Published online Feb 21, 2009. doi: 10.3748/wjg.15.836
Revised: December 13, 2008
Accepted: December 20, 2008
Published online: February 21, 2009
AIM: To investigate the effect of detachment of esophageal cancer cells from extracellular matrix on the localization of death receptor 5 (DR5) and apoptosis.
METHODS: Anchorage-dependent EC9706 cells of esophageal squamous cell carcinoma were pretreated or not treated with brefeldin A. Detached cells were harvested by ethylenediaminetetraacetic acid digestion. Expression and localization of DR5 in these cells were determined by immunocytochemical and immunofluorescence assays, as well as flow cytometry analysis. Apoptosis of EC9706 cells was detected by flow cytometry after stained with fluorescein isothiocyanate-labeled annexin V/propidium iodide. Activation of caspase 8 was detected by Western blot analysis.
RESULTS: Immunocytochemical assay indicated that DR5 was predominantly perinuclear in adherent cells but was mainly localized in cell membrane in detached cells. In addition, immunofluorescence assay also confirmed the above-mentioned results, and further demonstrated that DR5 was present in the form of coarse granules in detached cells, but in the form of fine granules in adherent cells. Cytometry analysis revealed higher levels of DR5 expression on the surfaces of brefeldin-A-untreated cells than on the surfaces of brefeldin-A-treated cells, but brefeldin A treatment did not affect the total DR5 expression levels. Moreover, nocodazole did not influence the extracelluar DR5 expression levels in EC9706 cells. Apoptosis assay revealed that detached cells were more sensitive to DR5 antibody-induced apoptosis than adherent cells. Western blotting showed that caspase 8 was activated in temporarily detached cells 4 h earlier than in adherent cells.
CONCLUSION: Progress from adhesion to detachment of EC9706 cells causes DR5 relocalization, and promotes cytoplasmic translocation of DR5 to cell surfaces via a Golgi-dependent pathway. Moreover, it might also result in DR5 aggregation to render apoptosis of detached cells.
- Citation: Liu GC, Zhang J, Liu SG, Gao R, Long ZF, Tao K, Ma YF. Detachment of esophageal carcinoma cells from extracellular matrix causes relocalization of death receptor 5 and apoptosis. World J Gastroenterol 2009; 15(7): 836-844
- URL: https://www.wjgnet.com/1007-9327/full/v15/i7/836.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.836
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) was identified in 1995 based on its sequence homology to FasL/Apo1L and TNF[1]. TRAIL is a type II membrane protein and can be cleaved from the cell surface to form a soluble ligand. Both membrane-bound and soluble TRAIL can rapidly induce apoptosis in a variety of tumor cells and transformed cells, with minimal adverse effects on normal cells[12]. In addition, it was reported that recombinant human TRAIL (rhTRAIL), in combination with chemotherapy or radiotherapy, has synergistic effects on several types of human cancer and can overcome resistance to both chemotherapy and radiotherapy[2]. Due to its highly selective tumoricidal activity, TRAIL is a promising agent for cancer therapy and mediates apoptotic effects by binding to its agonistic death receptors as a homotrimer. Several TRAIL receptors have been discovered to date, including TRAIL-R1, TRAIL-R2, TRAIL-R3, and TRAIL-R4. TRAIL-R1 and TRAIL-R2, which are death receptors, commonly known as DR4 and DR5, respectively, transduce TRAIL-mediated death signals to the intracellular apoptotic machinery. However, TRAIL-R3 and TRAIL-R4 are designated as anti-apoptotic decoy receptors that antagonize TRAIL-induced apoptosis[2]. It was reported that DR5 is probably the main TRAIL death receptor[23] because it exhibits a considerably higher affinity for TRAIL than DR4 in physiological conditions (37°C)[4], and may play a more prominent role than DR4 in mediating apoptotic signals emanating from TRAIL in cells expressing both death receptors[5]. Thus, DR5 is a good potential target for anti-tumor therapy.
Since TRAIL and agonistic antibodies against DR5 activate membrane-bound DR5 to trigger apoptosis, cellular localization sites of DR5 directly affect TRAIL-induced apoptosis[23]. Therefore, it is very important to elucidate the cellular localization of DR5 and the mechanisms underlying the regulation of DR5 expression and transport. Cellular localization of DR5 has been partially determined. There are numerous DR5s in cytoplasm and a few on the cell membrane of human melanoma cells, but DR5 is not present in nuclei[6]. Recently, Laguinge et al[7] reported that DR5 expression is increased in detached human colorectal carcinoma cells and DR5 mediates anoikis through a caspase 8-dependent pathway. In the development and progress of malignant tumors, as detachment from primary tumor is an obligatory step in metastasis, tumor cells must detach from their substrates at a distant site to create metastasis. Furthermore, high levels of TRAIL have been reported in tumor-infiltrating lymphocytes of cancer patients, thus high levels of DR5 in tumor cells will decrease the survival and metastasis of detached cells. Therefore, low levels of DR5 are frequently expressed in metastasized cells[89]. To date, the mechanisms underlying the expression and translocation of DR5 are still unclear.
