Published online Mar 28, 2010. doi: 10.3748/wjg.v16.i12.1495
Revised: January 19, 2010
Accepted: January 26, 2010
Published online: March 28, 2010
AIM: To establish a quantitative method to measure the amount of lipids.
METHODS: The livers of 53 male Wistar rats (225 g) with different degrees of hepatic steatosis were studied. This model of hepatic steatosis was based on a high carbohydrate, fat-free modified diet. Biopsies were classified into four grades depending on fat accumulation, using the Kleiner and Brunt classification. Total fat was studied by the Soxtec method (Soxtec™ 2050 Auto Fat Extraction System), and agreement between both assays was assessed by calculating the κ coefficient.
RESULTS: According to the histological classification, 38% of rats presented grade 0, 21% grade 1, 22% grade 2 and 20% grade 3. The amount of fat per 100 g tissue was 2.60 ± 0.64 g for grade 0, 3.87 ± 1.59 g for grade 1, 5.82 ± 1.37 g for grade 2 and 8.68 ± 2.30 g for grade 3. Statistically significant differences were found between the mean values for each of the histological grades (P < 0.05). The correlation for the quantification of fat in the liver between both assays was moderate (κ = 0.60).
CONCLUSION: The biochemical quantification of fat in liver tissue by the Soxtec method was correlated with the histological classification, although the agreement between the two tests was only moderate.
- Citation: Hijona E, Hijona L, Larzabal M, Sarasqueta C, Aldazabal P, Arenas J, Bujanda L. Biochemical determination of lipid content in hepatic steatosis by the Soxtec method. World J Gastroenterol 2010; 16(12): 1495-1499
- URL: https://www.wjgnet.com/1007-9327/full/v16/i12/1495.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i12.1495
Nonalcoholic fatty liver disease (NAFLD) covers a wide spectrum that ranges from fatty liver alone to steatohepatitis, fibrosis or cirrhosis. Fatty liver alone is the most common histological lesion in patients with NAFLD[1], and liver biopsy is the only diagnostic test that can reliably identify and quantify the degree of steatosis[2,3].
The gold standard for detecting steatosis is the histopathological analysis of a liver sample collected by biopsy. The biopsy procedure is invasive and painful and presents a risk for patients[4]. Actually, most hospitals use the histological method to determine the degree of steatosis. However, this method is subjective as it can vary, depending on the criteria for each pathologist. It is therefore necessary to develop a new method that is capable of determining the exact amount of fat in the liver.
Our aim was to compare a simple method for the isolation and purification of total lipids with the histological classification for quantifying fat deposits in a simple steatosis model in rats.
Male Wistar [CRL:Wi (Han)] rats (Charles River Laboratories) weighing approximately 225 g were studied. All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the United States Public Health Service. Animals were kept in separate cages with a regular 12-h light regime at a controlled temperature (25 ± 2°C). The modified diet (high carbohydrate, fat-free) consisted of carbohydrates (80%, as starch), protein (16%, as casein), and vitamins and minerals (4%) (PANLAB, Barcelona, Spain). The standard diet consisted of a balanced diet that contained carbohydrates (51%), protein (16%), vitamins and minerals (4%), and lipids (3%). The standard diet contained 2.9 kcal/g, and the modified diet 3.58 kcal/g. This model was based on that reported by Delzenne et al[5] and Bujanda et al[6].
