Published online Mar 7, 2009. doi: 10.3748/wjg.15.1072
Revised: January 19, 2009
Accepted: January 26, 2009
Published online: March 7, 2009
AIM: To investigate the inhibitory effects of RNA interference (RNAi) on expression of matrix metalloproteinase-2 (MMP-2) gene and invasiveness and adhesion of human pancreatic cancer cell line, BxPC-3.
METHODS: RNAi was performed using the vector (pGPU6)-based small interference RNA (siRNA) plasmid gene silence system to specifically knock down MMP-2 expression in pancreatic cancer cell line, BxPC-3. Four groups of different specific target sequence in coding region of MMP-2 and one non-specific sequence were chosen to construct four experimental siRNA plasmids of pGPU6-1, pGPU6-2, pGPU6-3 and pGPU6-4, and one negative control siRNA plasmid of pGPU6 (-). MMP-2 expression was measured by reverse transcription polymerase chain reaction (RT-PCR) and Western blot. Cell proliferation and apoptosis were examined by methyl thiazolyl tetrazolium (MTT) and flow cytometry, respectively. The abilities of adhesion and invasion were detected by cell adhesion assay and cell invasion assay using Transwell chambers.
RESULTS: The expression of MMP-2 was inhibited and the inhibitory effects of different sequence varied. pGPU6-1 group had the most efficient inhibitory effect, followed by pGPU6-2 and pGPU6-3 groups. Invasiveness and adhesion were more significantly reduced in pGPU6-1, pGPU6-2 and pGPU6-3 groups as compared with pGPU6 (-) and blank control groups. However, no difference concerning cell proliferation and apoptosis was observed after transfection between experiment groups and control groups.
CONCLUSION: RNAi against MMP-2 successfully inhibited the mRNA and protein expression of MMP-2 in the pancreatic cancer cell line, BxPC-3, leading to a potent suppression of tumor cell adhesion and invasion without affecting cell proliferation and apoptosis. These findings suggest that the RNAi approach towards MMP-2 may be an effective therapeutic strategy for the clinical management of pancreatic tumor.
-
Citation: Zhi YH, Song MM, Wang PL, Zhang T, Yin ZY. Suppression of matrix metalloproteinase-2
via RNA interference inhibits pancreatic carcinoma cell invasiveness and adhesion. World J Gastroenterol 2009; 15(9): 1072-1078 - URL: https://www.wjgnet.com/1007-9327/full/v15/i9/1072.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.1072
Pancreatic cancer is one of the most aggressive common tumors, most patients die within months as a result of rapid local spread of the tumor or metastatic dissemination[1]. The very poor prognosis may in part be attributed to the high invasive potential of this malignancy, and the invasion or metastasis of pancreatic cancer has been known to be a complex process involving many molecular mechanisms, of which proteolytic degradation of extracellular matrix (ECM) exerted by matrix metalloproteinases (MMPs) was considered to be an essential step[2]. Some data suggest that MMP-2 is involved in pancreatic cancer invasion and metastasis, and a high level of MMP-2 has been found to correlate with poor prognosis in patients with pancreatic cancer[3]. Therefore, inhibition of MMP-2 may be of great value in both preventing pancreatic cancer and blocking metastasis of established tumors.
RNA interference (RNAi) is a conserved biologic response to double-stranded RNA that results in the sequence-specific silencing of target gene expression. As a kind of highly efficient, specific and relatively stable tool, RNAi technology has already been used to silence specific target gene expression[4].
In this report, we used a vector-based MMP-2 siRNA expression system to suppress the expression of MMP-2 in pancreatic cancer cell line BxPC-3 and to evaluate its efficacy in the adhesion and invasion of pancreatic cancer cell. We found that specific down-regulation of MMP-2 by RNAi successfully inhibited the mRNA and protein expression of MMP-2, leading to significant inhibition of adhesion and invasion of pancreatic cancer cells without affecting cell proliferation and apoptosis. Thus, RNAi towards MMP-2 may be an effective therapeutic strategy for the treatment of patients with pancreatic cancer.
Human pancreatic cancer cell line BxPC-3, obtained from Shanghai Institute of Biochemistry and Cell Biology, were maintained in Dulbecco Modified Eagle Medium (DMEM) containing 10% fetal calf serum (FCS; Hyclone Co., Ltd.) and were incubated in a humidified (37°C, 5% CO2) incubator, grown in 75-cm2 culture flasks and passaged upon reaching 80% confluence.