In the present study, we investigated the expression, relocalization, and translocation of DR5 in esophageal cancer cells during the process of cell detachment. Our data suggest that detachment of EC9706 cells from extracellular matrix cause DR5 relocalization, promotes cytoplasmic translocation of DR5 to cell surfaces via a Golgi-dependent pathway, and might also result in DR5 aggregation. Our findings provide new insights into the mechanisms underlying the regulation of intracellular localization and translocation of DR5 and also indicate the potential clinical applications of DR5 antibodies and TRAIL in anticancer therapy.
Neutralizing monoclonal antibody 366 EC and functional monoclonal antibody mDRA-6 with apoptotic activity against DR5 were prepared as previously described[310]. Rabbit anti-mouse SP kit and DAB kit were from Zymed Laboratories (San Francisco, CA, USA). Endogenous peroxidase blocking kit was obtained from Vector Laboratories (Burlingame, CA, USA). Goat anti-human caspase-8 antibody was from R&D systems (Minneapolis, MN, USA). Fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG1, horseradish peroxidase (HRP)-conjugated goat anti-mouse and rabbit anti-goat IgG1, and ECL plus Western blotting detection system were from Amersham Pharmacia Biotech (Piscataway, NJ, USA). Annexin V-FITC/PI apoptosis detection kit was from BD Pharmingen (San Diego, CA, USA). Polyvinylidene difluoride membrane was from Millipore Corp (Bedford, MA, USA). Fetal bovine serum was from Tianjin Haoyang Biological Manufacture Co., Ltd (Tianjin, China). RPMI-1640 medium, trypsin, and ethylenediaminetetraacetic acid (EDTA) were from Gibco BRL (Gaithersburg, MD, USA). Brefeldin A and nocodazole were from Sigma (St. Louis, MO, USA). Other chemicals used were of analytical reagent grade.
Esophageal cancer cell line EC9706 was obtained from Cancer Institute, Chinese Academy of Medical Sciences (Beijing, China). EC9706 cells were cultured in RPMI-1640 medium supplemented with 100 mL/L fetal bovine serum, 50 IU/mL penicillin, and 50 mg/L gentamycin at 37°C under an atmosphere containing 50 mL/L CO2. The medium was changed every 2 d until 80%-90% confluence was achieved. The cells were harvested by 0.3 g/L trypsin-EDTA digestion for subculture or to prepare a single-cell suspension for subsequent experiments.
To detect the cell-surface expression of DR5, EC9706 cells (105/well) were seeded in a 24-well plate and incubated for 10 h at 37°C under an atmosphere containing 50 mL/L CO2. Adherent cells were incubated in a medium containing brefeldin A at a final concentration of 10 mg/L, or only in the culture medium, for 30 min, and then trypsinized to obtain a single-cell suspension for subsequent experiments. The adherent cells were incubated in the medium containing nocodazole at a final concentration of 10 mg/L, or only in the culture medium, for 8 h, followed by trypsinization to obtain a single-cell suspension. The cells were washed with a staining buffer (Hank’s solution into which 1 g/L BSA and 1 g/L NaN3 were added). One portion of the cells was used for extracellular staining. These cells were stained on ice with 4 mg/L 366EC antibody in 100 &mgr;L of staining buffer for 40 min, washed twice with PBS, and then incubated on ice with FITC-IgG for 40 min, avoiding direct light exposure. After two final washings with PBS, the cells were fixed in a 10 g/L paraformaldehyde (PFA) solution and finally resuspended in 500 &mgr;L PBS. Flow cytometry was performed using a FACSCalibur cytometer (BectonDickinson, San Jose, CA, USA). A minimum of 1 × 104 cells per sample was interrogated and the data were analyzed using the CellQuest software (BectonDickinson). The negative control sample used for flow cytometry experiments was treated with normal mouse serum instead of 366EC antibody. All experiments were repeated at least three times.
In order to measure DR5 intracellular protein levels as well as extracellular expression, the remaining cells were permeabilized by methanol and 1 g/L saponin after fixation using 10 g/L PFA. The cells were stained following the same procedure as the extracellular staining except for the lack of a final fixation step in 10 g/L PFA. Total DR5 expression level was analyzed by flow cytometry as described above.
