G, Bonvarlet P, Canva-Delcambre V, Paris JC, L‘Herminé C. Hepatic iron overload: diagnosis and quantification with MR imaging. AJR Am J Roentgenol. 1997;168:1205-1208. |
| 131. | Kaltwasser JP, Gottschalk R, Schalk KP, Hartl W. Non-invasive quantitation of liver iron-overload by magnetic resonance imaging. Br J Haematol. 1990;74:360-363. |
| 132. | Lecube A, Hernández C, Simó R. Glucose abnormalities in non-alcoholic fatty liver disease and chronic hepatitis C virus infection: the role of iron overload. Diabetes Metab Res Rev. 2009;25:403-410. |
| 133. | Isom HC, McDevitt EI, Moon MS. Elevated hepatic iron: a confounding factor in chronic hepatitis C. Biochim Biophys Acta. 2009;1790:650-662. |
| 134. | Wallace DF, Subramaniam VN. Co-factors in liver disease: the role of HFE-related hereditary hemochromatosis and iron. Biochim Biophys Acta. 2009;1790:663-670. |
| 135. | Adams PC, Passmore L, Chakrabarti S, Reboussin DM, Acton RT, Barton JC, McLaren GD, Eckfeldt JH, Dawkins FW, Gordeuk VR. Liver diseases in the hemochromatosis and iron overload screening study. Clin Gastroenterol Hepatol. 2006;4:918-923; quiz 807. |
| 136. | Kohgo Y, Ikuta K, Ohtake T, Torimoto Y, Kato J. Iron overload and cofactors with special reference to alcohol, hepatitis C virus infection and steatosis/insulin resistance. World J Gastroenterol. 2007;13:4699-4706. |
| 137. | Ito K, Mitchell DG, Gabata T, Hann HW, Kim PN, Fujita T, Awaya H, Honjo K, Matsunaga N. Hepatocellular carcinoma: association with increased iron deposition in the cirrhotic liver at MR imaging. Radiology. 1999;212:235-240. |
| 138. | Kim MJ, Mitchell DG, Ito K, Kim JH, Pasqualin D, Rubin R. Hepatic iron deposition on magnetic resonance imaging: correlation with inflammatory activity. J Comput Assist Tomogr. 2002;26:988-993. |
| 139. | Kim MJ, Mitchell DG, Ito K, Hann HW, Park YN, Kim PN. Hepatic iron deposition on MR imaging in patients with chronic liver disease: correlation with serial serum ferritin concentration. Abdom Imaging. 2001;26:149-156. |
| 140. | Ito K, Mitchell DG, Hann HW, Outwater EK, Kim Y, Fujita T, Okazaki H, Honjo K, Matsunaga N. Progressive viral-induced cirrhosis: serial MR imaging findings and clinical correlation. Radiology. 1998;207:729-735. |
| 141. | Sassa S. Modern diagnosis and management of the porphyrias. Br J Haematol. 2006;135:281-292. |
| 142. | Dereure O, Jumez N, Bessis D, Gallix B, Guillot B. Measurement of liver iron content by magnetic resonance imaging in 20 patients with overt porphyria cutanea tarda before phlebotomy therapy: a prospective study. Acta Derm Venereol. 2008;88:341-345. |
| 143. | Tanner MA, He T, Westwood MA, Firmin DN, Pennell DJ. Multi-center validation of the transferability of the magnetic resonance T2* technique for the quantification of tissue iron. Haematologica. 2006;91:1388-1391. |
| 144. | Westwood MA, Firmin DN, Gildo M, Renzo G, Stathis G, Markissia K, Vasili B, Pennell DJ. Intercentre reproducibility of magnetic resonance T2* measurements of myocardial iron in thalassaemia. Int J Cardiovasc Imaging. 2005;21:531-538. |
| 145. | Westwood MA, Anderson LJ, Firmin DN, Gatehouse PD, Lorenz CH, Wonke B, Pennell DJ. Interscanner reproducibility of cardiovascular magnetic resonance T2* measurements of tissue iron in thalassemia. J Magn Reson Imaging. 2003;18:616-620. |