Published online Feb 7, 2009. doi: 10.3748/wjg.15.561
Revised: October 24, 2008
Accepted: November 1, 2008
Published online: February 7, 2009
AIM: To investigate the effects of Tat-NEMO-binding domain (NBD) peptide on taurocholate-induced pancreatitis and lipopolysaccharide (LPS)-stimulated AR42J acinus cells inflammatory response in rats.
METHODS: Sodium taurocholate (5%) was used to induce the pancreatitis model. Forty-eight rats from the taurocholate group received an intravenous bolus of 13 mg/kg Tat-NBD (wild-type, WT) peptide, Tat-NBD (mutant-type, MT) peptide, NBD peptide or Tat peptide. The pancreatic histopathology was analyzed by hematoxylin staining. LPS was added to the culture medium to stimulate the AR42J cells. For pretreatment, cells were incubated with different peptides for 2 h before LPS stimulation. Expression of IL-1β and TNF-α mRNA was analyzed using a semi-quantitative reverse-transcript polymerase chain reaction (RT-PCR) method. IL-1β and TNF-α protein in culture medium were detected by enzyme linked immunosorbent assay (ELISA). NF-κB DNA-binding in pancreas was examined by electrophoretic mobility shift assays. P65 expression of AR42J was determined by Strept Actividin-Biotin Complex (SABC) method.
RESULTS: Pretreatment with Tat-NBD (WT) peptide at a concentration of 13 mg/kg body wt showed beneficial effect in pancreaitis model. LPS (10 mg/L) resulted in an increase of IL-1β mRNA, IL-1β protein, TNF-α mRNA and TNF-α protein, whereas significantly inhibitory effects were observed when cells were incubated with Tat-NBD (WT). Consisting with p65 expression decrease analyzed by SABC method, NF-κB DNA-binding activity significantly decreased in Tat-NBD (WT) pretreatment group, especially at the largest dose. No significant changes were found in the control peptide group.
CONCLUSION: Our result supports that active NF-κB participates in the pathogenesis of STC-induced acute pancreatitis in rats. Tat-NBD (WT) peptide has anti-inflammatory effects in this model and inhibits the inflammation of acinus simulated by LPS.
- Citation: Long YM, Chen K, Liu XJ, Xie WR, Wang H. Cell-permeable Tat-NBD peptide attenuates rat pancreatitis and acinus cell inflammation response. World J Gastroenterol 2009; 15(5): 561-569
- URL: https://www.wjgnet.com/1007-9327/full/v15/i5/561.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.561
Because of the systematic inflammatory response syndrome (SIRS) in severe acute pancreatitis (SAP), the mortality rate of SAP is very high, reported to reach about 30%[12]. Though it is known that several kinds of proinflammatory mediators, such as lipopolysaccharide (LPS), cytokine, etc., are closely related to the development of systematic complications, the exact mechanism of acute necrotic pancreatitis is still unclear. Therefore, it is very important to develop an effective therapy. Nuclear factor kappa B (NF-κB) is a member of the Rel family of transcriptional regulatory proteins including p50, p52, p65, c-Rel and Rel B[3]. It has been proved that in many inflammatory diseases, including rheumatoid arthritis, inflammatory bowel disease and SIRS, NF-κB is highly activated while the inflammatory mediators, such as LPS, can intermediate the activation of NF-κB[4]. Once activated, NF-κB is translocated into the nucleus from the cytosol. It binds the consensus sequence to promoter or enhancer region of related genes and regulates gene transcription.