We used the pGPU6 siRNA plasmid vector-based gene silence system to produce stable transfection, plasmid vector pGPU6 was purchased from Shanghai GenePharma Co., Ltd. Aiming at the sequence of MMP-2, four DNA chains with the following sense and antisense sequences were synthesized: no. 1, 5'-GGAGAGCTGCAACCTGTTTGT-3' (sense) and 5'-ACAAACAGGTTGCAGCTCTCC-3' (antisense); MMP-2 siRNA no. 2, 5'-GCTCCACCACCTACAACTTTG-3' (sense) and 5'-CAAAGTTGTAGGTGGTGGAGC-3' (antisense); MMP-2 siRNA no. 3, 5'-GCAAACAGGACATTGTATTTG-3' (sense) and 5'-CAAATACAATGTCCTGTTTGC-3' (antisense); MMP-2 siRNA no. 4, 5'-GGAGATACAATGAGGTGAAGA-3' (sense) and 5'-TCTTCACCTCATTGTATCTCC-3' (antisense). The target sequence of negative control group which is named pGPU6 (-) is 5'-GTTCTCCGAACGTGTCACGT-3' (sense) and 5'-ACGTGACACGTTCGGAGAAT-3' (antisense), which has no homology with that of human beings or mice. The cancer cells without any plasmid were defined as blank control group. All DNA chains were designed and synthesized by Shanghai GenePharma Co., Ltd., Shanghai, China. We contrived the structure of the DNA chains to be BamHI + sense chain + loop + antisense chain + termination signal + EcoRI + HindIII. The four DNA chains were annealed and ligated into (BamHI/EcoRI) sites of pGPU6 to generate the plasmid pGPU6/ MMP-2. The negative control plasmid pGPU6 (-) was constructed using the same procedure. As a result, four experimental groups of plasmids pGPU6-1, pGPU6-2, pGPU6-3, pGPU6-4, and negative control group plasmid pGPU6 (-) were generated. The plasmids were extracted and the accuracy of the constructs was confirmed by sequencing.
BxPC-3 cells were seeded in a 24-well culture plate and divided into blank control group, pGPU6 (-) group and positive experimental groups (pGPU6-1, pGPU6-2, pGPU6-3 pGPU6-4). Each group contained 3 culture wells. 2 × 105 cells were plated into each culture well 24 h before transfection, and cultured in a humidified (37°C, 5% CO2) incubator. BxPC-3 cells were stably transfected with pGPU6-1, pGPU6-2, pGPU6-3, pGPU6-4, or pGPU6 (-) in the presence of Lipofectamine 2000 (Invitrogen Co., Ltd.) following the manufacturer’s instructions. No plasmid was introduced in the blank control plates; only Lipofectamine 2000 was used for the transfection in the blank control group. The cells were transfected with plasmid DNA (2 &mgr;g) and transfection reagent (4 &mgr;L) at a DNA: reagent ratio of 1:2, and then incubated at 37°C in a CO2 incubator for 24 h prior to testing for gene expression.
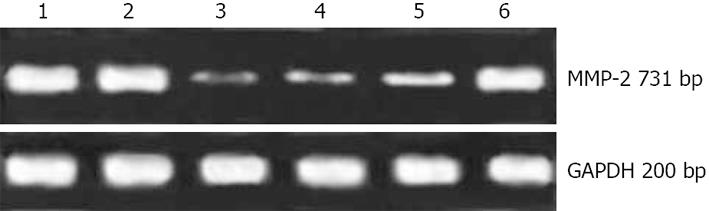
Twenty-four hours after the transfection, 5×105 cells were collected and total RNA was extracted using the Trizol reagent (Invitrogen Co., Ltd.) following the manufacturer’s instructions. The concentration and purity of the total RNA were detected with ultraviolet spectrophotometer. Glutaraldehyde-3-phosphate dehydrogenase (GAPDH) was used as internal control. The primer sequences for the genes and expected product sizes were as follows: 5'-TGATCTTGACCAGAATACCA-3' (sense), 5'-TGCCATACTTCTTGTCGCGGT-3' (antisense) for MMP-2 (731 bp), 5'-CCATGGAGAAGGCCGGGG-3' (sense), 5'-CAAAGTTGTCATGGATGACC-3' (antisense) for GAPDH (200 bp). The RT reaction was performed at 25°C for 10 min, then 37°C for 60 min. PCR amplification was performed under the following reaction conditions: 94°C for 30 s, 55°C (MMP-2) or 53°C (GAPDH) for 30 s, 72°C for 1 min, and a final extension at 72°C for 7 min. The amplification used 28 cycles for MMP-2, and 26 cycles for GAPDH. PCR products were analyzed by electrophoresis on 1% agarose gel and were visualized by ethidium bromide staining under ultraviolet light. The expression intensity of MMP-2 was denoted with the ratio of the photodensity of the RT-PCR products of MMP-2 to GAPDH. The inhibition ratio of MMP-2 expression was calculated with the following formula: inhibition ratio of MMP-2 expression = (1-the expression intensity of MMP-2 in the experiment group/the expression intensity of MMP-2 in the blank or negative control group) × 100%.
