Published online Mar 7, 2009. doi: 10.3748/wjg.15.1126
Revised: January 22, 2009
Accepted: January 29, 2009
Published online: March 7, 2009
Pruritis with abnormal liver function tests is the classical presentation of intrahepatic cholestasis of pregnancy (ICP), a condition associated with significant fetal complications. Although the etiology of ICP is unclear in many cases, certain features of the clinical presentation should alert the practitioner to the possibility of an underlying metabolic defect, which may not only affect subsequent pregnancies, but may be an indicator of more serious subsequent liver disease. We report a kindred of Anglo-Celtic descent, among whom many members present with ICP, gallstones or cholestasis related to use of oral contraception. Genetic studies revealed a novel mutation in the ABCB4 gene, which codes for a phospholipid transport protein. The clinical significance of this mutation and the importance of identifying such patients are discussed.
- Citation: Hardikar W, Kansal S, Elferink RPJO, Angus P. Intrahepatic cholestasis of pregnancy: When should you look further? World J Gastroenterol 2009; 15(9): 1126-1129
- URL: https://www.wjgnet.com/1007-9327/full/v15/i9/1126.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.1126
Intrahepatic cholestasis of pregnancy (ICP) is characterized by pruritis, jaundice and raised serum bile salts, which typically starting in the third trimester of pregnancy, resolve after delivery and recur in subsequent pregnancies[1]. It is associated with an increased incidence of fetal distress, premature delivery and stillbirth[2].
ICP is thought to be caused by abnormal biliary transport, which may result from a number of factors, including hormonal, environmental and genetic. Recently it has been recognized that up to 15% of ICP may be associated with mutations in the MDR3 (ABCB4) gene[1]. The ATP binding cassette subfamily B, member 4 (ABCB4) gene codes for a protein responsible for the translocation of phosphatidylcholine (PC) from the inner to the outer leaflet of the canalicular membrane of the hepatocyte[3]. It is now becoming increasingly clear that disruption in the ABCB4 gene can present a spectrum of clinical disorders ranging from ICP and low-phospholipid-associated cholestasis (LPAC), to progressive familial intrahepatic cholestasis type III (PFIC III), depending on the location of the new mutation[3].
LPAC is a condition characterized by gallstones, high serum gamma glutamyl transferase (GGT), intrahepatic microlithiasis and recurrent biliary symptoms despite cholecystectomy[4], while PFIC type III is characterized by chronic cholestasis that presents early in life, which often progresses to end-stage liver disease that requires liver transplantation[3].
We report a kindred of Anglo-Celtic descent, who present with features of ICP and gallstones (LPAC), and have been found to have a novel mutation in the MDR3 gene. The clinical aspects of the presentation that prompted further investigation are discussed.
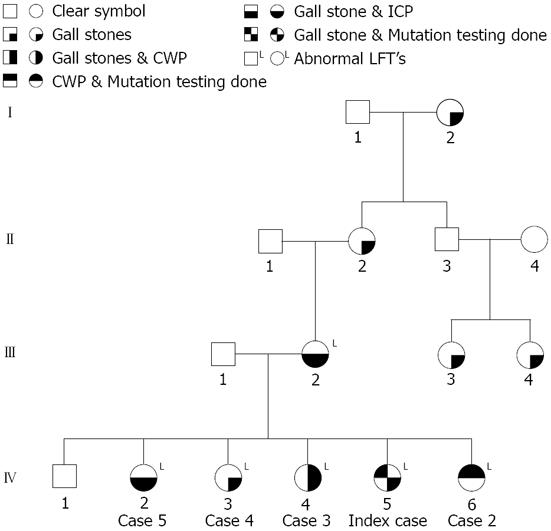
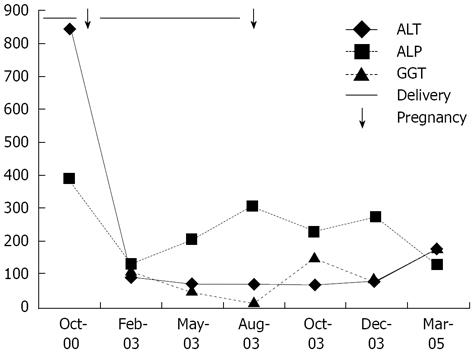
The family (Figure 1) came to medical attention when the index case (case 1) was referred with persistently abnormal liver function tests following her second pregnancy. She was a 33-year-old healthy G2P2 who had pruritus, but no jaundice during her first pregnancy, and recovered. During the second pregnancy, she again developed pruritus at 32 wk gestation, associated with abnormal liver function tests (Figure 2). She received ursodeoxycholic acid supplements, but with only mild relief, and abnormalities in her liver function tests persisted. She delivered at 37 wk through induced labor because of worsening jaundice and gestational diabetes. Various investigations including serum ceruloplasmin, serum bile salts, alpha-1 antitrypsin assays and viral serology for hepatitis viruses were performed, and were all normal. Further questioning revealed several members of the family with similar symptoms (Table 1).
