Published online Feb 14, 2009. doi: 10.3748/wjg.15.732
Revised: December 7, 2008
Accepted: December 14, 2008
Published online: February 14, 2009
AIM: To explore a method to establish an animal model of ischemic type intrahepatic biliary lesion in rabbits.
METHODS: Forty Japanese white rabbits of clean grade were divided randomly into four groups (10 rabbits per group) including sham operation (SO) group, and artery-bile obstruction (ABO)-1 h group, ABO-2 h group and ABO-3 h group. All the rabbits in this study underwent the same initial surgical procedure in which the liver was prepared as for graft removal during liver transplantation. Subsequently in the SO group, no additional vascular intervention was performed, while in groups ABO-1 h, ABO-2 h and ABO-3 h, the animals underwent combined clamping of the hepatic artery and common bile duct with microvascular clips for 1, 2 and 3 h, respectively. After the scheduled occlusion time, the clip was removed to recover blood supply. The animals were killed 4 wk after operation. The survival rate, liver function, cholangiography and histopathological manifestation of the rabbits in each group were observed.
RESULTS: The survival rate was 100% in groups SO, ABO-1 h and ABO-2 h, while it was 60% in group ABO-3 h. At each observation time, the change degree of the indexes of liver function was proportional to the clamping time (ABO-3 h > ABO-2 h > ABO-1 h > SO, P < 0.05). Cholangiographical and histopathologic manifestations both showed that intrahepatic biliary lesion aggravated proportionally with the increase of the clamping time.
CONCLUSION: An animal model of ischemic type intrahepatic biliary lesion in rabbits is successfully established, which may provide a reliable technique for basic and clinical research into the etiology, development and prophylaxis of ischemic type intrahepatic biliary lesion after liver transplantation.
- Citation: Sheng QS, Chen DZ, Lang R, He Q, Yang YJ, Qu ZW, Zhao DF, Zhang XS. Establishment of an animal model of ischemic type intrahepatic biliary lesion in rabbits. World J Gastroenterol 2009; 15(6): 732-736
- URL: https://www.wjgnet.com/1007-9327/full/v15/i6/732.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.732
Biliary complications, which occur at a rate of approximately 6%-35%[1], have long been recognized as a major cause of morbidity and graft failure in patients after orthotopic liver transplantation (OLT). The most troublesome is the so-called ischemic type biliary lesion (ITBL), with an incidence varying between 5% and 15%[2]. ITBL is a special biliary complication with non-anastomotic biliary tree destruction, which is one of the most important reasons for liver re-transplantation. For intrahepatic ITBL, especially in the presence of extensive intrahepatic ITBL, endoscopic and radiological techniques and surgical approaches are usually unsuccessful and re-transplantation is mostly unavoidable[2]. Therefore, it is urgent to establish an animal model of ischemic type intrahepatic biliary lesion to study the etiology, development and prophylaxis of ITBL. In the present study, by combined clamping of the common bile duct and hepatic artery for 2 h, producing the biliary ischemia reperfusion injury, and raising the animals for 4 wk, an animal model of ischemic type intrahepatic biliary lesion in rabbits was successfully established.
Animal care and experimental procedures were carried out strictly in accordance with the guide for the care and use of laboratory animals (National Research Council of USA, 1996) and the related ethical regulations of our university. Forty Japanese white rabbits of clean grade, weighing 2.0-2.5 kg, were selected (provided by Institute of Laboratory Animal Science), irrespective of male or female gender. All of the rabbits were raised under the same condition including a temperature of 18-23°C, the relative humidity of 50%-60%, 12 h diurnal rhythm and freedom to eat and drink. The rabbits were divided randomly into four groups (10 rabbits per group) including sham operation (SO) group, and artery-bile obstruction (ABO)-1 h group, ABO-2 h group and ABO-3 h group.
The rabbits were prohibited to eat for 12 h and drink for 6 h before operation. They were anesthetized by injecting 1% pentobarbital (1-2 mL/kg) intravenously. The skin was prepared and disinfected routinely. Then, a median incision of the epigastrium about 6 cm in length was formed. All the rabbits in this study underwent the same initial surgical procedure in which the liver was prepared as for graft removal during liver transplantation. Upon the completion of this procedure, the liver was isolated from all vascular supply except for the main hepatic artery, the extra-hepatic peribiliary plexus and the portal vein. In the SO group, no additional vascular intervention was performed. In groups ABO-1 h, ABO-2 h and ABO-3 h, the animals underwent combined clamping of the hepatic artery and common bile duct with microvascular clips for 1, 2 and 3 h, respectively. After the scheduled occlusion time, the clip was removed to recover blood supply. All the animals from these groups were killed 4 wk after operation.