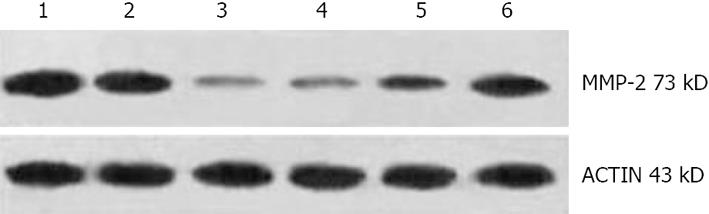
Cells were collected 24 h after transfection, washed twice with phosphate buffered solution (PBS), and the supernatant was scraped off. Cell pellets were then lysed in iced bath for 30 min. The lysates were transferred to new tubes and centrifuged at 12 000 r/min for 30 min at 4°C. Proteins were separated by 8% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and transferred to a hybond enhanced chemiluminescence (ECL) nitrocellulose membrane (Amersham Pharmacia Biotech, Germany). The membranes were blocked for 1 h in 3% bovine serum albumin (BSA) in PBS and then incubated with monoclonal antibodies recognizing MMP-2 or actin (Santa Cruz, USA). Second antibody incubations were carried out using peroxidase-conjugated goat anti-rabbit antibody, and reactive bands were detected by chemiluminescence. The expression levels of MMP-2 and actin protein were quantified by densitometry. The signal strength of each MMP-2 signal was normalized against the corresponding actin control. The inhibition ratio of MMP-2 expression was calculated with the following formula: inhibition ratio of MMP-2 expression = (1-the relative intensity of MMP-2 expression in the experiment group/the relative intensity of MMP-2 in the blank or negative control group) × 100%.
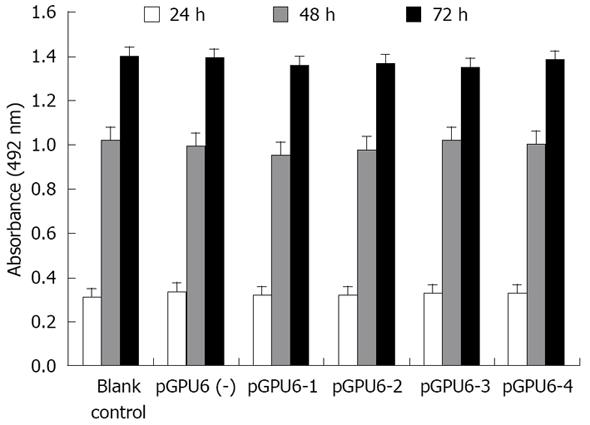
Cell proliferation was assessed by using the MTT assay. Cells were collected 24 h after transfection, pancreatic cancer cells were plated at 1 × 104 cells/well in 96-well plates in DMEM containing 10% FBS. Five duplicate wells were set up for each group. Blank control cells served as control. After 24, 48 and 72 h of incubation, 200 &mgr;L of 5 mg/&mgr;L MTT solution (Sigma Co., Ltd.) in PBS was added to each well for 4 h. Absorbance of each well was measured on a microplate reader at a wave length of 492 nm.