For immunocytochemical staining, the EC9706 cells were cultured as a monolayer in six-well dishes containing sterilized cover-slips at 37°C for 12 h. The adherent cells were treated with brefeldin A at a final concentration of 10 mg/L, or with culture medium only, for 30 min. The adherent cells plated in six-well dishes were digested by EDTA buffer to obtain a single-cell suspension. After two final washings with PBS, the detached cells were spun on coverslips. After treatment, all cells on the coverslips were fixed in cold methnol: water (1:1) for 10 min and dried for 5 min at room temperature. Endogenous peroxidase was blocked with 3 mL/L H2O2 in a PBS solution for 30 min. The coverslips were then incubated with avidin and biotin blocking solutions, pre-incubated with 50 mL/L normal mouse serum and 10 g/L bovine serum albumin (BSA) in PBS for 15 min, and then incubated with primary antibodies (4 mg/L 366EC) at 37°C for 1 h. After washing with PBS, the coverslips were incubated with biotinylated goat-antimouse antibody at room temperature for 40 min, and then with streptavidin-peroxidase at room temperature for 15 min. Peroxidase activity was observed by incubating the slides for 3 min in a DAB solution. After washed several times, the coverslips were counterstained with hematoxylin (or not counterstained), dehydrated with ethanol, rinsed in xylene, and then mounted with gum for microscopic examination and photography. The negative control sample was treated with normal mouse serum instead of 366EC antibody.
For immunofluorescence staining, the cells were treated as described above. After the cells were incubated with primary antibody, secondary antibody FITC-IgG diluted at 1:200 was added, and incubated at 4°C in the dark and at room temperature for 1 h, respectively. The staining specificity was analyzed under an Axioskop 2 plus fluorescence microscope (Carl Zeiss, Oberkochen, Germany) using a 40 × objective lens. Image capture was performed with a Spot RT color CCD camera and the Spot RT software (Diagnostic Instruments, Sterling Heights, MI, USA). All experiments were repeated at least three times. The negative control sample was treated with non-immune normal rabbit IgGs instead of primary antibodies.
Apoptosis was evaluated using an Annexin V-FITC apoptosis detection kit. Briefly, EC9706 cells (105/well) were seeded in a 24-well tissue culture plate and incubated for 10 h. The cells were randomly divided into adhesion group, detachment group, and negative control group. Cells in the adhesion group were treated with 2 &mgr;g/mL mDRA-6 antibody for 12 h, cells in the detachment group were trypsinized to prepare a single-cell suspension (detached cells) and then detached cells were treated with 2 mg/L mDRA-6 antibody for 12 h, and cells in the negative control group were incubated in a medium without mDRA-6 antibody for 12 h. Subsequently, floating and adherent cells in the medium were collected and centrifuged at 1800 r/min for 6 min at 4°C. Cell pellets were washed twice with cold PBS and then resuspended in a binding buffer at a concentration of 1 × 106 cells/mL, and 100-&mgr;L aliquots of this cell suspension (1 × 105 cells) were then transferred to a 5-mL culture tube. Using an Annexin V-FITC apoptosis detection kit, the cells were stained with AnnexinV- FITC and propidium iodide (PI) according to the manufacturer’s instructions. Five microliters of annexin V-FITC and 5 &mgr;L of PI were added to each 100-&mgr;L aliquot of the above-mentioned cell suspension, and the cells were gently vortexed and incubated for 15 min at room temperature in the dark. Each sample, to which 400 &mgr;L of 1 × binding buffer was added, was analyzed using a FACSCalibur cytometer within 1 h. A minimum of 1 × 104 cells was detected in each flow cytometry sample and the data were analyzed using the CellQuest software.
To assess the caspase-8 activation, after required treatments, cells (3 × 106) were washed once with PBS and lysed in the sample buffer (200 &mgr;L) for SDS-PAGE and immediately boiled for 4 min. Each sample was subjected to 15 % SDS-PAGE, and proteins separated on the gel were subsequently electrotransferred onto a polyvinylidene difluoride membrane which was blocked with 50 g/L non-fat dry milk in TBS-T (20 mmol/L Tris-HCl at pH 7.4, 8 g/L NaCl, and 1 mL/L Tween 20) for 2 h at room temperature. The membrane was then incubated with goat anti-human caspase-8 antibody in TBS-T containing 50 g/L non-fat dry milk at 4°C overnight, washed three times with TBS-T and probed with peroxidase-conjugated rabbit anti-goat antibody at room temperature for 2 h. After washing four times with TBS-T, the protein was observed using the ECL Plus Western blotting detection system according to its manufacturer’s instructions.