| Index case | Case II | Case III | Case IV | Case V | Mother | |
| Age of start of cholestasis | 30 | 30 | 17 | 28 | 28 | NA |
| Cholestasis with contraceptive pill | _ | + | - | - | - | NA |
| Cholestasis with pregnancy 1 | + | NA | + | - | + | + |
| Pregnancy 2 | + | NA | + | - | + | + |
| Pregnancy 3 | NA | NA | NA | NA | NA | + |
| Pregnancy 4 | NA | NA | NA | NA | NA | + |
| Pregnancy 5 | NA | NA | NA | NA | NA | + |
| Pregnancy 6 | NA | NA | NA | NA | NA | + |
| Gallstones | + | + | + | + | - | + |
| Cholecystectomy | + | + | + | + | - | + |
| Abnormal LFTs | + | + | + | + | + | + |
| Improvement with ursodeoxycholic acid | + | NA | NA | NA | NA | NA |
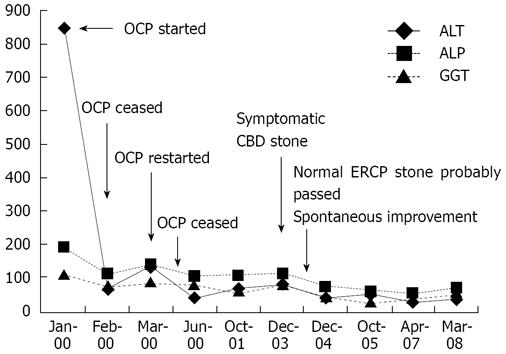
Case II was a 30-year-old, single, healthy, woman who developed pruritus and abnormal liver function tests after she commenced taking oral contraceptives (Figure 3). These symptoms improved after discontinuation of contraceptives; however, they recurred when she re-commenced the contraceptives. She had undergone a cholecystectomy at the age of 26 years following a prolonged history of episodic abdominal pain. Extensive investigations including viral serology, serum ceruloplasmin and copper levels, bile salt and alpha 1 antitrypsin assays for other causes of hepatitis were all normal. She was found to have a stone in her common bile duct upon ultrasound examination; but, subsequent endoscopic retrograde cholangiopancreatography was normal. Her liver function tests started improving spontaneously, but never normalized.
Subsequently, a third sister (case III) was referred for persistently abnormal liver biochemistry that was detected incidentally during routine screening of liver biochemistry while she was taking anti-convulsant drugs for post-traumatic epilepsy. She had a cholecystectomy at the age of 17 years for gallstones. However, 4 years later, she had a severe head injury following which she developed post- traumatic epilepsy. She was treated with sodium valproate and her abnormal liver function tests were initially attributed to this drug. However, drug level monitoring revealed her anti-convulsants to be well within the therapeutic range. Further investigations including viral serology, copper studies and abdominal ultrasound were negative. She also had a liver biopsy that showed very mild chronic lobular inflammation.
Case IV had a cholecystectomy for gallstones associated with persistently abnormal liver function tests, and case V had cholestasis during both her pregnancies. Their mother had cholecystectomy for gallstones and also cholestasis during all her pregnancies.
Interestingly, their brother had normal liver function tests and was asymptomatic. A maternal grandmother had gallstones, but had not undergone cholecystectomy, and a maternal great grandmother died of a secondary liver cancer, the nature of which was uncertain. Two maternal cousins also had gallstones. There was no history suggestive of cholestatic disorders on their father’s side. There was also a history of miscarriages in the family; the mother having had two and the index case and one of the sisters having had one each. The children of all the sisters are below 10 years of age and have not yet shown any features suggestive of cholestatic liver disease.
DNA of the index cases I and II was analyzed at the Academic Medical Centre, Amsterdam. Both the sisters were found to be positive for an 1102T > A mutation that converts phenylalanine at position 368 into isoleucine. This phenylalanine is highly conserved and the mutation has not been found in 150 control alleles. The mutation is located between the sixth transmembrane helix and the ATP-binding cassette, and is, therefore, likely to be of functional significance.
Pruritus associated with abnormal liver function tests and raised bile acids during pregnancy are the classical presentation of ICP. As this syndrome carries a risk of premature delivery and sudden intrauterine fetal death, most practitioners commence ursodeoxycholic acid, which reduces pruritus, transaminases and probably prematurity, without adverse side effects[15]. If the pregnancy proceeds uneventfully, many practitioners do not pursue further follow-up or investigation to look for a cause of the ICP.
However, it is now apparent that, a significant minority of patients with ICP will have underlying MDR3 mutations, which may predispose to further high-risk pregnancies and possibly serious liver disease later in life[3]. The percentage of patients with ICP caused by MDR3 mutations varies in different populations. In a recent Italian study, 7/96 (7.2%) women with ICP had MDR3 mutations identified while other studies have reported up to 15%[1].