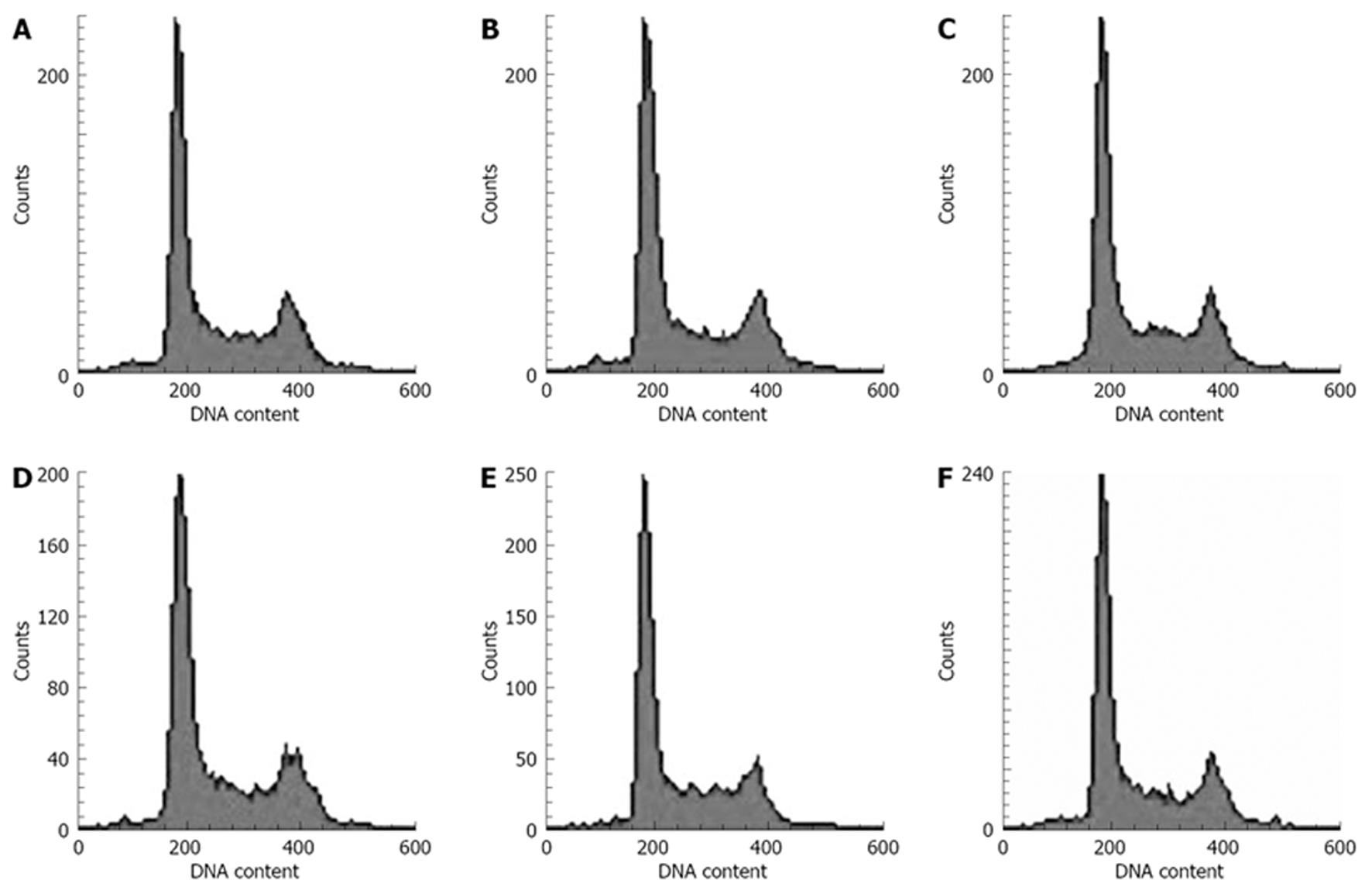
Flow cytometry was used to estimate the apoptosis,. The harvested cells were washed with PBS, fixed with cold 75% ethanol at -20°C for 24 h, treated with 0.1 mL RNase A (Sigma Co., Ltd.) and then stained with propidium iodide (Sigma Co., Ltd.). Finally, cell cycle analysis was carried out using flow cytometer.
Cells were collected 24 h after transfection. The cancer cells were trypsinized and seeded at 1 × 104 cells/well in 96-well plates. Five duplicate wells were set up for each group. The cells were cultured for 60 min, and washed twice with PBS to remove the cells not adherent. The MTT assay as above was performed to assess A value of the adhesive cells. The inhibition ratio was calculated with the following formula: inhibition ratio = (1-A value of the experiment group/A value of the control group) × 100%.
