Published online Feb 28, 2009. doi: 10.3748/wjg.15.907
Revised: January 12, 2009
Accepted: January 19, 2009
Published online: February 28, 2009
Complicated changes occur in hemodynamics of hepatic artery and vein, and portal vein under various kinds of pathologic status because of distinct double hepatic blood supply. This article reviews the clinical application of hepatic computed tomography perfusion in some liver diseases.
- Citation: Zhong L, Wang WJ, Xu JR. Clinical application of hepatic CT perfusion. World J Gastroenterol 2009; 15(8): 907-911
- URL: https://www.wjgnet.com/1007-9327/full/v15/i8/907.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.907
Since Miles et al[1] first described liver perfusion computed tomography (CT) imaging (CTP). It has been generally used with the development of imaging techniques and post-processing software. People are no longer satisfied with the diagnosis of diseases due its functional imaging features. Demands of therapeutic effect evaluation, prognosis analysis, etc, are rapidly increasing.
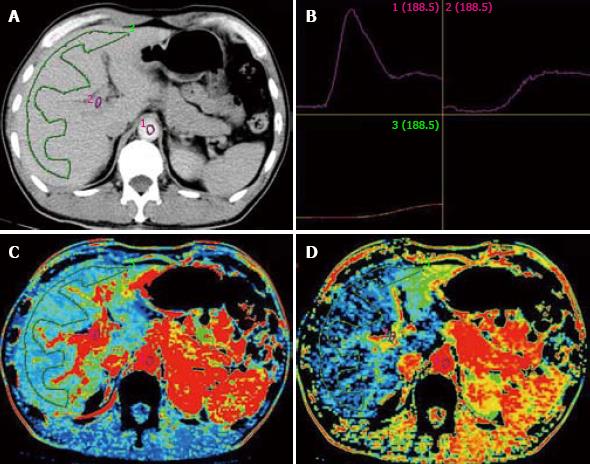
There are some disagreements in normal liver perfusion parameters from different sources due to different choices of computational model and people (Table 1). However, they are all verged about 1/4-1/3 as the ratio of HAP/PVP, in rough approximation of the ratio of blood supply from hepatic artery and portal vein. Pseudo-color images can also be obtained from CT workstation (Figure 1).
Chronic liver diseases include chronic hepatitis, liver fibrosis and cirrhosis. Previous studies used liver biopsy as gold standard to assess the degree of liver cirrhosis[67]. CTP can show pathological changes in the liver before cirrhosis, even at the early stage of fibrosis, and it can make non-invasive assessment of the degree of chronic liver disease (Table 2).
| Liver disease | Sources | Object | n | HAP | PVP | HPI | HBF | HBV | MTT |
| Chronic | Guan et al[7] | Rat | 14 | ↑ | ↓ | ↓ | ↑ | ||
| Liver | Van Beers et al[8] | Patient | 34 | ↑ | ↑ | ||||
| Disease | Hashimoto et al[6] | Patient | 38 | ↑ | |||||
| Primary | Zhou et al[5] | Patient | 62 | ↑ | ↑ | ↑ | |||
| Hepatic | Tsushima et al[11] | Patient | 11 | ↑ | |||||
| Carcinoma | Komemushi et al[12] | Patient | 37 | ↑ | ↑ | ||||
| Sahani et al[13] | Patient | 25 | ↑ | ↑ | ↓ | ||||
| Liver | Miles et al[2] | Patient | 4 | ↑ | ↑ | ||||
| Metastatic | Tsushima et al[11] | Patient | 17 | ↑ | |||||
| Disease | Leggett et al[16] | Patient | 20 | ↑ | |||||
| Shi et al[18] | Rat | 19 | ↑ | ↓ |
Guan et al[7] induced liver diffuse lesions in rats with diethylnitrosamine. They divided the processes of hepatic diffuse lesions into three stages of hepatitis, hepatic fibrosis, and cirrhosis. In the test group, hepatic arterial perfusion index (HPI) tended to increase gradually, mean transit time (MTT) prolonged obviously, hepatic blood volume (HBV) and hepatic blood flow (HBF) decreased at the same time. The results are consistent with the gradually increased portal vein pressure due to pathological changes.
