Published online Mar 7, 2009. doi: 10.3748/wjg.15.1141
Revised: February 1, 2009
Accepted: February 8, 2009
Published online: March 7, 2009
Here, we report a case of intrapancreatic accessory spleen confirmed by pathologic diagnosis and discuss its differential diagnosis and surgical management with a review of the literature.
- Citation: Guo W, Han W, Liu J, Jin L, Li JS, Zhang ZT, Wang Y. Intrapancreatic accessory spleen: A case report and review of the literature. World J Gastroenterol 2009; 15(9): 1141-1143
- URL: https://www.wjgnet.com/1007-9327/full/v15/i9/1141.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.1141
Accessory spleen, a relatively common congenital defect, found in 10%-30% of patients at autopsy, is due to the fusion failure of the splenic anlage, which is located in the dorsal mesogastrium[1–3]. The splenic hilus is the most common site of an accessory spleen followed by pancreatic tail. When an accessory spleen is located in the pancreas, it may mimic a hypervascular pancreatic tumor. Because an accessory spleen does not usually require treatment, accurate preoperative diagnosis is important. Here, we report an intrapancreatic accessory spleen and discuss its differential diagnosis and surgical management.
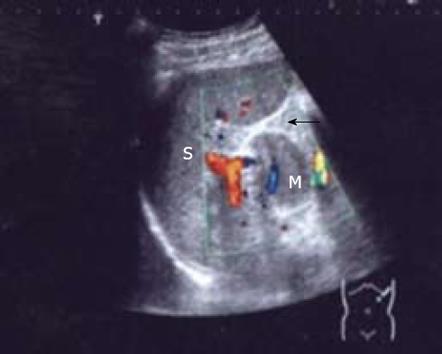
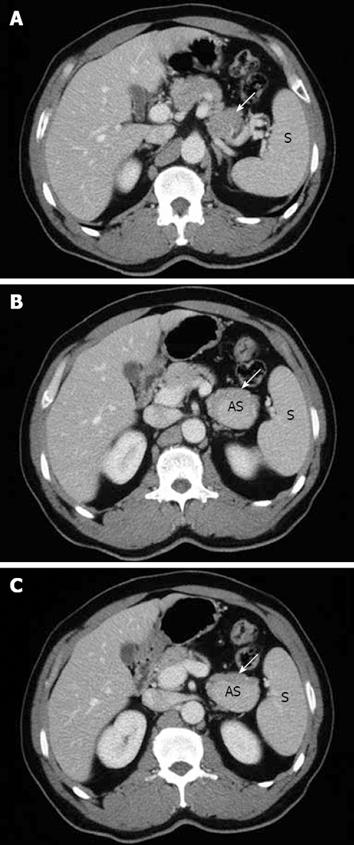
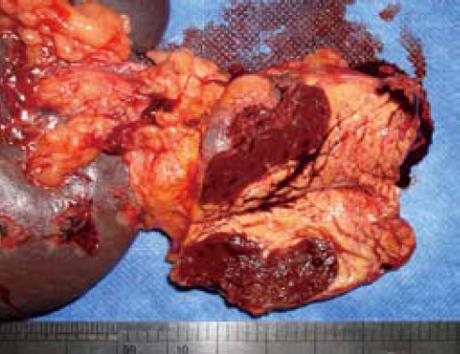
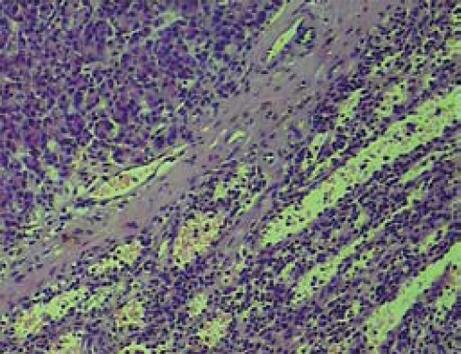
A 51-year-old man without any abdominal discomfort or complaints had a health examination in our hospital in January 2008. Physical examination and laboratory data including peripheral blood counts, blood sugar and liver function tests were all unremarkable. Tumor markers, including CA19-9, CA125, carcino-embryonic antigen (CEA) and alpha-fetoprotein (AFP), were within the normal range. Abdominal sonography was performed, and a well-defined 4.2 cm × 5.2 cm focal lesion was noted in pancreatic tail. The echogenicity of the lesion was homogeneous and lower than that of the pancreatic parenchyma. On color Doppler sonography images, blood echo was observed in the lesion (Figure 1). Computed tomography (CT) confirmed the 4.0 cm × 5.1 cm mass in pancreatic tail (Figure 2). A nonfunctioning islet cell tumor or a solid pseudopapillary neoplasm of pancreatic tail was suspected on January 14, 2008, a laparotomic exploration was arranged to treat the lesion. After anesthesia, a left subcostal incision was performed and the well-defined mass was palpated in pancreatic tail. Because it was very close to the splenic hilus, excision of pancreatic tail and spleen was performed (Figure 3). An accessory spleen was confirmed by pathologic diagnosis (Figure 4).