Cell invasion assay was performed using Transwell chambers (Corning Co., Ltd.). After 24 h transfection, 5 × 105 cells were suspended in 100 &mgr;L serum-free medium and placed into the upper compartment of the Transwell chambers. The lower compartment of the chambers was filled with 200 &mgr;L serum-containing medium and the cells were allowed to migrate for 24 h. After a 24-h incubation, cells on the lower surface of the filter were fixed in cold ethanol and stained with 0.5% crystal violet (CV) for 30 min, and 5 random fields were counted at 200 × magnification. Data represent the average cells of 5 fields were compared between the experimental groups and control group.
All results were expressed as mean ± SD. All statistical analyses were performed using one-way ANOVA. P < 0.05 was considered significant.
Four MMP-2 siRNA–expressing plasmids (pGPU6-1, pGPU6-2, pGPU6-3 and pGPU6-4) and one negative plasmid pGPU6 (-) were constructed using the pGPU6 vectors. Twenty-four hours after the transfection, we observed significant inhibition of MMP-2 mRNA expression in the experimental groups (pGPU6-1, pGPU6-2 and pGPU6-3) compared with blank control and pGPU6 (-) group (P < 0.05) whereas the slight inhibition was observed in pGPU6-4 cells (P > 0.05) (Table 1). The inhibition ratio was 75.3% (pGPU6-1), 64.5% (pGPU6-2), 51.6% (pGPU6-3) with respect to blank control and 74.7% (pGPU6-1), 63.7% (pGPU6-2), 50.5% (pGPU6-3) compared to pGPU6 (-). The transfection with pGPU6 (-) had no significant inhibitory effect on the expression of MMP-2 mRNA compared to the blank group (P > 0.05) (Figure 1).
The levels of MMP-2 protein in the total cell lysates were assessed by Western blot. Western blot analyses using the anti-MMP-2 antibody revealed significant decreases in MMP-2 expression after transfection with siRNA in positive experimental groups (pGPU6-1, 2, 3) compared with blank control and pGPU6 (-) group (P < 0.05). However, no change in MMP-2 expression was observed after transfection with siRNA in pGPU6-4 and pGPU6 (-) groups when compared with the blank control (P > 0.05) (Table 2). Quantitative analysis of MMP-2 protein by densitometry revealed a decrease in protein expression with pGPU6-1 by 79.1%, pGPU6-2 by 64%, pGPU6-3 by 52.3% compared with blank control group and pGPU6-1 by 78%, pGPU6-2 by 62.2% and pGPU6-3 by 50.5% compared with pGPU6 (-) group (Figure 2).
To address whether siRNA directed against MMP-2 has an inhibitory effect on pancreatic cancer cell proliferation, cell proliferation was assessed using the MTT assay. We found that treatment of pancreatic cancer with MMP-2 siRNA did not cause any significant inhibitory effect in tumor cell proliferation. And the pGPU6 (-) group did not significantly decrease tumor cell proliferation compared with blank control group (P > 0.05) (Figure 3).
The effects of MMP-2 siRNA molecules on the induction of apoptosis in pancreatic cancer cells were inspected by flow cytometry. However, no discrepancy was observed after transfection with siRNA in positive experimental groups (pGPU6-1, 2, 3, 4) compared with blank control and pGPU6 (-) group (P > 0.05). Besides, the pGPU6 (-) group did not significantly increase cell apoptosis compared with blank control group (P > 0.05) (Figure 4).
Cell adhesion assay revealed significant decreases in cancer cell adhesion after transfection with siRNA in experimental groups (pGPU6-1, 2, 3) compared with blank control and pGPU6 (-) group (P < 0.05). However, no change was observed after transfection with siRNA in pGPU6-4 and pGPU6 (-) groups when compared with the blank control (P > 0.05) (Table 3). The inhibition ratio were 63.1% (pGPU6-1), 42.9% (pGPU6-2) and 25.6% (pGPU6-3) compared with the blank control, and 62.2% (pGPU6-1), 41.6% (pGPU6-2) and 23.9% (pGPU6-3) compared with pGPU6 (-) group.