Van Beers et al[8] reported that the total liver perfusion (TLP) is decreased in patients with cirrhosis and non-cirrhotic chronic liver disease, while HPI and MTT are increased. The severity of liver disease, which was categorized into five classes (normal, non-cirrhotic liver disease, Child A, Child B, Child C), was correlated significantly with TLP, HPI, and MTT. The best cutoff point to differentiate patients with cirrhosis from those without cirrhosis was considered a MTT of 22.6 s, with a sensitivity of 81% and a specificity of 81%, respectively.
Hashimoto et al[6] reported that HBF decreases with the severity of chronic liver disease. The HPI of patients without liver disease was significantly lower than that of those with Child B and C liver disease. However, the HBV and MTT did not show any correlation between the groups. At the same time, the HPI was correlated significantly with the degree of fibrosis.
Different study parameters may have a certain discrepancy. Selection of patients, scan parameters and other factors may cause inconsistency.
Li et al[9] induced early, intermediate, and advanced stages of liver cirrhosis in rats, while healthy rats received CT hepatic perfusion. At the same time, free portal pressure (FPP) measured with a multiplying channel instrument was closely related with portal vein pressure (PVP), suggesting that CT perfusion is a new non-invasive and efficient modality for the assessment of portal pressure in patients with liver cirrhosis at various stages.
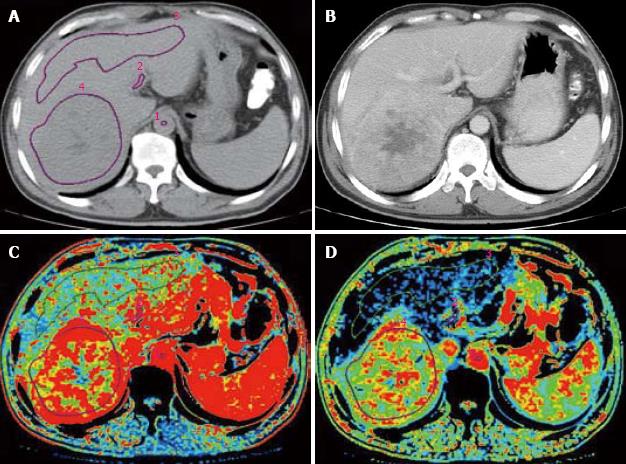
CT angiography (CTA) and CT arterial portography (CTAP) can be used to detect the blood flow of hepatic artery and portal vein to study the changes in HCC hemodynamics. Tajima et al[10] used CTA and CTAP to detect hepatic nodus confirmed by biopsy. By comparing the ratio of blood vessels in different degrees of dysplasia nodus, HCC and liver parenchyma, they found that the blood flow in artery is increased while that in portal vein is decreased in the nodus. The CTP has gradually substituted the CTA and CTAP in the study of HCC hemodynamics (Figure 2).
Miles et al[2] reported that HAP is obviously increased in perimeter of liver tumors, but decreased in the center zone of necrosis. Zhou et al[5] showed that the HBF and HPI are higher in primary hepatic carcinoma than in normal liver. The HAP, acquired by analog computation using HBF and HPI, is also higher in primary hepatic carcinoma than in normal liver. Tsushima et al[11] showed that the HAP is significantly increased in HCC (0.94 ± 0.26 mL/min per millilitre). Analysis of region of interest (ROI) showed no perfusion of portal vein in the tumor. After transcatheter arterial embolization (TAE), arterial perfusion of tumors was significantly decreased. Komemushi et al[12] obtained the pure arterial perfusion of tumor and liver parenchyma by hepatic arteriography, and found that increased arterial BF and BV in tumors are significantly tumors are significantly correlated with rich blood supply in the tumor, revealing that blood-supply in primary hepatic carcinoma is mainly provided by hepatic artery (Table 2).
Sahani et al[13] studied perfusion changes in progressive HCC and background liver tissue, showing that the HBF, HBV and permeability-surface area product (PS) are higher in HCC tissue than in background liver tissue, while MTT is decreased. No significant difference was observed in tumor perfusion in the presence and absence of portal vein invasion, confirming that blood supply of primary hepatic carcinoma is mainly provided by hepatic artery.