Statistical analysis was performed using SAS® 6.12 (SAS Institute, Cary, NC, USA). All values were expressed as mean ± SD. The F test was used to compare pairs of means. P < 0.05 was considered statistically significant.
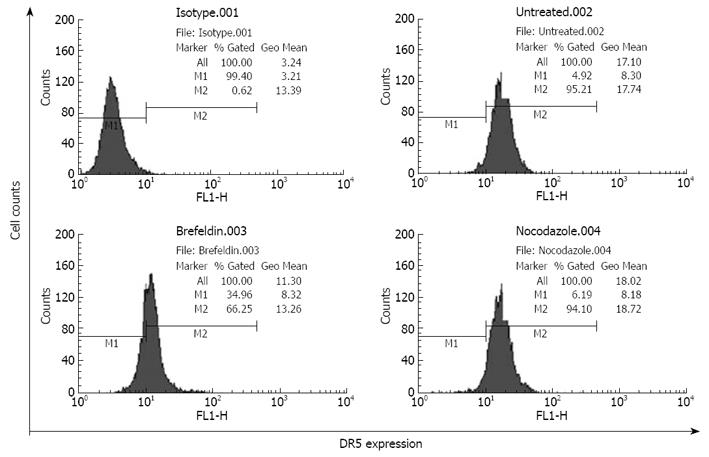
In the progress of malignant tumors, detachment is very important to the metastasis of tumor cells and tumor cells must detach from their substrates frequently at a distant site to create metastasis. As the expression level of DR5 receptors on cell surfaces directly affects TRAIL-induced apoptosis, it is thus essential to investigate the relation between DR5 expression on cell surfaces and cell detachment. To determine DR5 expression on the surfaces of detached cells, detached cells were harvested after pretreatment with or without brefeldin A, which can specifically disrupt the functions of Golgi apparatus[11] and block proteins trafficking to cell membranes. The detached cells labeled with FITC-IgG were detected by flow cytometry. The DR5 expression rate on cell surfaces was 96.21% in brefeldin A-untreated EC9706 cells. However, it was decreased to 66.25% (P < 0.01) and the expression intensity was decreased from 17.34 to 13.26 (P < 0.05) in brefeldin-A-treated cells (Figure 1), suggesting that transformation of EC9706 cells from a spreading adherent to a detached status is accompanied with translocation of DR5 to the cell surface.
Brefeldin A treatment did not change the total DR5 expression, indicating that detachment does not influence DR5 synthesis, and increased DR5 expression on the cell surface could not be simply explained by DR5 protein synthesis. Since cytoplasmic DR5 is mainly localized within the Golgi apparatus[6] and brefeldin A can specifically inhibit the functions of the Golgi network[11], the present results indicate that Golgi network is involved in DR5 translocation from the cell interior to the plasma membrane, and DR5 shuttles to the cell surface in a classic protein secretory route. It has been reported that DR5 translocation from the Golgi to plasma membrane occurs along microtubules, and nocodazole inhibits Fas translocation to plasma membrane by disrupting microtubules[1213]. In the present study, nocodazole should have attenuated DR5 expression on the surface of detached cells, but DR5 expression remained unchanged in nocodazole-treated cells (Figure 1), indicating that microtubules are not involved in DR5 translocation.
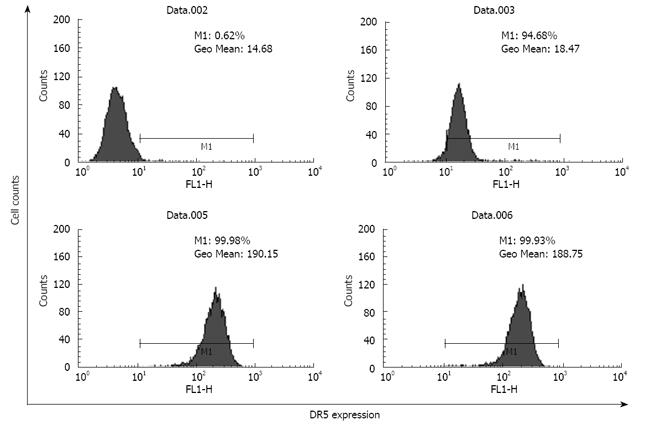
The total DR5 expression level was 10-fold higher than the cell surface expression of DR5 in brefeldin-A-untreated EC9706 cells (Figure 2), demonstrating that the majority of total cellular DR5 was sequestered within intracellular compartments and the minority was associated with the plasma membrane.