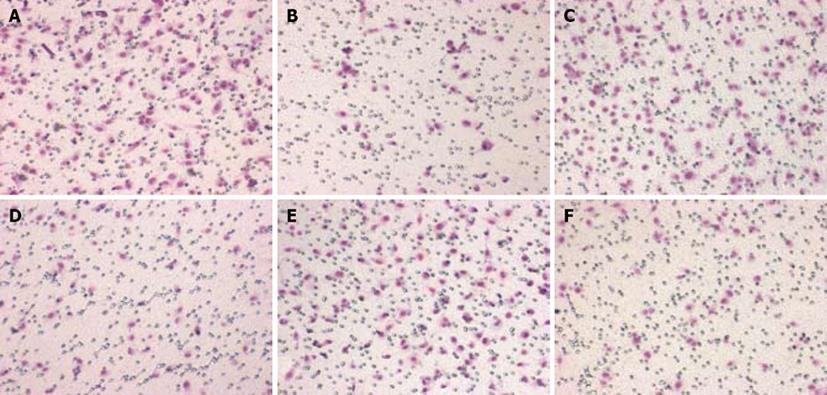
Cell invasion was assessed using Transwell chambers. As shown in Table 4, for each 200 × field under microscope, the average migrated cell number of 5 fields of experimental groups (pGPU6-1, 2, 3) were observed to be significantly lower than the number of blank control and the pGPU6 (-) groups (P < 0.05), which was not found in the pGPU6-4 group. In addition, there was little difference between blank control and the pGPU6 (-) groups (P > 0.05) (Figure 5).
MMPs are a group of enzymes, which degrade the macromolecules of connective tissue, ECM and basement membrane. These enzymes are believed to play important roles in tumor metastasis, invasion and angiogenesis[5–7]. As a subgroup of MMPs, MMP-2 seems to play an important role in the progression of pancreatic cancer. Bramhall et al[8] found MMP-2 messenger RNA was the most commonly expressed MMP in pancreatic tumor specimens (93%), but was not seen in normal pancreas. Apparently, MMPs, particularly MMP-2 play an important role in the pathogenesis of pancreatic cancer. Consequently, a tumor therapy targeting MMP-2 would be particularly efficacious in the treatment of pancreatic cancer. As a very potent tool, RNAi technology can generate a cellular knockdown of a desired gene utilizing a plasmid-based system that stably expresses siRNA molecules to target specific mRNAs for degradation[910]. In this study, we developed a siRNA sequence, when stably integrated into cellular DNA, which can selectively target MMP-2 expression. Our study demonstrated that RNAi against MMP-2 successfully inhibited the mRNA and protein expression of MMP-2 in the pancreatic cancer cell line BxPC-3. In contrast, MMP-2 expression was unchanged in the control groups. By using a stably integrating plasmid to express our siRNA molecule, we obtained at the most a 75% mRNA reduction and a 79% protein reduction in MMP-2 expression. Some studies are similar to our findings, which also draw a conclusion that MMP-2 mRNA and protein levels can be significantly inhibited by RNAi in solid tumors[1112].
On the basis of significant inhibitory effects of MMP-2 mRNA and protein, we investigated the effects of MMP-2 siRNA on tumor adhesion and invasion. We found that the MMP-2 siRNA not only suppressed the adhesion of the cancer cell, but also their ability to migrate and invade. Similar to our findings, Sun et al[13] also found that MMP-2 silencing by RNAi could inhibit invasion and growth of laryngeal cancer, and MMP-2 might be a potential target for gene therapy in laryngeal cancer. Moreover, a study in vivo showed that tumors implanted in MMP-2-deficient mice had decreased invasive properties, which further suggest that MMP-2 is important for pancreatic cancer invasion[14].