Changes in perfusion parameters are valuable in qualitative and differential diagnosis of primary hepatic carcinoma. CTP can detect the abnormality of liver perfusion before morphologic change occurs. Fournier et al[14] induced primary hepatic carcinoma in a rat model by chemistry, and performed CTP after 11 and 18 wk, respectively, showing that the induced hepatic carcinoma has a high arterial blood flow and a low portal blood flow, with a sensitivity of 87% and a specificity of 80% at week 18, a sensitivity of 86% and a specificity of 65% at week 11, respectively. The data indicate that CTP can detect hepatic carcinoma at its early stage.
In addition, CTP can evaluate tumor in vivo. Tumor tissues with the highest malignant grade can be taken by biopsy under CT guidance, thus avoiding errors of grading occurred in selection of biopsy region.
Diagnosis of liver metastatic disease is very important for its staging, prognosis and treatment.
CT perfusion imaging can show the increased arterial perfusion in patients with liver metastatic disease with the slope-ratio analytic methods[2]. Tsushima et al[11] showed that HAP of liver metastasis from colon carcinoma is 0.67 ± 0.33 mL/min per millilitre, while that of metastasis from other carcinomas is fluctuant. In a study by Shi et al[15], 20 patients with liver metastatic tumors received CTAP via SMA and multi-slice spiral CT perfusion imaging, and found that no tumor vessel can be found in the lesions at portal venous phase of SMA-digital subtraction angiography (DSA). The BF, BV and PS in portal vein in metastatic lesions were lower than those in normal liver tissues, showing that portal vein is not involved in blood supply of liver metastatic tumors (Table 2).
Leggett et al[16] also reported that HAP is increased (> 0.25 mL/min per millilitre) in patients with overt colorectal metastasis examined by CTP with slope-ratio analytic methods. PVP showed no statistical variance. However, PVP was decreased in 3 patients as metastasis progressed, indicating that the threshold value of HAP (> 0.25 mL/min per millilitre) can be used to diagnose occult metastasis with a sensitivity of 82%, and no metastasis with a sensitivity of 38%, respectively. Decreased PVP may prognosticate the progression of disease.
Routine CT and MRI are insensitive to occult and early stage hepatic micro metastasis of tumors. Although there is no apparent abnormality in morphology, CTP can display changes in hemodynamics through its functional imaging. Both of increased HAP and HPI can declare the possibility of liver micro metastasis. Cuenod et al[17] used the deconvolution method to study liver hemodynamic changes caused by occult hepatic micro metastasis in rat, and found micro metastases in normal liver leads to a 34% decrease in portal blood flow and a 25% increase in MTT, suggesting that resistance is increased in sinusoidal capillaries. Shi et al[18] reported that HAP is higher and PVP is lower in rats with micro metastasis.
Hemangioma is one of the common benign liver tumors; however, it is sometimes misdiagnosed as a malignant tumor.
Wang et al[19] used CTP to discriminate liver carcinoma and hemangioma and showed that the HAP in center and edge of liver carcinoma is 0.70 ± 0.23 mL/min per millilitre and 0.39 ± 0.22 mL/min per millilitre, respectively, which are significantly higher than those in normal liver. In addition, the HAP in center and edge of hemangioma is 0.14 ± 0.09 mL/min per millilitre and 0.50 ± 0.21 mL/min per millilitre, respectively, suggesting that comparison between the HAP in center and edge of liver carcinoma and hemangiom is important in discriminating liver carcinoma and hemangioma.
It is very important to evaluate non-invasive liver perfusion after liver transplantation. CTP can monitor the tendency of hemodynamic changes in portal vein and hepatic artery, contributing to the early diagnosis of blood vessel complications after transplantation.
Huang et al[20] detected perfusion parameters in 9 patients after liver transplantation for end stage liver disease, and found that the PVP in transplanted and control groups is 1.5763 ± 0.4540 mL/min per millilitre and 1.1885 ± 0.3899 mL/min per millilitre, respectively, while the TLP is 1.9594 ± 0.5727 mL/min per millilitre and 1.4712 ± 0.4451 mL/min per millilitre, respectively. The PVP and TLP in transplanted group was significantly increased (P = 0.044 and 0.036). No significant deviation was found in HAP and HPI. However, different data have also been reported[21]. HAP and HPI in transplanted and control group were 0.16 mL/min per millilitre, 0.25 mL/min per millilitre and 0.12, 0.16, respectively. Both parameters had a statistical significance. Huang et al[20] hold that it is related to the selection of patients, and examination time after transplantation, etc. Therefore, hemodynamic change after transplantation is a dynamic process, and different patients have different characteristics of hemodynamics.