Accessory spleen is a congenital focus of healthy splenic tissue that is separated from the main body of spleen[1]. It results from the fusion failure of splenic anlage, which is located in the dorsal mesogastrium to fuse[23]. Accessory spleen, a relatively common congenital defect, is seen in 10%-30% of patients at autopsy[124]. Splenic hilus is the most common site of an accessory spleen followed by pancreatic tail. In an autopsy study of 3000 patients, 61 of 364 (17%) accessory spleens identified were found in pancreatic tail[5]. Although an accessory spleen usually appears as an isolated asymptomatic abnormality, it may have clinical significance in some situations. When an accessory spleen is located in the pancreas, it may mimic an islet cell tumor or a pseudopapillary neoplasm[6–8]. Because an accessory spleen does not usually require treatment, accurate preoperative diagnosis is important.
At present, the main preoperative diagnostic technique for intrapancreatic accessory spleen is radiological. On baseline sonography, intrapancreatic accessory spleens are well defined and round, ovoid or lobulated. The echogenicity of most intrapancreatic accessory spleens is low compared with pancreatic parenchyma. In all intrapancreatic accessory spleens, the echogenicity is homogeneous and identical to that of the main spleen. On color or power Doppler sonography images, blood supply to intrapancreatic accessory spleens from splenic artery or vein can be seen in some patients.
In recent years, contrast-enhanced ultrasound has been more frequently used to diagnose spleen abnormalities. Levovist is an ultrasound contrast agent containing air microbubbles. In the vascular phase, it increases signal intensities in both grey-scale and Doppler modes. In the delayed phase, also known as “hepatosplenic phase”, microbubbles are trapped almost exclusively by the hepatic and splenic parenchyma. By increasing the acoustic pressure, the trapped microbubbles are disrupted and produce a non-correlated Doppler signal, which can be visualized as a strong transient enhancement. This method is also known as sonoscintigraphy, loss of correlation, stimulated acoustic emission and transient scattering[9]. Kim et al[10] used this technique to study 6 patients with accessory spleen. In the vascular phase, the vascular pedicle was clearly visualized in 3 patients, including a patient with a suspected vascular pedicle on color Doppler sonography images. In the arterial phase, there was an inhomogeneous enhancement in 3 patients and a homogeneous enhancement in the other three patients. In all the 6 patients, the intrapancreatic accessory spleen became homogeneous in the portal phase, showing a dense and persistent enhancement for 3-5 min. In comparison with pancreatic parenchyma, the intrapancreatic accessory spleen appeared to be hyperechoic during all dynamic sonography phases. The echo enhancement of all intrapancreatic accessory spleens, however, was identical to that of the spleen in all phases.
Mortelé et al[11] performed abdominal CT scans on 1000 consecutive patients. Of these patients, 156 (15.6%) had at least one accessory spleen, and 21 of these patients (13%) had more than one accessory spleen, with a maximum of three accessory spleens per patient, resulting in a total of 180 accessory spleens. Their anteroposterior diameter ranged 4-29 mm, with a mean of 11.9 mm. Their transverse diameter ranged 4-25 mm, with a mean of 11.6 mm. All accessory spleens were well defined, round in 141 patients (78.3%), ovoid in 27 (15%) and triangular in 12 (6.7%). The location of accessory spleens was variable. Most accessory spleens were located at the hilus of spleen. Intrapancreatic accessory spleens were seen in 2 patients. The findings suggest that most accessory spleens have a characteristic appearance on CT, and are well-defined, round masses that are smaller than 2 cm in diameter. Homogeneous enhancement on contrast-enhanced images is another important feature. However, in these case series, 32% of the accessory spleens were hypodense compared with the main spleen. Because all the accessory spleens were smaller than 1 cm in diameter, their attenuation may have been caused by partial volume effects. It is likely that, when thinner collimation (e.g. ≤ 5 mm) is used, accessory spleens appear similar to the spleen.