Survival rate: The animals in each group were raised for 4 wk after operation, and the survival rates of each group were observed.
Examination of liver function: biochemical liver tests were monitored at different time points up to 4 wk including preoperation, and the 1 d, the 1, 2, 3 and 4 wk post-operation. Serum concentrations of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), γ-glutamyl transpeptidase (GGT), total bilirubin (TBIL) and direct bilirubin (DBIL) were measured, using standard analytical methods.
Cholangiography: Four weeks after operation, all the rabbits in the four groups underwent cholangiography. Under routine anesthesia, the abdominal cavity was opened according to the former incision. Three to five milliliters of 30% meglucamine diatrizoate solution was injected slowly through the distal end of the common bile duct, and a X-ray photograph was taken to observe the intrahepatic biliary lesion.
Histopathologic examination: After cholangiography, the animals were killed to collect liver tissue samples at the hepatic hilum for histopathological examination. Serial 4-&mgr;m-thick sections of formalin-fixed, paraffin-embedded liver tissues were stained with hematoxylin-eosin.
Quantitative data were shown as mean ± SD. SPSS statistical software 13.0 was used to conduct analysis of variance of multiple means. For all analyses, a P value less than 0.05 was considered statistically significant.
The animals in groups SO, ABO-1 h and ABO-2 h lived throughout the period of the experiment with a survival rate of 100%. However, four rabbits in group ABO-3 h died on the days 5, 7, 12 and 14 after operation, respectively, with a survival rate of 60%. The causes of death included hepatic ischemic necrosis, bile leakage and abdominal infection, which was supported by autopsy and histopathological examination.
All the biochemical indexes (AST, ALT, ALP, GGT, TBIL and DBIL) in groups ABO-1 h, ABO-2 h and ABO-3 h increased with different degrees after operation. No biochemical abnormality was observed in group SO during the entire follow-up. The indexes reached a peak on the day 1 after operation, and then decreased gradually. At the end of the observation period (4 wk after operation), all biochemical abnormalities were spontaneously resolved except for the ALP and GGT in group ABO-2 h and ABO-3 h, which were still higher than that in group SO. At each observation time, the change in the indexes was proportional to clamping time (ABO-3 h > ABO-2 h > ABO-1 h > SO, P < 0.05) (Table 1).
| Groups | Preoperation | 1 d post-operation | 1 wk post-operation | 2 wk post-operation | 3 wk post-operation | 4 wk post-operation |
| AST (U/L) | ||||||
| SO | 38.3 ± 2.4 | 38.1 ± 2.6 | 38.0 ± 2.4 | 38.7 ± 2.3 | 38.5 ± 2.1 | 39.1 ± 3.3 |