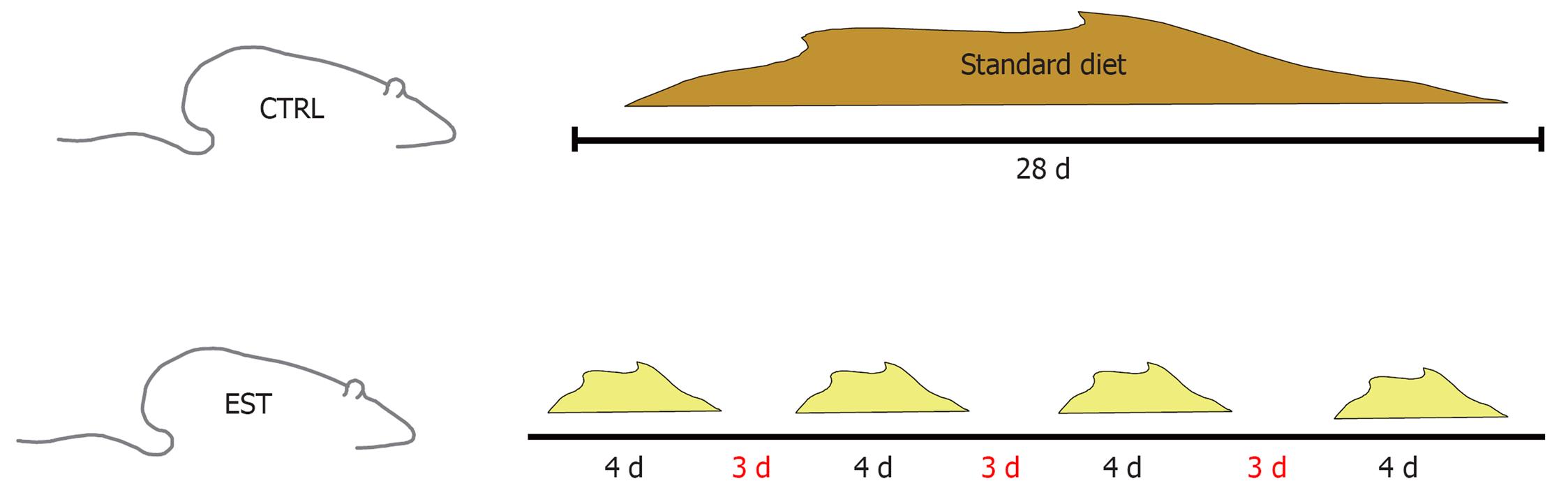
The rats were separated into two groups: control (20 rats) and steatosis (33 rats). The control group was given free access to food and drink and was fed a standard diet during 28 d. The steatosis group was given free access to food and water 4 d/wk and fasted for the remaining 3 d (only access to water was allowed). They were fed a modified diet during the dietary restriction cycles (Figure 1).
All rats were killed after completing four cycles of feeding and fasting, i.e. 28 d after study start. The amount of food taken by the rats and the weight of the animals were controlled. At the end of the study, all rats were anesthetized using isoflurane (Forane®). The animals were placed in an induction chamber with 5% isoflurane and administered 0.2 mL/200 g buprenorphine (Buprex®) as an analgesic. Once asleep, they were maintained with 3% isoflurane and 0.5-0.75 L oxygen.
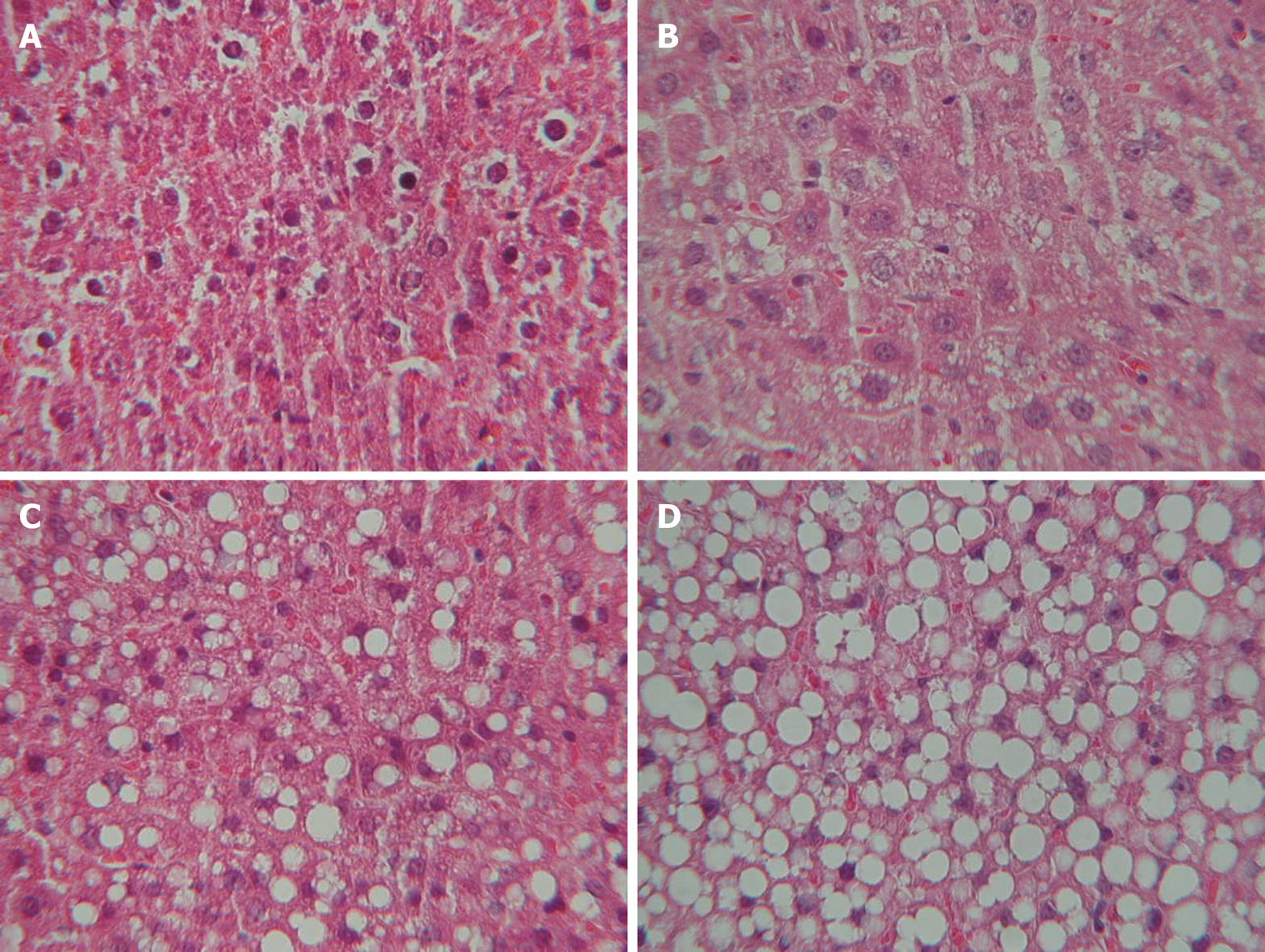
A histological study was performed following a midline laparotomy to remove the liver. The hepatic index was obtained by dividing the liver weight by rat weight and multiplying by 100. Liver tissue samples taken at the time the rat was killed were immediately placed in 10% buffered formalin and subsequently embedded in paraffin. Liver sections were stained with hematoxylin and eosin using standard techniques. The sections were viewed without prior knowledge of the treatment group to which each animal belonged. Biopsies were classified into four grades, depending on fat accumulation, using the classification proposed by Kleiner et al[2], whereby a patient is classified as grade 0 when < 5% of hepatocytes are affected by fat vacuoles, grade 1 when fat vacuoles are seen in 5%-33% of hepatocytes, grade 2 when 33%-66% of hepatocytes are affected by fat vacuoles, and grade 3 when fat vacuoles are found in > 66% of hepatocytes. Two experienced pathologists blinded to the experiment evaluated all samples, and agreement between both pathologists was determined. In case of discrepancy, the opinion of a third histopathologist reviewed the biopsies.
The Soxtec™ 2050 Auto Fat Extraction (Foss® Analytical, Hilleroed, Denmark) apparatus consisted of an extraction unit, a control unit and a drive unit. One gram of liver tissue was inserted into the extraction unit, solvent was added to the extraction cups in a closed system, and the cups were heated with an electric heating plate. The four-step extraction consisted of boiling, rinsing, solvent recovery and pre-drying. The results were calculated as total amount of fat (g) per 100 g tissue.
Samples were classified into four grades, according to the amount of fat detected in the steatosis group, using the data obtained for the control group and the maximum amount of fat detected in the steatosis rats as a reference.