The current report highlights a number features in the history of a patient with presumed ICP, which should have alerted the practitioner to the possibility of an underlying mutation and the need for further investigation. Clues that could be readily obtained from the history included a cholestatic reaction to oral contraception, ICP in previous pregnancies and a strong family history of gallstones under the age of 40 years, and recurrence of symptoms after cholecystectomy[4].
In our pedigree, the index case presented with typical ICP symptoms. However, it soon became apparent from the family history that most members had a history consistent with ICP during one or more pregnancies, and that all but one had gallstones at a young age. Whilst a high GGT level is observed in 30% patients with ICP, its presence increases the likelihood of an underlying MDR3 mutation[6]. Importantly, the first clue to the diagnosis and the reason for referral of our index case was that her obstetrician was concerned when GGT levels did not return to normal in the post-partum period.
The MDR3 (ABCB4 transporter) is responsible for the translocation of PC from the inner to the outer leaflet of the canalicular membrane of hepatocytes. PC is extracted from the membrane by bile salts and then mixes with bile salts to form mixed micelles. These mixed micelles are known to solubilize cholesterol more efficiently than simple bile-salt micelles. On the other hand, the mixed micelles extract phospholipids less well from the membrane than simple bile-salt micelles and this protects the cells lining the biliary tree from membrane solubilization[3]. The rate of phospholipid secretion is an important factor in the prevention of gallstone formation and partial defects in phospholipid secretion may predispose to gallstone formation[4].
Mutations in MDR3 in ICP have been described in multiple exons[7]. The mutation described in this report adds to an expanding group of mutations that have been shown to cause familial cholestatic syndromes including ICP. However, there is still little understanding of why and how various mutations in the MDR3 gene produce different clinical syndromes.
Factors that occur during pregnancy, which might lead to the development of cholestasis in previously asymptomatic individuals with these disorders, include generalized impairment of bile formation in the third trimester[8] and the effects of sex hormones that are known to promote cholestasis, possibly by inhibition of the bile salt export pump. The administration of exogenous progesterone in the third trimester may also precipitate ICP.
This kindred is especially interesting as different members have presented with various features of the clinical spectrum of ABCB4 transporter defects over many years, before the significance of the family history was finally recognized. This highlights the importance of obtaining a family history in all patients with ICP. Even if a positive family history is not obtained, patients who present with abnormal liver tests during pregnancy should have follow-up testing to ensure the abnormality resolves post-partum. The possibility of a bile acid transport defect should be considered in all patients who have a family history of ICP or other symptoms suggestive of a familial cholestatic disorder. Of note, patients presenting with gallstones in young adulthood (under the age of 40 years) should be evaluated further, particularly if there is a suspicious family history. Early detection of such patients should ensure that there is adequate monitoring of subsequent pregnancies, early treatment with ursodeoxycholic acid, and that there is ongoing follow-up to detect and prevent the development of significant liver disease.
| 1. | Hay JE. Liver disease in pregnancy. Hepatology. 2008;47:1067-1076. |
| 2. | Paus TC, Schneider G, Van De Vondel P, Sauerbruch T, Reichel C. Diagnosis and therapy of intrahepatic cholestasis of pregnancy. Z Gastroenterol. 2004;42:623-628. |
| 3. | Oude Elferink RP, Paulusma CC. Function and pathophysiological importance of ABCB4 (MDR3 P-glycoprotein). Pflugers Arch. 2007;453:601-610. |
| 4. | Rosmorduc O, Poupon R. Low phospholipid associated cholelithiasis: association with mutation in the MDR3/ABCB4 gene. Orphanet J Rare Dis. 2007;2:29. |
| 5. | Mazzella G, Rizzo N, Azzaroli F, Simoni P, Bovicelli L, Miracolo A, Simonazzi G, Colecchia A, Nigro G, Mwangemi C. Ursodeoxycholic acid administration in patients with cholestasis of pregnancy: effects on primary bile acids in babies and mothers. Hepatology. 2001;33:504-508. |
| 6. | Milkiewicz P, Gallagher R, Chambers J, Eggington E, Weaver J, Elias E. Obstetric cholestasis with elevated gamma glutamyl transpeptidase: incidence, presentation and treatment. J Gastroenterol Hepatol. 2003;18:1283-1286. |
| 7. | Floreani A, Carderi I, Paternoster D, Soardo G, Azzaroli F, Esposito W, Montagnani M, Marchesoni D, Variola A, Rosa Rizzotto E. Hepatobiliary phospholipid transporter ABCB4, MDR3 gene variants in a large cohort of Italian women with intrahepatic cholestasis of pregnancy. Dig Liver Dis. 2008;40:366-370. |
| 8. | Pusl T, Beuers U. Intrahepatic cholestasis of pregnancy. Orphanet J Rare Dis. 2007;2:26. |