CTP also plays an important role in therapeutic effect evaluation of liver diseases. Weidekamm et al[4] determined the perfusion parameters of liver parenchyma with dynamic single-section CT in patients with liver cirrhosis before and after transjugular intrahepatic portosystemic shunt (TIPS) and found that TIPS significantly increases HAP and TLP, but does not increase PVP.
Li et al[22] evaluated the changes in hepatic perfusion after interventional obliteration in patients with cirrhosis and portal hypertension using spiral CT perfusion imaging, demonstrating that partial spleen embolization (PSE) only increases HAP, while PSE in combination with percutaneous transhepatic obliteration (PTO) significantly increase HAP and TLP.
Tsushima et al[23] observed hemodynamic changes in hepatic parenchyma induced by transcatheter arterial embolization (TAE) for hepatocellular carcinoma in 22 patients. The HAP was increased 2-6 d after TAE (0.146 ± 0.073 mL/min per millilitre, P < 0.0002) compared with that before TAE (0.064 ± 0.039 mL/min per millilitre), but was decreased one month after TAE (0.086 ± 0.038 mL/min per millilitre). The PVP was decreased 2-6 d after TAE (0.541 ± 0.180 mL/min per millilitre, P < 0.001) compared with that before TAE (0.733 ± 0.263 mL/min per millilitre) and remained almost unchanged one month after TAE (0.651 ± 0.214 mL/min per millilitre), suggesting that such perfusion changes are due to acute inflammatory responses.
Kan et al[24] quantified tumor perfusion in rats after TAE, and found that functional CT can detect changes in tumor perfusion after TAE, and MTT is increased because of obstruction of tumor vessels and reduced BF after TAE. TAE-associated hypoxia stimulates tumor angiogenesis and increases vascular permeability. Changes in permeability-surface area products are similar to those in BF, suggesting that measurement of tumor permeability after TAE is an important means for assessing the therapeutic effect and tumor angiogenic response that may help design a novel antivascular therapy that combines TAE and antiangiogenic therapy.
In summary, liver CT perfusion imaging has opened up a new area for its clinical application. One single CT scan can provide both morphologic and functional information, so that clinicians can detect the disease before morphological changes and evaluate the effect of treatment.
Multi-slice CT has a high time and spatial resolution for the measurement of perfusion. As an effective non-invasive method, CT perfusion imaging is safe, reproducible, easy to operate, etc. Whole liver perfusion will come true due to the development of multi-slice CT. We can get complete perfusion parameters of the entire liver. Therefore, CT perfusion imaging will be increasingly used in clinical practice.
| 1. | Miles KA, Hayball M, Dixon AK. Colour perfusion imaging: a new application of computed tomography. Lancet. 1991;337:643-645. |
| 2. | Miles KA, Hayball MP, Dixon AK. Functional images of hepatic perfusion obtained with dynamic CT. Radiology. 1993;188:405-411. |
| 3. | Blomley MJ, Coulden R, Dawson P, Kormano M, Donlan P, Bufkin C, Lipton MJ. Liver perfusion studied with ultrafast CT. J Comput Assist Tomogr. 1995;19:424-433. |
| 4. | Weidekamm C, Cejna M, Kramer L, Peck-Radosavljevic M, Bader TR. Effects of TIPS on liver perfusion measured by dynamic CT. AJR Am J Roentgenol. 2005;184:505-510. |
| 5. | Zhou ZF, Huang HL, Xu B, Lin BJ, Liu ZH, Yang JY, Qiu YC. Clinic application with MSCT perfusion in liver tumor. Linchuang Fangshexue Zazhi. 2006;25:233-237. |
| 6. | Hashimoto K, Murakami T, Dono K, Hori M, Kim T, Kudo M, Marubashi S, Miyamoto A, Takeda Y, Nagano H. Assessment of the severity of liver disease and fibrotic change: the usefulness of hepatic CT perfusion imaging. Oncol Rep. 2006;16:677-683. |