Quantitative data were expressed as the mean ± SD for the different animals in each group, and comparisons were made using a two-sided Student’s t test. P < 0.05 was considered statistically significant. The agreement between biochemical assessments (grades) and histological steatosis grades (gold standard) was assessed by calculating the κ coefficient, which assessed how much better the agreement was than it would have been by chance alone (κ = 1 indicates perfect agreement; 0.80 < κ < 1, excellent; 0.60 < κ < 0.80, good; 0.40 < κ < 0.60, moderate; 0.20 < κ < 0.40, fair; 0 < κ < 0.20, poor agreement). Weighted κ coefficients were calculated for ordered categorical data, whereas simple κ values were determined for other data.
Rat weight increased significantly in the control group (221 ± 10 to 355 ± 16 g), but remained similar in the group with fatty liver disease (222 ± 12 to 226 ± 14 g). The rats in the control group ate 31.11 ± 10.1 g/d and rats in the steatosis group ate 14.78 ± 3.2 g/d. The hepatic index was 4.47 ± 0.63 and 3.9 ± 0.31 in the steatosis and control groups, respectively.
No fatty infiltration was seen in the control group (Figure 1). Eleven rats (34%) in the steatosis group presented histological grade 1, 12 (36%) grade 2, and 10 (30%) grade 3. Inter-observer agreement was 0.89 and intra-observer agreement 0.92 (Figure 2).
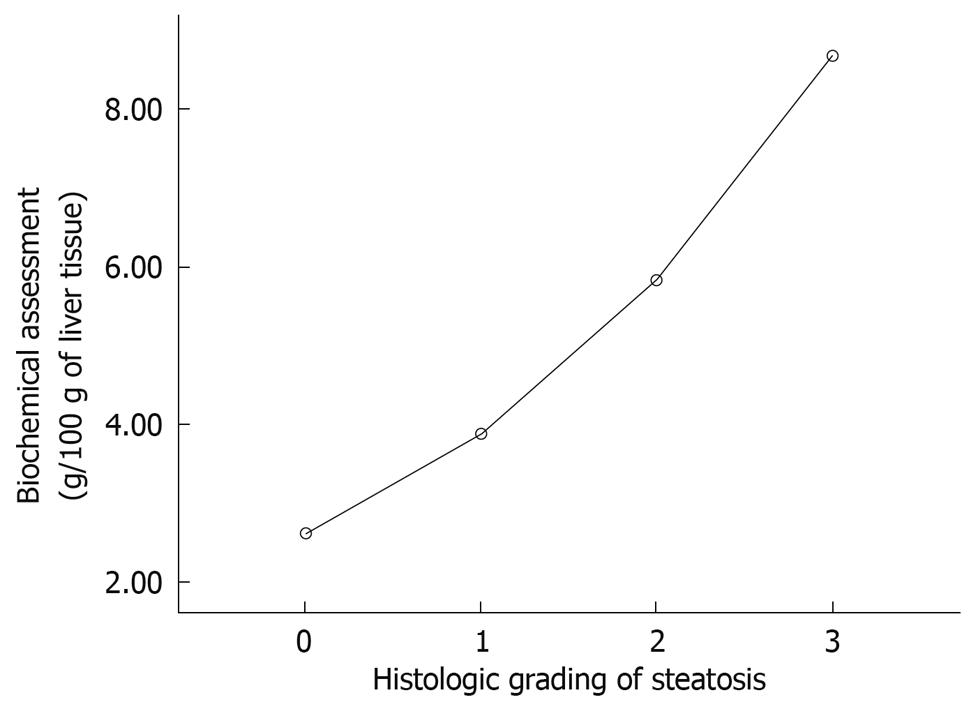
The amount of fat per 100 g tissue was 2.60 ± 0.64 g for histological grade 0, 3.87 ± 1.59 g for grade 1, 5.82 ± 1.37 g for grade 2, and 8.68 ± 2.30 g for grade 3. Statistically significant differences were found between the mean values for each of the histological grades (P < 0.05), except between grades 0 and 1 (Figure 2). The following biochemical classification was proposed on the basis of the amount of fat observed in the control group (2.6 g/100 g tissue) and the maximum amounts of fat observed in the steatosis group (11.16 g/100 g tissue). If we group the rats for each histological grade and the average and SD we obtain the cut-off. The animals were classified as grade 0, when the amount of fat per 100 g tissue was < 2.6 g; grade 1, when the amount of fat was 2.6-5 g; grade 2, when the amount of fat was 5.1-8 g; and grade 3, when the amount of fat was > 8.1 g (Figure 3). The κ correlation between the histological and biochemical classifications was calculated to be 0.6 (moderate).