The mechanism of MMP-2 involvement in the tumor invasion and metastasis is complex, some studies suggest that MMP-2 is associated with the degradation of type IV collagen which is the main component of basement membranes. Furthermore, Kim et al[15] found that one additional mechanism by which MMP-2 was proposed to increase the local invasiveness was to facilitate angiogenesis. Interestingly, we got the suppressing effects of tumor cell adhesion and invasion by RNAi targeting the MMP-2 gene, but we did not find any significant change in tumor cell proliferation and apoptosis between the experimental groups and the control group. Some studies support our data showing that down-regulation of MMP-2 can inhibit cell invasion without affecting cell proliferation[1617]. Based on these findings, we hypothesize that the ability of the pancreatic tumor cell adhesion and migration, but not the quantity of tumor cell proliferation, is the crucial factor for MMP-2 involved in pancreatic tumor cell invasion and metastasis. Some findings agreed with our hypothesis, which also observed a decrease in pancreatic cancer cell invasion through a reconstituted matrix in a dose dependent fashion without affecting cell proliferation in vitro[1819]. Matrix metalloproteinases are enzymes responsible for extracellular matrix degradation, a critical component influencing metastatic potential of cancer. When endothelial cell MMP-2 gene is silenced, cell growth cycle will change correspondingly, which will be blocked in G1 stage. This may explain why MMP-2 gene affects only the ability of pancreatic cancer cells to modify the extracellular matrix to facilitate invasion and growth without affecting cell proliferation.
At time of diagnosis, 75%-85% of patients with pancreatic cancer can not accept resectable operation and conventional therapies which virtually are ineffective[20]. Therefore, there is clearly a need for new approaches to the treatment of this cancer. The over-expression of MMP-2 in pancreatic tumor is a mark of poor prognosis with respect to disease progression as well as survival[21]. Our data has provided evidence that RNAi against MMP-2 successfully inhibited the mRNA and protein expression of MMP-2 in the pancreatic cancer cell line BxPC-3, leading to a potent suppression of tumor cell adhesion and invasion without affecting cell proliferation and apoptosis. These findings suggest that the RNAi approach towards MMP-2 may be an effective therapeutic strategy for the clinical management of pancreatic tumor. Although the leap to clinical practice remains elusive, gene therapy targeting MMP-2 is attractive and warrants further investigations.
Pancreatic carcinoma is an aggressive malignancy with an extremely poor prognosis. Most patients with pancreatic carcinoma have extremely poor prognosis, and the reason may in part be attributed to the high invasive potential of this malignancy leading to early metastasis. However, either invasion or metastasis of pancreatic carcinoma has been known to be a complex process involving molecular mechanisms. Activation of matrix metalloproteinase-2 (MMP-2) has been implicated in the progression, invasion, and metastasis of various cancers, but little information is available with regard to its role in pancreatic carcinoma with poor prognosis.
MMP-2 has an activity to degrade type IV collagen and is associated with invasion angiogenesis of malignant tumors. It seems that MMP-2 plays an important role in the progression of pancreatic carcinoma. In the area of pancreatic carcinoma gene therapy, one of the research hotspots is how to down-regulate MMP-2. As a kind of highly efficient, specific and relatively stable tool, RNA interference technology has already been used to silence specific target gene expression. Thus, RNA interference towards MMP-2 may be an effective therapeutic strategy for the treatment of patients with pancreatic cancer.
A gene silencing system using the vector (pGPU6)-based small interference RNA (siRNA) plasmid has been established to specifically knock down MMP-2 expression in pancreatic cancer cells. MMP-2 expression was measured by reverse transcription polymerase chain reaction (RT-PCR) and Western blot. Cell proliferation and apoptosis were examined by MTT and flow cytometry, respectively. The abilities of adhesion and invasion were detected by cell adhesion assay and cell invasion assay using Transwell chambers. RNA interference against MMP-2 successfully inhibited the mRNA and protein expression of MMP-2 in the pancreatic cancer cell line, BxPC-3, leading to a potent suppression of tumor cell adhesion and invasion without affecting cell proliferation and apoptosis.
RNA interference towards MMP-2 may be an effective therapeutic strategy for the clinical management of pancreatic tumors. Although the leap to clinical practice remains elusive, gene therapy targeting MMP-2 is attractive and warrants further investigations.
The MMPs are a family of zinc-dependent endopeptidases. Their primary function is degradation of proteins in the extracellular matrix. RNA interference is a process of post-transcriptional gene silencing in which double-stranded RNA inhibits gene expression in a sequence dependent manner via degradation of the corresponding mRNA.