| 7. | Guan S, Zhao WD, Zhou KR, Peng WJ, Mao J, Tang F. CT perfusion at early stage of hepatic diffuse disease. World J Gastroenterol. 2005;11:3465-3467. |
| 8. | Van Beers BE, Leconte I, Materne R, Smith AM, Jamart J, Horsmans Y. Hepatic perfusion parameters in chronic liver disease: dynamic CT measurements correlated with disease severity. AJR Am J Roentgenol. 2001;176:667-673. |
| 9. | Li JP, Yang JY, Chen W, Hang YH. A relative study of hepatic perfusion and portal vein pressure in rats with liver cirrhosis. Yingxiang Zhenduan Yu Jieru Fangshexue. 2006;15:280-283. |
| 10. | Tajima T, Honda H, Taguchi K, Asayama Y, Kuroiwa T, Yoshimitsu K, Irie H, Aibe H, Shimada M, Masuda K. Sequential hemodynamic change in hepatocellular carcinoma and dysplastic nodules: CT angiography and pathologic correlation. AJR Am J Roentgenol. 2002;178:885-897. |
| 11. | Tsushima Y, Funabasama S, Aoki J, Sanada S, Endo K. Quantitative perfusion map of malignant liver tumors, created from dynamic computed tomography data. Acad Radiol. 2004;11:215-223. |
| 12. | Komemushi A, Tanigawa N, Kojima H, Kariya S, Sawada S. CT perfusion of the liver during selective hepatic arteriography: pure arterial blood perfusion of liver tumor and parenchyma. Radiat Med. 2003;21:246-251. |
| 13. | Sahani DV, Holalkere NS, Mueller PR, Zhu AX. Advanced hepatocellular carcinoma: CT perfusion of liver and tumor tissue--initial experience. Radiology. 2007;243:736-743. |
| 14. | Fournier LS, Cuenod CA, de Bazelaire C, Siauve N, Rosty C, Tran PL, Frija G, Clement O. Early modifications of hepatic perfusion measured by functional CT in a rat model of hepatocellular carcinoma using a blood pool contrast agent. Eur Radiol. 2004;14:2125-2133. |
| 15. | Shi GF, Wang Q, Li ZG, Wang SJ, DU Y, Wang YN. [Blood supply of liver metastatic tumors observed by both CT arterial portography via superior mesenterica arterial and multi-slice spiral CT perfusion imaging.]. Ai Zheng. 2007;26:1257-1262. |
| 16. | Leggett DA, Kelley BB, Bunce IH, Miles KA. Colorectal cancer: diagnostic potential of CT measurements of hepatic perfusion and implications for contrast enhancement protocols. Radiology. 1997;205:716-720. |
| 17. | Cuenod C, Leconte I, Siauve N, Resten A, Dromain C, Poulet B, Frouin F, Clement O, Frija G. Early changes in liver perfusion caused by occult metastases in rats: detection with quantitative CT. Radiology. 2001;218:556-561. |
| 18. | Shi GF, Wang SJ, Wang Q, Xu Q, Li RX, Du Y, Wang YN, Li YK, Yang L, Zhang JJ. [Effect of perfusion CT scan on hepatic hemodynamic changes in rats with liver micrometastases]. Ai Zheng. 2006;25:849-854. |
| 19. | Wang JY, Wang SQ, Chen L, Dong D. Application of CT perfusion imaging in discrimination of liver carcinoma and hemangioma. Linchuang Gandan Bing Zazhi. 2006;22:455-457. |
| 20. | Huang YH, Yang JY, Chen W, Zhuang WQ, Kong J, Jiang L. Assessment of hepatic hemodynamics with dynamic computed tomography in end stage liver disease after transplantation. Linchuang Fangshexue Zazhi. 2005;24:40-45. |
| 21. | Bader TR, Herneth AM, Blaicher W, Steininger R, Muhlbacher F, Lechner G, Grabenwoger F. Hepatic perfusion after liver transplantation: noninvasive measurement with dynamic single-section CT. Radiology. 1998;209:129-134. |
| 22. | Li JP, Chen W, Huang YH, Liang LJ, Zhou Q, Yang JY. [Hepatic perfusion changes after interventional disconnection evaluated by CT perfusion imaging]. Zhonghua Waike Zazhi. 2007;45:913-916. |
| 23. | Tsushima Y, Unno Y, Koizumi J, Kusano S. Hepatic perfusion changes after transcatheter arterial embolization (TAE) of hepatocellular carcinoma: measurement by dynamic computed tomography (CT). Dig Dis Sci. 1998;43:317-322. |