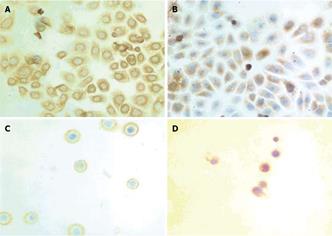
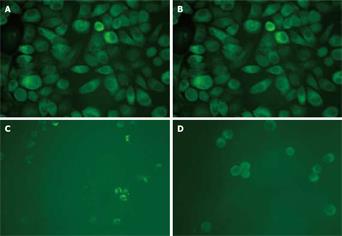
To further investigate the cellular localization and confirm the apparent translocation of DR5 from cell interior to plasma membrane, EC9706 cells were examined with immunostaining. In adherent cells, the results of both immunocytochemical and immunofluorescence staining showed that DR5 had a predominantly perinuclear localization but no nuclear localization (Figures 3A and 4A) and DR5 localization was not significantly different between brefeldin-A-treated and untreated cells (Figure 3A and B and Figure 4A and B). In detached cells, DR5 was predominantly localized in cytoplasm but not in nuclei (Figure 3C and D and Figure 4C and D). Furthermore, in brefeldin-A-untreated detached cells, DR5 tended to localize in the cell membrane and became exactly consistent with aggregation or capping of DR5 (Figure 4C). However, in brefeldin-A-treated cells, DR5 exhibited perinuclear localization (Figures 3D and 4D) and diffused (Figure 4D). These findings suggest that loss of cell adhesion enhanced DR5 translocation to the membrane of detached cells, leading to DR5 relocalization and aggregation.
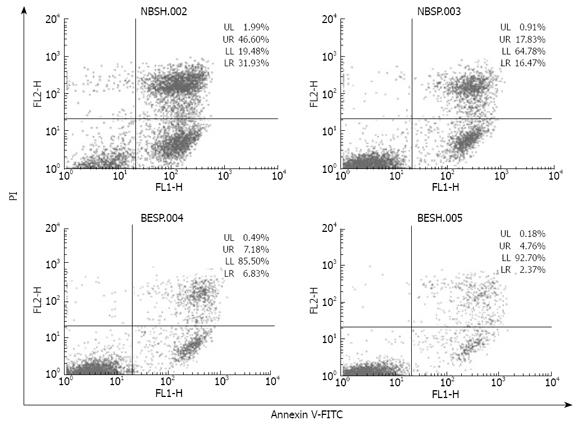
Since binding of DR5 agonist antibody mDRA-6 to DR5 triggers apoptotic cascades[10], we reasoned that by increasing cell surface expression of DR5, detached cells might become more susceptible to apoptosis induced by mDRA-6. Indeed, apoptosis induced by mDRA-6 was significantly increased in detached cells (Figure 5). The ability of detachment to sensitize EC9706 cells to apoptosis induced by mDRA-6 was inhibited by brefeldin A, suggesting that DR5 trafficking from cell interior to surface is necessary for detachment sensitization of EC9706 cells to mDRA-6-induced apoptosis. Interestingly, the spontaneous apoptotic rate for detached cells was higher than that for adherent cells (P < 0.05), demonstrating that increased cell surface expression of DR5 in detached cells is functional in transducing death signals and loss of cell adhesion may trigger apoptotic cascades via DR5 aggregation.
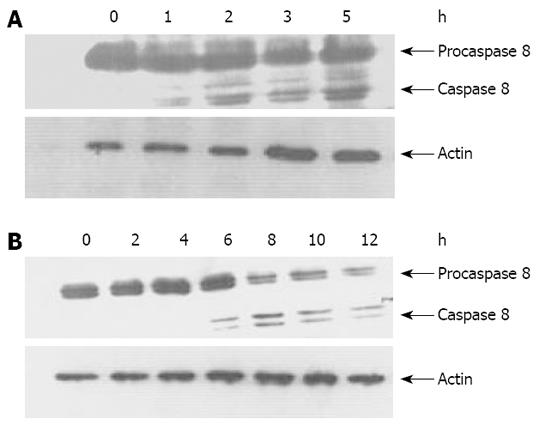
It has been shown that TRAIL and anti-DR4/DR5 antibodies could trigger apoptotic signaling pathway of death receptor by activating caspase 8[210], which is implicated in apoptosis induced by cell detachment[714]. To characterize the signaling pathways through which detachment treatment substantially enhances mDRA-6-induced killing of EC9706 cells, activation of caspase 8 was determined by Western bloting in EC9706 cells. Consequently, time-dependent activation of caspase 8, as determined by decreased procaspase 8 (p55/53) levels and cleavage of caspase 8 (p43/p41), was observed after mDRA-6 treatment in spreading and temporarily detached EC9706 cells, respectively. Caspase 8 was activated 4 h earlier in temporarily detached cells than in spreading cells (Figure 6). However, even if the level of spontaneous apoptosis was much higher in temporarily detached EC9706 cells than in spreading EC9706 cells, caspase 8 activation was not detectable by Western bloting (data not shown), suggesting that activation of caspase 8 induced by cell detachment via DR5 oligomerization activation is very weak and is thus difficult to be assessed by Western bloting.