| ABO-1 h | 38.2 ± 2.3 | 260.3 ± 19.51 | 85.0 ± 11.11 | 39.0 ± 1.6 | 38.2 ± 2.5 | 39.0 ± 3.3 |
| ABO-2 h | 40.1 ± 1.7 | 359.9 ± 11.92 | 154.8 ± 14.42 | 86.9 ± 5.72 | 39.1 ± 1.7 | 38.8 ± 1.9 |
| ABO-3 h | 39.8 ± 4.6 | 461.9 ± 11.43 | 199.2 ± 16.93 | 112.0 ± 16.73 | 82.7 ± 9.73 | 40.1 ± 3.6 |
| ALT (U/L) | ||||||
| SO | 42.2 ± 2.2 | 42.0 ± 2.3 | 43.2 ± 2.0 | 40.0 ± 3.7 | 40.7 ± 3.4 | 42.0 ± 2.3 |
| ABO-1 h | 42.0 ± 2.4 | 200.5 ± 13.01 | 169.8 ± 14.31 | 65.9 ± 11.51 | 42.3 ± 2.8 | 43.2 ± 2.0 |
| ABO-2 h | 43.9 ± 2.3 | 400.1 ± 12.32 | 200.2 ± 12.02 | 92.2 ± 8.62 | 42.7 ± 3.1 | 42.2 ± 3.7 |
| ABO-3 h | 43.8 ± 4.6 | 505.9 ± 14.03 | 292.0 ± 15.83 | 116.6 ± 9.03 | 87.7 ± 8.83 | 44.6 ± 2.5 |
| ALP (U/L) | ||||||
| SO | 117.0 ± 14.7 | 115.9 ± 15.9 | 114.3 ± 14.8 | 110.3 ± 9.6 | 113.3 ± 12.7 | 111.4 ± 11.7 |
| ABO-1 h | 117.6 ± 14.5 | 203.9 ± 18.81 | 139.8 ± 10.31 | 119.6 ± 9.01 | 112.5 ± 9.3 | 109.5 ± 7.5 |
| ABO-2 h | 118.8 ± 14.2 | 304.8 ± 16.82 | 173.1 ± 11.22 | 136.2 ± 6.62 | 138.8 ± 6.32 | 139.6 ± 7.42 |
| ABO-3 h | 113.9 ± 11.7 | 440.0 ± 29.43 | 226.0 ± 13.73 | 183.7 ± 9.83 | 149.4 ± 10.23 | 149.7 ± 11.53 |
| GGT (U/L) | ||||||
| SO | 16.7 ± 2.1 | 17.5 ± 2.5 | 18.0 ± 2.1 | 18.5 ± 2.6 | 18.8 ± 3.9 | 17.3 ± 2.3 |
| ABO-1 h | 18.7 ± 2.2 | 62.8 ± 10.21 | 20.3 ± 3.4 | 17.3 ± 2.8 | 19.7 ± 3.5 | 17.5 ± 2.5 |
| ABO-2 h | 17.7 ± 2.8 | 111.5 ± 9.22 | 106.5 ± 11.32 | 81.9 ± 7.62 | 84.7 ± 9.42 | 59.6 ± 13.62 |
| ABO-3 h | 17.9 ± 3.0 | 214.6 ± 18.63 | 182.5 ± 8.63 | 153.7 ± 14.43 | 155.9 ± 19.33 | 98.0 ± 6.83 |
| TBIL (&mgr;mol/L) | ||||||
| SO | 10.0 ± 2.0 | 9.5 ± 1.7 | 10.4 ± 2.0 | 10.1 ± 1.9 | 10.6 ± 2.2 | 10.8 ± 2.3 |
| ABO-1 h | 10.1 ± 1.8 | 21.0 ± 3.11 | 10.6 ± 2.4 | 9.8 ± 1.6 | 9.5 ± 2.0 | 10.5 ± 2.3 |
| ABO-2 h | 9.9 ± 2.2 | 30.7 ± 2.32 | 19.8 ± 1.92 | 10.5 ± 2.2 | 9.3 ± 1.5 | 9.9 ± 2.0 |
| ABO-3 h | 9 .7 ± 1.9 | 40.7 ± 4.33 | 30.6 ± 2.43 | 18.1 ± 2.23 | 10.3 ± 1.8 | 11.2 ± 2.3 |
| DBIL (&mgr;mol/L) | ||||||
| SO | 3.9 ± 1.0 | 3.8 ± 1.5 | 3.9 ± 1.3 | 4.9 ± 1.6 | 4.5 ± 1.1 | 5.5 ± 1.2 |
| ABO-1 h | 4.7 ± 1.3 | 13.5 ± 2.51 | 4.2 ± 1.3 | 4.8 ± 0.8 | 4.1 ± 1.2 | 4.6 ± 0.8 |
| ABO-2 h | 4.3 ± 1.3 | 20.4 ± 2.82 | 13.4 ± 2.22 | 4.9 ± 1.1 | 3.9 ± 1.1 | 4.6 ± 1.3 |
| ABO-3 h | 4.5 ± 1.1 | 33.7 ± 3.03 | 19.6 ± 1.73 | 11.2 ± 2.03 | 5.0 ± 1.4 | 4.8 ± 1.5 |
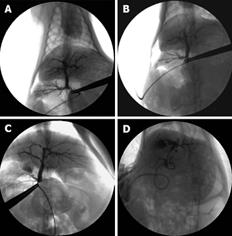
Four weeks after operation, all the rabbits in the four groups underwent cholangiography. The images of the intra-hepatic bile duct indicated that it was normal and no intrahepatic biliary lesion was visualized in groups of SO and ABO-1 h. However, a biliary lesion was observed obviously in groups ABO-2 h and ABO-3 h. It showed that intrahepatic biliary lesion aggravated proportionally with the increase in the clamping time (Figure 1).