This is an interesting study. The invasiveness is usually related to cell growth. The manuscript is well written, but it needs explanation of the discrepancy in the study.
| 1. | Warshaw AL, Fernández-del Castillo C. Pancreatic carcinoma. N Engl J Med. 1992;326:455-465. |
| 2. | Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst. 1997;89:1260-1270. |
| 3. | Ellenrieder V, Alber B, Lacher U, Hendler SF, Menke A, Boeck W, Wagner M, Wilda M, Friess H, Büchler M. Role of MT-MMPs and MMP-2 in pancreatic cancer progression. Int J Cancer. 2000;85:14-20. |
| 5. | Davies B, Waxman J, Wasan H, Abel P, Williams G, Krausz T, Neal D, Thomas D, Hanby A, Balkwill F. Levels of matrix metalloproteases in bladder cancer correlate with tumor grade and invasion. Cancer Res. 1993;53:5365-5369. |
| 6. | Duffy MJ. The role of proteolytic enzymes in cancer invasion and metastasis. Clin Exp Metastasis. 1992;10:145-155. |
| 7. | Liotta LA, Tryggvason K, Garbisa S, Hart I, Foltz CM, Shafie S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. 1980;67-68. |
| 8. | Bramhall SR, Neoptolemos JP, Stamp GW, Lemoine NR. Imbalance of expression of matrix metalloproteinases (MMPs) and tissue inhibitors of the matrix metalloproteinases (TIMPs) in human pancreatic carcinoma. J Pathol. 1997;182:347-355. |
| 9. | Blackburn JS, Rhodes CH, Coon CI, Brinckerhoff CE. RNA interference inhibition of matrix metalloproteinase-1 prevents melanoma metastasis by reducing tumor collagenase activity and angiogenesis. Cancer Res. 2007;67:10849-10858. |
| 10. | Yuan J, Dutton CM, Scully SP. RNAi mediated MMP-1 silencing inhibits human chondrosarcoma invasion. J Orthop Res. 2005;23:1467-1474. |
| 11. | Tsung AJ, Kargiotis O, Chetty C, Lakka SS, Gujrati M, Spomar DG, Dinh DH, Rao JS. Downregulation of matrix metalloproteinase-2 (MMP-2) utilizing adenovirus-mediated transfer of small interfering RNA (siRNA) in a novel spinal metastatic melanoma model. Int J Oncol. 2008;32:557-564. |
| 12. | Wang A, Zhang B, Huang H, Zhang L, Zeng D, Tao Q, Wang J, Pan C. Suppression of local invasion of ameloblastoma by inhibition of matrix metalloproteinase-2 in vitro. BMC Cancer. 2008;8:182. |
| 13. | Sun YN, Yang BF, Liu M, Guo YL, Tian LL, Jiao H. [The experimental investigation of the invasion and growth of laryngeal cancer by matrix metalloproteinase-2 and matrix metalloproteinase-9 gene silence together]. Zhonghua Yixue Zazhi. 2008;88:36-39. |
| 14. | Itoh T, Tanioka M, Yoshida H, Yoshioka T, Nishimoto H, Itohara S. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58:1048-1051. |
| 15. | Kim YM, Jang JW, Lee OH, Yeon J, Choi EY, Kim KW, Lee ST, Kwon YG. Endostatin inhibits endothelial and tumor cellular invasion by blocking the activation and catalytic activity of matrix metalloproteinase. Cancer Res. 2000;60:5410-5413. |
| 16. | Hu XH, Fan L, Ruan CG. [Function of matrix metalloprotenase-2 by RNA interference]. Zhongguo Shiyan Xueyexue Zazhi. 2008;16:381-386. |
| 17. | Canel M, Secades P, Garzón-Arango M, Allonca E, Suarez C, Serrels A, Frame M, Brunton V, Chiara MD. Involvement of focal adhesion kinase in cellular invasion of head and neck squamous cell carcinomas via regulation of MMP-2 expression. Br J Cancer. 2008;98:1274-1284. |
| 18. | Jimenez RE, Hartwig W, Antoniu BA, Compton CC, Warshaw AL, Fernández-Del Castillo C. Effect of matrix metalloproteinase inhibition on pancreatic cancer invasion and metastasis: an additive strategy for cancer control. Ann Surg. 2000;231:644-654. |
| 19. | Zervos EE, Norman JG, Gower WR, Franz MG, Rosemurgy AS. Matrix metalloproteinase inhibition attenuates human pancreatic cancer growth in vitro and decreases mortality and tumorigenesis in vivo. J Surg Res. 1997;69:367-371. |
| 21. | Duffy MJ, McCarthy K. Matrix metalloproteinases in cancer: prognostic markers and targets for therapy (review). Int J Oncol. 1998;12:1343-1348. |