Since DR5 is the key death receptor of TRAIL[2–4], it is very important to investigate its cellular expression and localization at the protein level. DR5, mainly localized in Golgi apparatus of cytoplasm, is a type of membrane protein[6] and translocated to cell membrane through the Golgi network. Brefeldin A can specifically inhibit the function of the Golgi apparatus[11]. This study showed that after EC9706 cells were treated with brefeldin A, the proportion of cells expressing DR5 on cell surface was significantly reduced in detached EC9706 cells, indicating that cell detachment is accompanied with translocation of DR5 from the cytoplasm to cell membrane (Figure 1), but a slight translocation occurred since DR5 was mainly localized in the cytoplasm (Figure 2), and total protein expression of DR5 did not increase in detached cells. However, it was recently reported that suspension culture enhances gene transcription and protein synthesis in DR5, but cell detachment does enhance DR5 expression on the cell surface[7]. Cell detachment from extracellular matrix had no effect on DR5 protein synthesis and the reason for this discrepancy was that cell detachment was temporary. To probe into DR5 translocation, morphological changes were observed with immunostaining in the present study. DR5 exhibited predominantly perinuclear localization in spreading EC9706 cells, while DR5 receptors were localized near the cell membrane of detached cells (Figures 3 and 4), revealing that cell detachment is mainly responsible for intracellular relocalization of DR5 and promotes translocation of DR5 to the cell membrane. It has been shown that DR5 receptors are mainly localized in the plasma membrane of some suspension cells[15], and in the cytoplasm of some spreading cells[616], suggesting that cellular localization of DR5 is closely related to the growth pattern and adhesion status of cells.
It was reported that the Golgi complex and cytoskeletal system are directly involved in translocation of membrane proteins and secretory proteins[111317]. Cell detachment influences cellular localization of DR5 and promotes translocation of DR5 to the cell membrane, indicating that the cytoskeleton may be involved in cellular localization and translocation of DR5. It has been shown that glycyl-chenodeoxycholic acid may enhance Fas expression on the surface of human liver cells, but nocodazole which can disrupt microtubules, inhibits the function of bile acids, suggesting that microtubules are involved in translocation of Fas[13]. However, results of the current study show that there was no difference in DR5 expression rate and intensity between nocodazole-treated and untreated esophageal cancer cells (Figure 1), demonstrating that microtubules are not implicated in DR5 translocation. The reason for this discrepancy still remains unclear and the inconsistent results might be attributed to the types of death receptors, cells, and stimulating period of time.
It has been shown that DR5 expression on the surface of tumor cells is closely related to the sensitivity of tumor cells to TRAIL-induced apoptosis[23]. Therefore, increased DR5 expression on the cell surface might be accompanied with increased cell sensitivity to TRAIL-induced apoptosis. mDRA-6 is an anti-DR5 agonist antibody and exhibits tumoricidal activities by inducing apoptosis[10]. This study demonstrated that when spreading esophageal cancer cells were transformed to detached cells, the rate of mDRA-6-induced apoptosis was increased by 44.23% (Figure 5). Although this was definitely attributed to the high cell-surface-expression levels of DR5 caused by cell detachment, it was more likely due to the aggregation of DR5. Loss of adhesion in esophageal cancer cells was found to be responsible for the granular distribution of DR5 in these cells (Figures 3 and 4), indicating that cell detachment leads to DR5 aggregation. Moreover, it was reported that DR5 aggregation might cause ligand-independent activation of DR5, thus resulting in activation of caspase 8 and consequently apoptosis[18]. It was also reported that detachment could activate caspase-8, leading to anoikis[14]. It has been recently shown that activation of caspase-8 mediated by detachment contributes prominently to DR5[7]. In this study, the spontaneous apoptotic rate for detached EC9706 cells was higher than that for adherent cells (P < 0.05) (Figure 5), and caspase 8 was activated 4 h earlier in temporarily detached cells than in spreading detached cells in the presence of DR5 agonist antibody (Figure 6), suggesting that cell detachment may lead to activation of caspase 8 due to DR5 aggregation. However, Western blot analysis revealed that no activated caspase 8 was detected in temporally detached EC9706 cells in the absence of DR5 agonist antibody (Figure 6), and it seems that the period of cell detachment was not long enough, so that activation of caspase 8 induced by DR5 oligomerization was too weak to be detected by Western bloting. DR5 oligomerization activation may exist in detached cells, thus effectively reducing the threshold of apoptosis, which increases the apoptotic sensitivity of detached cells to DR5 agonist antibody.