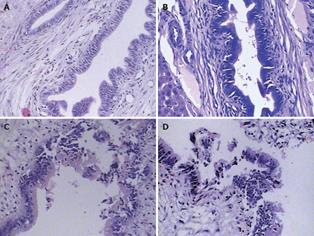
After cholangiography, the sample of liver tissues at the hepatic hilum was taken for histopathological examination. The results indicated that the morphology of the intrahepatic bile duct epithelial cells was normal and there was no cell necrosis in groups SO and ABO-1 h. However, epithelial cells were obviously damaged and sloughed into the bile duct lumen in groups ABO-2 h and ABO-3 h. It also showed that the intrahepatic biliary lesion was aggravated proportionally with the clamping time (Figure 2).
ITBL is defined as non-anastomotic destruction of the graft’s biliary tree after OLT and characterized by the formation of sludge or stone, bile duct destruction, and even non-function of the allograft[34]. A classification of ITBL has been proposed based on the localization of the abnormalities, distinguishing type I (extrahepatic lesions), type II (intrahepatic lesions) and type III (intra- and extrahepatic alterations)[1]. For type II and type III, especially for multiple and diffuse ITBL, there is poor prognosis and graft survival, with the result of liver function failure and inevitable re-transplantation in most patients. Therefore, it is of clinical significance to establish an animal model of ischemic type intrahepatic biliary lesion for studying the etiology, development and prophylaxis of ITBL.
Over the past years, several risk factors of this complication have been identified, strongly suggesting a multi-factorial origin, although the exact pathophysiological mechanism of ITBL is still unknown. The generally accepted risk factors include prolonged cold ischemic time warm ischemic time, reperfusion injury, disturbed blood flow in the peribiliary vascular plexus, ABO incompatibility, cytomegalovirus infection, chemokine polymorphism CCR5 delta 32, and bile salt-induced injury[25]. Obviously, ischemic injury is of vital importance for the occurrence of ITBL.
Several studies[6–8] have suggested that the peribiliary plexus (PBP) and hepatic artery branches are both the blood supply of the intrahepatic bile duct. Furthermore, it is demonstrated by scanning electron microscopy that there is no arterio-portal anastomosis in the liver of rabbits[9]. That is to say, the intrahepatic bile duct of rabbits do not receive their blood supply from portal vein. Therefore, in this study, by combined clamping of common bile duct and hepatic artery, as well as isolating the liver from all peripheral vascular connections, the blood supply of the intrahepatic bile duct was occluded nearly completely. After removing the clip, the intrahepatic bile duct underwent warm ischemia-reperfusion, which better simulated the clinical procedure of intrahepatic biliary warm ischemia and reperfusion injury in liver transplantation.
In this study, with the increase of the clamping time (1 h, 2 h, 3 h), it was found that intrahepatic biliary lesions were aggravated proportionally, as observed by biochemical indexes, cholangiography and histopathologicial examination. As for the clamping time, in group ABO-1 h, biliary lesions were mild and no imaging and histopathological changes were found, while in group ABO-3 h, the success rate of the animal model was only 60%, in spite of the obvious intrahepatic biliary lesion. However, significant biliary lesions and a high success rate of the model (100%) were both observed in group ABO-2 h. Therefore, combined clamping of the common bile duct and hepatic artery for 2 h was considered the optimal clamping time to establish the model of ischemic-type intrahepatic biliary lesion in rabbits.
Generally, the time from biliary ischemic necrosis and fibrosis to stricture is about 30 d after clinical liver transplantation. Zhao et al[10] established an animal model of biliary ischemic stenosis with clamping in mice and observed the significant extrahepatic biliary ischemic stenosis on day 21 after operation. Therefore, the time interval of 4 wk was chosen in this study for the initial observation time. It was not confirmed that the animals in group ABO-2 h underwent more significant intrahepatic stricture when prolonging the observation time after operation.
Overall, the advantages of this animal model included the following. (1) By combined clamping of the common bile duct and hepatic artery, as well as isolating the liver from all peripheral vascular connections, the intrahepatic bile duct is in complete warm ischemia, which can better reflect the clinical procedure of intrahepatic biliary warm ischemia and reperfusion injury in liver transplantation. (2) Surgery with occlusion of the common bile duct and hepatic artery and without that of portal vein, is easy to perform, and has less trauma and higher survival rate. (3) The common bile duct and hepatic artery of all the animals are clamped by the same microvascular clip, which can control the strength and time precisely. (4) The animal model excluded the influence of other surgical operations (biliary anastomosis, hepatic artery anastomosis, T tube detaining), rejection, biliary cold conservation and drug toxicity. (5) The intrahepatic biliary anatomy, structure and microcirculation of the rabbits are similar to those of humans. Moreover, this animal is cheap and easy to obtain sufficient samples[11].