Activation of NF-κB dimers is regulated by the inhibitory proteins, IκBα, IκBβ, and IκBepsilon. The phosphorylation sites of IκBα have been identified, and the kinases have been found involved in the process, namely the IκB kinase (IKK)[56]. The IKK complex comprised primarily three IKKs, α, β, and γ. IKKγ, the third protein within the IKK complex, which is also named NEMO (NF-B essential modulator), was deemed regulatory of the other IKKs. NEMO is critical for pro-inflammatory activation of the IKK complex[78]. Although the precise mechanism of NEMO action is poorly understood, it was speculated that it recruits the IKK complex to ligate cytokine receptors and facilitate transphosphorylation events. More intriguingly, NEMO may facilitate the recruitment of upstream IKK activators such as kinases that specifically target the activation loops within the catalytic domains of the IKK subunits. NEMO interacts with a COOH-terminal sequence within the both IKKs termed the NEMO-binding domain (NBD)[9]. Importantly, short cell-permeable peptide spanning the IKKβ NBD disrupted the association of NEMO with IKKβ, blocked NF-κB activation, and ameliorated responses in animal models of inflammation. These observations defined the IKK NBD as a viable target for the development of anti-inflammatory drugs[71011]. Such peptide interacting the NBD had been confirmed to attenuate the severity of cerulein-induced mouse pancreatitis which ameliorated inflammation in the pancreas, reduced hemorrhage in the lungs, and lowered the myeloperoxidase activity in both pancreas and lung. It indicates that this novel compounds that selectively target NF-κB may be useful in the treatment of AP and AP-associated lung injury[12]. In rat pancreatitis induced by taurocholate, activation of NF-κB in pancreas has been reported[13]. It was not clear whether or not the peptide could have anti-inflammatory effects in taurocholate-induced SAP model and inhibited the inflammation of acinus stimulated by LPS directly. In this paper, we report the effects of Tat-NBD peptide on STC-induced pancreatitis and LPS-stimulated AR42J acinus cell inflammatory response in rats.
Sodium taurocholate and LPS were purchased from Sigma Company (America); AR42J cells, from American Type Culture Collection (ATCC N.O: CRL-1492); agarose, inhibitor of RNase and M-MLV retroviridase, from Promega Company (America); F12 culture medium, from Gibco Company (America); DNA marker, from Beijing Huamei Biological Company (China); primers for reverse transcription-polymerase chain reaction (RT-PCR) from Shanghai Boya Biological Company (China); TRIzolTM Reagent from Invitrogen Company (America); and EX Taq enzyme from TaKaRa Company (Japan). Peptide was synthesized by Xian Meilian Biological Company (China). TNF-α kit for enzyme-linked immunosorbent assay (ELISA) is a product of Guangzhou Jingmei Biological Company (China). Immunohistochemistry (IH) kit of steptavidin-biotin complex method (SABC) was obtained from Wuhai Boshide Company (China). Anti-rat monoclonal antibody of p65 is from Santa Crutz Company (America). All other chemicals were obtained from either Sigma or Beijing Dingguo Company. Female and male Sprague-Dawley (S-D) rats (200 ± 10 g) were from specific pathogeny free unit in the Animal Center of Guangdong Medical College.
According to Dai et al[7], the wild Tat-NBD peptide was synthesized with a 23-amino acid sequence (YGRKKRRQRRR-G-TTLDWSWLQME). A mutant peptide was called Tat-NBD (MT) with TrpAla mutations (YGRKKRRQRRR-G-TTLDASALQME). NBD sequence (TTLDWSWLQME) and Tat sequence (YGRKKRRQRRR) were synthesized respectively as controls. The peptide concentrations of 0.1, 1, 10 and 100 mg/L were prepared with F12 culture medium (no serum and antibody).
Seventy-two Sprague-Dawley rats were randomized into different groups. All rats were maintained at
23°C on a 12 h light/dark cycle and allowed free access to water and standard laboratory chow. From 12 h before start of the experiments, the animals were deprived of food, except access to water. Acute taurocholate pancreatitis was induced according to Aho et al[14]. Under anesthesia with a combination of xylazine (10 mg/kg) and ketamine (100 mg/kg), a midline laparotomy was performed and a PE-50 catheter (inside diameter 0.58 mm, outside diameter 0.96 mm) was inserted in the pancreatic duct through a puncture of the duodenum. The biliopancreatic duct was occluded transiently by a silk ligature at the liver hilus to prevent regurgitation into the liver. Sodium taurocholate solution (1 mL/kg of 5%; STC group, n = 60) or the same volume of saline (sham-operated group, n = 12) was retrogradely infused in the pancreatic duct at a flow rate of 0.07 mL/min by a microinfusion pump. Upon the completion of the infusion, the catheter and hilar ligature were removed and the abdomen was closed with suture. For pretreatment with peptide, 48 rats from the TC group received an intravenous bolus of 13 mg/kg Tat-NBD (WT) peptide [Tat-NBD (WT) group, n = 12], Tat-NBD (MT) peptide [Tat-NBD (MT) group, n = 12], NBD peptide (NBD group, n = 12) or Tat peptide (Tat group, n = 12). Peptide in F12 culture medium was incubated 2 h before intraductal infusion of taurocholate, followed by a continuous intravenous infusion. Controls received an intravenous saline infusion. These groups of rats were killed 6 and 12 h after taurocholate administration.