Cellular localization of DR5 may influence cell survival, and may change from cell adhesion to cell detachment. Since most types of human cells exhibit adhesion growth, DR5 receptors are mainly located in the cytoplasm, and only a few DR5s are present on the cell surface. Cytoplasmic localization of DR5 effectively reduces its interaction with TRAIL, which may lead to apoptosis, thus leading to cell survival. Furthermore, high levels of TRAIL have been reported in tumor-infiltrating lymphocytes of cancer patients[19], and DR5 maybe highly express on the surface of detached tumor cells. Thus, interaction between tumor and immune cells may increase apoptosis of metastatic cells, and inhibit metastasis of tumor cells. Consequently, low expression of DR5 augments tumor metastasis. In addition, if DR5 receptors are mainly located in the cytoplasm of adherent cells, the localization of DR5 may lead to resistance of tumor cells to TRAIL-induced apoptosis and prevent tumor cells from TRAIL-induced apoptosis. However, some drugs can up-regulate DR5 expression and enhance translocation of DR5 to the cell membrane[1820], and these drugs may increase the sensitivity of tumor cells to TRAIL-induced apoptosis and enhance the efficacy of TRAIL-induced apoptosis. Thus, TRAIL or agonistic anti-DR5 antibody in combination with subtoxic doses of chemotherapeutic agents and radiotherapy may provide a promising effective therapeutic strategy for resistant tumors.
In summary, detachment of EC9706 cells from extracellular matrix causes DR5 relocalization, promotes cytoplasmic translocation of DR5 to the cell surface via a Golgi-dependent pathway, and also results in DR5 oligomerization and apoptosis. Our findings provide new insights into the mechanisms underlying the regulation of intracellular localization and translocation of DR5, and also show the potential clinical applications of DR5 antibodies and TRAIL in anticancer therapy. How cellular detachment influences DR5 translocation and aggregation needs to be further studied. Furthermore, additional works are needed to determine whether DR5 expression varies with the adherent phase of cells in all solid tumors.
DR5, the main agonist death receptor of TRAIL, is a good potential target for anti-tumor therapy. Since TRAIL and agonistic antibody against death receptor 5 (DR5) can activate membrane-bound DR5 to trigger apoptosis, cellular localization of DR5 receptors directly affects TRAIL-induced apoptosis. In the development and progress of malignant tumors, detachment from primary tumor is an obligatory step in metastasis; tumor cells must detach from their substrates at a distant site to create metastasis. To date, DR5 expression caused by cell detachment remains poorly understood. In the present study, the effect of cell detachment on DR5 relocalization and translocation in esophageal carcinoma cells was observed.
This study was focused on the relocalization and translocation of DR5 and apoptosis during the process of cell detachment.
This study showed that detachment of EC9706 cells from extracellular matrix caused DR5 relocalization, promoted cytoplasmic translocation of DR5 to the cell surface via a Golgi-dependent pathway, and also resulted in DR5 oligomerization. These findings will provide new insights into the mechanisms underlying DR5 translocation regulation and anoikis, and also indicate the potential clinical applications of DR5 antibodies and TRAIL in anticancer therapy.
The authors investigated the potential effect of detachment of esophageal squamous carcinoma cells from extracellular matrix on the localization and translocation of DR5, and the results demonstrate that detachment of EC9706 cells from extracellular matrix could cause DR5 relocalization, promote cytoplasmic translocation of DR5 to the cell surface via a Golgi-dependent pathway, and result in DR5 oligomerization. The study was well designed with interesting and informative findings.