The amount of total fat determined by the Soxtec method correlates well with the Kleiner and Brunt histological classification[2,3]. Quantifying fat biochemically is a more objective and accurate method of determining the fat content in liver tissue. In the Soxtec method, the crude fat is extracted from the liver by the Randall method[7], a two-step extraction procedure that reduces fat-extraction times to < 1 h per sample. This method for fat extraction has previously been used for meat and meat products and is accepted as an Association of Analytical Communities Official Method of Analysis (991.36)[8]. It is recommended for Official First Action. This method is now automated and allows several samples to be analyzed simultaneously and rapidly (< 1 h). The approximate cost per sample is low, at around 22 euros. The Soxtec method is easier to perform than the classical biochemical method described by Folch in 1957 and is automated, which in our opinion, means it is the method of choice.
The diagnostic gold standard in another cumulative liver disease, namely hemochromatosis, is to determine the amount of iron present in a tissue sample obtained by liver biopsy. Other techniques, such as magnetic resonance imaging (MRI), which allow iron to be determined without the need for a liver biopsy, have been established based on this gold standard. In light of this, we are of the opinion that the best method for determining the amount of fat deposited in the liver is the biochemical method described herein, and that all other techniques, especially histological classification, are less accurate.
One of the main limitations of this biochemical technique, which is also inherent to histological classification, is that the amount of fat detected depends on the region where the biopsy is taken, which means that incorrect results can be obtained in cases of localized steatosis. Another limitation of biochemical determination is that it does not provide any information regarding other types of lesion that may be present, such as fibrosis or inflammation. The main limitations of histological classification include the subjective evaluation of the tissue sample by a pathologist, which can lead to incorrect results in the case of localized hepatic steatosis, and the higher cost with respect to biochemical analysis. The main advantage of histological classification with respect to biochemical analysis is that it provides information regarding other types of liver lesions. Despite its subjective nature, the majority of studies concerning the histological classification of fat in the liver have reported a good (κ = 0.60-0.80) or excellent (κ > 0.80) inter- and intra-individual correlation[2,9]. Indeed, the correlation between the pathologists in our hospital was excellent (κ = 0.89). Another limitation for both tests is that both techniques (Soxtec method and histological) are invasive and therefore need a liver biopsy.
Several noninvasive imaging techniques, including ultrasonography, computed tomography, and MRI, can identify hepatic steatosis and have been advocated as diagnostic tests for NAFLD[10-15] but sometimes cannot distinguish between simple steatosis and steatohepatitis, or stage degree of fibrosis accurately[16]. However, the gold standard for diagnosing hepatic steatosis is still liver biopsy, and the most objective technique is biochemical determination.
In the not too distant future, we must be able to diagnose steatosis, without using invasive methods. Today, for the diagnosis, liver biopsy is used, but it would be very interesting to be able to perform diagnosis using noninvasive techniques. For example, determining the amount of liver fat by magnetic resonance techniques. It would be very useful to perform a predictive test, but for this, we need much more research about the interaction of different factors, molecules and genes[17]. The identification of the molecular mechanism that leads to fat accumulation and oxidative imbalance in steatotic liver, as well as genome and proteome studies from patients at various stages of the disease, is expected to improve the diagnostic and therapeutic approaches. In this way, attractive pharmacological designs include new molecules that can decrease lipid levels in the liver and improve insulin sensitivity.
In summary, the biochemical determination of fat in liver tissue by the Soxtec method is the most objective and direct technique available and should therefore be considered the gold standard for this assay. The results obtained by this method complement those obtained from histological studies. Further studies are needed to confirm our findings and their application in humans.
The prevalence of nonalcoholic fatty liver disease is high, and histological classification is currently considered to be the gold standard for quantifying fat deposits in the liver.
Histological analysis is the gold standard to determine the amount of fat in the liver. However, this method is subjective because it can vary depending on the pathologist’s criteria. We therefore propose a new biochemical method, the Soxtec method, to determine exactly the amount of fat in the liver.