The most specific imaging method for diagnosing ectopic splenic tissue is nuclear scintigraphy using technetium-99m-labeled sulfur colloid or 99mTc-labeled heat-damaged RBCs. However, this technique offers far inferior anatomic resolution to CT or MRI, increasing likelihood of misdiagnosis[7].
RES-specific contrast media are particles, such as superparamagnetic iron oxides, that are phagocytosed by the reticuloendothelial system (liver, spleen, lymph nodes, and bone marrow). Resovist-enhanced imaging can show an uptake of the contrast agent in normal splenic parenchyma because of its endothelial and Kupffer’s cells. Negative enhancement or loss of signal intensity of normal splenic parenchyma is delineated using T2-weighted images because of the more effective T2 shortening in these sequences[12].
Endoscopic ultrasound (EUS)-guided fine-needle aspiration (FNA) biopsy is a very sensitive test, particularly for the evaluation of pancreatic lesions. Schreiner[13] and his colleagues reported three cases of intrapancreatic accessory spleen diagnosed by EUS-guided FNA, which revealed predominantly small lymphocytes with a subset of histiocytes, conspicuous eosinophils, and plasma cells. There was also characteristic CD8 positive immunostaining of endothelial cells in cell block sections.
In conclusion, when an asymptomatic intra-pancreatic mass is detected, the possibility of an accessory spleen should be considered. Although some radiological techniques are not widely used in clinics, well-defined round masses in pancreas should be considered accessory spleens, especially when its contrast-enhanced images are similar to those of the splenic parenchyma during all dynamic phases.
| 1. | Freeman JL, Jafri SZ, Roberts JL, Mezwa DG, Shirkhoda A. CT of congenital and acquired abnormalities of the spleen. Radiographics. 1993;13:597-610. |
| 2. | Dodds WJ, Taylor AJ, Erickson SJ, Stewart ET, Lawson TL. Radiologic imaging of splenic anomalies. AJR Am J Roentgenol. 1990;155:805-810. |
| 3. | Chin S, Isomoto H, Mizuta Y, Wen CY, Shikuwa S, Kohno S. Enlarged accessory spleen presenting stomach submucosal tumor. World J Gastroenterol. 2007;13:1752-1754. |
| 4. | Gayer G, Zissin R, Apter S, Atar E, Portnoy O, Itzchak Y. CT findings in congenital anomalies of the spleen. Br J Radiol. 2001;74:767-772. |
| 5. | Halpert B, Gyorkey F. Lesions observed in accessory spleens of 311 patients. Am J Clin Pathol. 1959;32:165-168. |
| 6. | Hamada T, Isaji S, Mizuno S, Tabata M, Yamagiwa K, Yokoi H, Uemoto S. Laparoscopic spleen-preserving pancreatic tail resection for an intrapancreatic accessory spleen mimicking a nonfunctioning endocrine tumor: report of a case. Surg Today. 2004;34:878-881. |
| 8. | Harris GN, Kase DJ, Bradnock H, Mckinley MJ. Accessory spleen causing a mass in the tail of the pancreas: MR imaging findings. AJR Am J Roentgenol. 1994;163:1120-1121. |
| 9. | Calliada F, Campani R, Bottinelli O, Bozzini A, Sommaruga MG. Ultrasound contrast agents: basic principles. Eur J Radiol. 1998;27 Suppl 2:S157-S160. |
| 10. | Kim SH, Lee JM, Lee JY, Han JK, Choi BI. Contrast-enhanced sonography of intrapancreatic accessory spleen in six patients. AJR Am J Roentgenol. 2007;188:422-428. |
| 11. | Mortelé KJ, Mortelé B, Silverman SG. CT features of the accessory spleen. AJR Am J Roentgenol. 2004;183:1653-1657. |
| 12. | Boraschi P, Donati F, Volpi A, Campori G. On the AJR viewbox. Intrapancreatic accessory spleen: diagnosis with RES-specific contrast-enhanced MRI. AJR Am J Roentgenol. 2005;184:1712-1713. |
| 13. | Schreiner AM, Mansoor A, Faigel DO, Morgan TK. Intrapancreatic accessory spleen: mimic of pancreatic endocrine tumor diagnosed by endoscopic ultrasound-guided fine-needle aspiration biopsy. Diagn Cytopathol. 2008;36:262-265. |