| 1. | Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673-682. |
| 2. | LeBlanc HN, Ashkenazi A. Apo2L/TRAIL and its death and decoy receptors. Cell Death Differ. 2003;10:66-75. |
| 3. | Ma YF, Zhang J, Zhao YP, Yang DL, Chen YH. [Correlation between sensitivity to TRAIL and expression level of DR5 on surface of tumor cells.]. Zhonghua Zhongliu Zazhi. 2004;26:528-530. |
| 4. | Truneh A, Sharma S, Silverman C, Khandekar S, Reddy MP, Deen KC, McLaughlin MM, Srinivasula SM, Livi GP, Marshall LA. Temperature-sensitive differential affinity of TRAIL for its receptors. DR5 is the highest affinity receptor. J Biol Chem. 2000;275:23319-23325. |
| 5. | Kelley RF, Totpal K, Lindstrom SH, Mathieu M, Billeci K, Deforge L, Pai R, Hymowitz SG, Ashkenazi A. Receptor-selective mutants of apoptosis-inducing ligand 2/tumor necrosis factor-related apoptosis-inducing ligand reveal a greater contribution of death receptor (DR) 5 than DR4 to apoptosis signaling. J Biol Chem. 2005;280:2205-2212. |
| 6. | Zhang XD, Franco AV, Nguyen T, Gray CP, Hersey P. Differential localization and regulation of death and decoy receptors for TNF-related apoptosis-inducing ligand (TRAIL) in human melanoma cells. J Immunol. 2000;164:3961-3970. |
| 7. | Laguinge LM, Samara RN, Wang W, El-Deiry WS, Corner G, Augenlicht L, Mishra L, Jessup JM. DR5 receptor mediates anoikis in human colorectal carcinoma cell lines. Cancer Res. 2008;68:909-917. |
| 8. | Zhuang L, Lee CS, Scolyer RA, McCarthy SW, Zhang XD, Thompson JF, Screaton G, Hersey P. Progression in melanoma is associated with decreased expression of death receptors for tumor necrosis factor-related apoptosis-inducing ligand. Hum Pathol. 2006;37:1286-1294. |
| 9. | Grosse-Wilde A, Voloshanenko O, Bailey SL, Longton GM, Schaefer U, Csernok AI, Schutz G, Greiner EF, Kemp CJ, Walczak H. TRAIL-R deficiency in mice enhances lymph node metastasis without affecting primary tumor development. J Clin Invest. 2008;118:100-110. |
| 10. | Liu GC, Ma YF, Zhang J, Li SL, Lu F, Bai HL, Zhao YP. [Cytotoxic mechanism of anit-human death receptor 5 monoclonal antibody mDRA-6]. Xibao Yu Fenzimian Yixue Zazhi. 2006;22:790-793. |
| 11. | Chardin P, McCormick F. Brefeldin A: the advantage of being uncompetitive. Cell. 1999;97:153-155. |
| 12. | Schrader M, Burkhardt JK, Baumgart E, Luers G, Spring H, Volkl A, Fahimi HD. Interaction of microtubules with peroxisomes. Tubular and spherical peroxisomes in HepG2 cells and their alterations induced by microtubule-active drugs. Eur J Cell Biol. 1996;69:24-35. |
| 13. | Sodeman T, Bronk SF, Roberts PJ, Miyoshi H, Gores GJ. Bile salts mediate hepatocyte apoptosis by increasing cell surface trafficking of Fas. Am J Physiol Gastrointest Liver Physiol. 2000;278:G992-G999. |
| 14. | Rytomaa M, Martins LM, Downward J. Involvement of FADD and caspase-8 signalling in detachment-induced apoptosis. Curr Biol. 1999;9:1043-1046. |
| 15. | Min YJ, Lee JH, Choi SJ, Chi HS, Lee JS, Kim WK, Lee KH. Prognostic significance of Fas (CD95) and TRAIL receptors (DR4/DR5) expression in acute myelogenous leukemia. Leuk Res. 2004;28:359-365. |
| 16. | Reesink-Peters N, Hougardy BM, van den Heuvel FA, Ten Hoor KA, Hollema H, Boezen HM, de Vries EG, de Jong S, van der Zee AG. Death receptors and ligands in cervical carcinogenesis: an immunohistochemical study. Gynecol Oncol. 2005;96:705-713. |
| 17. | Bennett M, Macdonald K, Chan SW, Luzio JP, Simari R, Weissberg P. Cell surface trafficking of Fas: a rapid mechanism of p53-mediated apoptosis. Science. 1998;282:290-293. |
| 18. | Higuchi H, Gores GJ. Bile acid regulation of hepatic physiology: IV. Bile acids and death receptors. Am J Physiol Gastrointest Liver Physiol. 2003;284:G734-G738. |
| 19. | Koyama S, Koike N, Adachi S. Expression of TNF-related apoptosis-inducing ligand (TRAIL) and its receptors in gastric carcinoma and tumor-infiltrating lymphocytes: a possible mechanism of immune evasion of the tumor. J Cancer Res Clin Oncol. 2002;128:73-79. |
| 20. | Nagane M, Pan G, Weddle JJ, Dixit VM, Cavenee WK, Huang HJ. Increased death receptor 5 expression by chemotherapeutic agents in human gliomas causes synergistic cytotoxicity with tumor necrosis factor-related apoptosis-inducing ligand in vitro and in vivo. Cancer Res. 2000;60:847-853. |