This is the first study to report the comparison of histological and biochemical (Soxtec) methods in steatosis. This report highlights the importance of the new method to determine exactly the fat content.
Being able to determine the exact amount of fat is important because it can be very useful in the treatment of these patients. This represents a future therapeutic intervention.
The authors have proposed a novel biochemical method for evaluating hepatic steatosis. Its reliability was assessed compared with that of histopathological analysis. This study is interesting and significant, although more clinical and laboratory data are needed to determine whether the Soxtec method is practically available.
Peer reviewer: Munechika Enjoji, MD, PhD, Department of Clinical Pharmacology, Fukuoka University, 8-17-1 Nanakuma, Jonan-ku, Fukuoka 814-0180, Japan
S- Editor Wang YR L- Editor Kerr C E- Editor Lin YP
| 1. | Gholam PM, Flancbaum L, Machan JT, Charney DA, Kotler DP. Nonalcoholic fatty liver disease in severely obese subjects. Am J Gastroenterol. 2007;102:399-408. |
| 2. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. |
| 3. | Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467-2474. |
| 4. | d'Assignies G, Ruel M, Khiat A, Lepanto L, Chagnon M, Kauffmann C, Tang A, Gaboury L, Boulanger Y. Noninvasive quantitation of human liver steatosis using magnetic resonance and bioassay methods. Eur Radiol. 2009;19:2033-2040. |
| 5. | Delzenne NM, Hernaux NA, Taper HS. A new model of acute liver steatosis induced in rats by fasting followed by refeeding a high carbohydrate-fat free diet. Biochemical and morphological analysis. J Hepatol. 1997;26:880-885. |
| 6. | Bujanda L, Hijona E, Larzabal M, Beraza M, Aldazabal P, García-Urkia N, Sarasqueta C, Cosme A, Irastorza B, González A. Resveratrol inhibits nonalcoholic fatty liver disease in rats. BMC Gastroenterol. 2008;8:40. |
| 7. | Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497-509. |
| 8. | Thiex NJ, Anderson S, Gildemeister B. Crude fat, diethyl ether extraction, in feed, cereal grain, and forage (Randall/Soxtec/submersion method): collaborative study. J AOAC Int. 2003;86:888-898. |
| 9. | Vuppalanchi R, Cummings OW, Saxena R, Ulbright TM, Martis N, Jones DR, Bansal N, Chalasani N. Relationship among histologic, radiologic, and biochemical assessments of hepatic steatosis: a study of human liver samples. J Clin Gastroenterol. 2007;41:206-210. |
| 10. | Schwenzer NF, Springer F, Schraml C, Stefan N, Machann J, Schick F. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol. 2009;51:433-445. |
| 11. | Browning JD. New imaging techniques for non-alcoholic steatohepatitis. Clin Liver Dis. 2009;13:607-619. |
| 12. | Myers RP. Noninvasive diagnosis of nonalcoholic fatty liver disease. Ann Hepatol. 2009;8 Suppl 1:S25-S33. |
| 13. | Reeder SB, Robson PM, Yu H, Shimakawa A, Hines CD, McKenzie CA, Brittain JH. Quantification of hepatic steatosis with MRI: the effects of accurate fat spectral modeling. J Magn Reson Imaging. 2009;29:1332-1339. |
| 14. | Hamaguchi M, Kojima T, Itoh Y, Harano Y, Fujii K, Nakajima T, Kato T, Takeda N, Okuda J, Ida K. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol. 2007;102:2708-2715. |
| 15. | Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, Mullen KD, Cooper JN, Sheridan MJ. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745-750. |
| 16. | Mehta SR, Thomas EL, Bell JD, Johnston DG, Taylor-Robinson SD. Non-invasive means of measuring hepatic fat content. World J Gastroenterol. 2008;14:3476-3483. |
| 17. | Bell LN, Theodorakis JL, Vuppalanchi R, Saxena R, Bemis KG, Wang M, Chalasani N. Serum proteomics and biomarker discovery across the spectrum of nonalcoholic fatty liver disease. Hepatology. 2010;51:111-120. |