At 6 or 12 h after taurocholate infusion, animals were killed and blood was obtained with heparinized tubes for amylase and TNF-α determinations. Portions of the gland were saved for morphological examination, which were fixed in 10% zinc-buffered formalin, embedded in paraffin, and cut into 3 to 5 &mgr;m thick sections. The microscopic damage score was calculated according to Spormann et al [15].
The AR42J cell line was obtained from American Type Culture Collection. Cells were grown in 75-cm2 flasks containing 12 mL medium, consisting of Ham’s F-12 nutrient medium (F12K) with 2 mmol/L L-glutamine, 1% antibiotic, 1.5% sodium bicarbonate, and 10% FBS. Flasks were placed into an incubator maintained at 37°C and a 5% CO2-95% air atmosphere. Cells were plated at a density of 105 cells/mL in 12-well plates. LPS was added to culture media at a dose of 10 mg/kg for
2 h to stimulate the cells. For pretreatment, cells were incubated with different peptides [Tat-NBD (WT), n = 3; Tat-NBD (MT), n = 3; NBD, n = 3; Tat, n = 3] for 2 h before LPS stimulation.
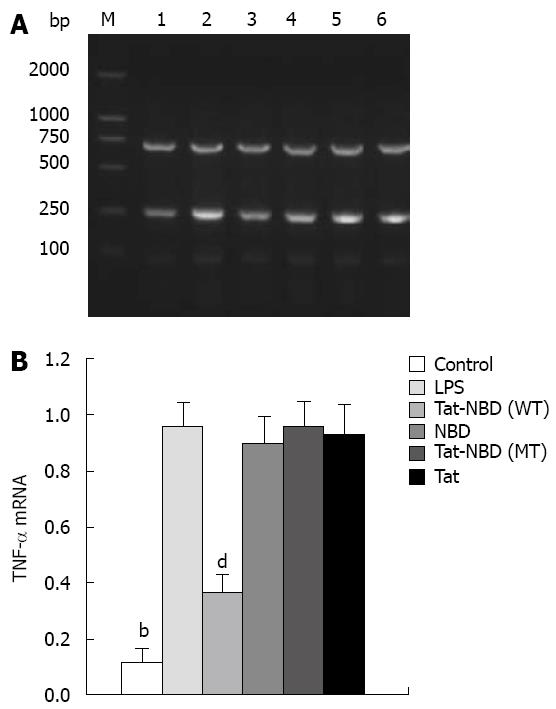
Analysis of TNF-α mRNA expression was conducted by a semiquantitative RT-PCR method. Total RNA from cells was extracted using the TRIzol reagent (Invitrogen Life Technologies). One microgram of total RNA was used for amplification using the Invitrogen One Step RT-PCR System according to the manufacturer’s instructions. The following primers were used for: IL-1β (307 bp) forward: 5'-GGATGATGACGACCTGCT AGTGT-3', reverse: 5'-CTTCTTTGGGTGTTTG GGA-3'; TNF-α (231 bp) forward: 5'-GAACTCCAGGCGGTGTCT-3', reverse: 5'-TCTGCTTGGTGGTTT GC-3'. Fragments were amplified using 25-30 cycles of PCR; each cycle consisted of 15 s at 94°C, 30 s at 55°C, and 1 min at 72°C. The resulting RT-PCR products were electrophoresed on 2% agarose gels with DNA markers, stained with ethidium bromide, and visualized under UV light. Beta-Actin (701 bp) was used as an internal control for stable expression (housekeeping gene) in all experiments. The forward primer was 5'-GCCAACCGT GAAAAGATGA-3', and the reverse primer was 5'- GCCAGGATAGAGCCACCAAT-3.