In conclusion, in the present study, by combined clamping of the common bile duct and hepatic artery for 2 h, producing the biliary ischemia-reperfusion injury, and raising the rabbits for 4 wk, the animal model of ischemic-type intrahepatic biliary lesion in rabbits was successfully established, which may provide a reliable technique for basic and clinical research into the etiology, development and prophylaxis of ischemic type intrahepatic biliary lesion after liver transplantation.
Biliary complications are a major cause of morbidity and graft failure in patients after orthotopic liver transplantation (OLT). The most troublesome is the so-called ischemic type biliary lesion (ITBL), which is one of the most important reasons for liver re-transplantation. Therefore, it is of clinical significance to establish an animal model of ischemic type intrahepatic biliary lesion for studying the etiology, development and prophylaxis of ITBL.
ITBL, with an incidence varying between 5% and 15% after OLT, is defined as non-anastomotic destruction of the graft’s biliary tree after OLT. Although the exact pathophysiological mechanism of ITBL is still unknown, several risk factors of this often cumbersome complication have been identified, strongly suggesting a multi-factorial origin. Therefore, the etiology, development and prophylaxis of ITBL have been research hotspots.
This animal model of ITBL is easy to establish, and has less trauma and a higher survival rate. Moreover, it can better reflect the clinical procedure of intrahepatic biliary warm ischemia and reperfusion injury in liver transplantation. In addition, the model excluded the influence of other operations, rejection and biliary cold conservation.
This animal model of ITBL may provide a reliable technique for basic and clinical research into the etiology, development and prophylaxis of ischemic type intrahepatic biliary lesion after liver transplantation.
The manuscript is of interest as a measure of assessing ischemia of the liver and bile ducts, and the methods appear acceptable. It is an interesting study.
| 1. | Verdonk RC, Buis CI, Porte RJ, Haagsma EB. Biliary complications after liver transplantation: a review. Scand J Gastroenterol Suppl. 2006;89-101. |
| 2. | Buis CI, Hoekstra H, Verdonk RC, Porte RJ. Causes and consequences of ischemic-type biliary lesions after liver transplantation. J Hepatobiliary Pancreat Surg. 2006;13:517-524. |
| 3. | Boraschi P, Donati F, Gigoni R, Urbani L, Femia M, Cossu MC, Filipponi F, Falaschi F. Ischemic-type biliary lesions in liver transplant recipients: evaluation with magnetic resonance cholangiography. Transplant Proc. 2004;36:2744-2747. |
| 4. | Hoffman A, Kiesslich R, Moench C, Bittinger F, Otto G, Galle PR, Neurath MF. Methylene blue-aided cholangioscopy unravels the endoscopic features of ischemic-type biliary lesions after liver transplantation. Gastrointest Endosc. 2007;66:1052-1058. |
| 5. | Moench C, Uhrig A, Lohse AW, Otto G. CC chemokine receptor 5delta32 polymorphism-a risk factor for ischemic-type biliary lesions following orthotopic liver transplantation. Liver Transpl. 2004;10:434-439. |
| 6. | Beaussier M, Wendum D, Fouassier L, Rey C, Barbu V, Lasnier E, Lienhart A, Scoazec JY, Rosmorduc O, Housset C. Adaptative bile duct proliferative response in experimental bile duct ischemia. J Hepatol. 2005;42:257-265. |
| 7. | Gaudio E, Franchitto A, Pannarale L, Carpino G, Alpini G, Francis H, Glaser S, Alvaro D, Onori P. Cholangiocytes and blood supply. World J Gastroenterol. 2006;12:3546-3552. |
| 8. | Nishida S, Nakamura N, Kadono J, Komokata T, Sakata R, Madariaga JR, Tzakis AG. Intrahepatic biliary strictures after liver transplantation. J Hepatobiliary Pancreat Surg. 2006;13:511-516. |
| 9. | Motta PM. The three-dimensional microanatomy of the liver. Arch Histol Jpn. 1984;47:1-30. |
| 10. | Zhao DF, Chen DZ, Lv JS, Lang R, Jin ZK, Qing H. Establishment of an animal model of biliary ischemic stenosis with clamping in mice. Transplant Proc. 2008;40:1303-1305. |
| 11. | Kanoria S, Glantzounis G, Jalan R, Davies NA, Seifalian AM, Williams R, Davidson BR. A model to study total hepatic ischemia-reperfusion injury. Transplant Proc. 2004;36:2586-2589. |