The activity of NF-κB DNA-binding was detected by EMSA as descried by Molloy[16] with the following modifications. The double-stranded oligonucleotide sequence of 5'-AGTTGAGGGGACTTTCCCAGGC-3, 3'-TCAACTCCCCTGAAAGGGTCCG-5', which corresponds to the κB binding site, was end-labeled with [γ-32P] ATP by T4 polynucleotide kinase (Promega). Labeled probes were purified and CPM detected with Whatman DE81 (> 5000 cpm/min). The nuclear extract equivalent to 15 &mgr;g was mixed with 5 × binding buffer 2 &mgr;L (room temperature, 10 min), then labeled probe (0.0175 pmoL) added for incubation (room temperature, 20 min). The mixture was subjected to electrophoresis on 4% polyacrylamide gel at 150 V in 0.5 × TBE buffer for 40 min at 4°C. After being dried, the gel was exposed to imaging system of FX P screen (Bio-Rad, America) for 24 h.
In immunohistochemical monitoring of NF-κB-p65 subunit to determine p65 expression, AR42J cells were incubated in coverslips overnight at 37°C under 5% CO2 in air. Cells were treated first with peptides or saline for 2 h, then LPS was added to the culture medium for 2 h. Cells were fixed with 4% formaldehyde in phosphate-buffered saline for 20 min at 4°C and permeabilized with 0.1% Triton in phosphate-buffered saline for 5 min at room temperature. Saturation was obtained by incubating cells with 5% bovine serum albumin in phosphate-buffered saline for 30 min at room temperature. Cells were stained with anti-p65 NF-κB antibody (Santa Cruz). Staining step was referred to the SABC kit description. Slides’ staining degree was scanned by imaging system (BIO-RAD) and semi-quantitated by software package of quantity.
The detection was performed according to the manufacturer’s instructions.
Parametric data were presented and statistical analyses were performed using two-way ANOVA with SPSS8.0. Results were considered significant when P < 0.05.
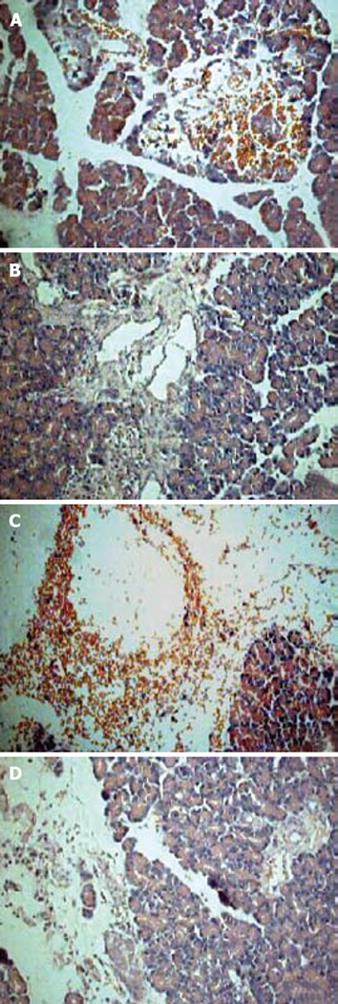
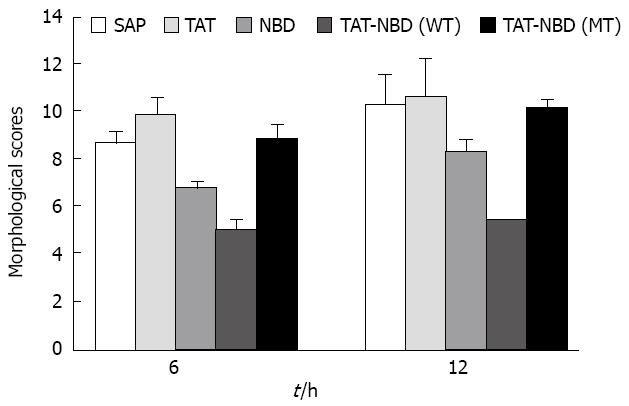
Histomorphology after sodium taurocholate-induced acute pancreatitis was analyzed semiquantatively using the Spormann score. Pancreatitis without treatment was characterized by severe interstitial edema formation, considerable necrosis of fatty tissue, distinct sublobular and lobular parenchymal necrosis, as well as hemorrhage, resulting in a Spormann score of 8.71 ± 0.45 at 6 h and 10.31 ± 1.23 at 12 h, which reflects severe, acute, necrotizing pancreatitis (Figure 1A and C). Pretreatment with Tat or Tat-NBD (MT) peptide at a concentration of 13 mg/kg body weight had no beneficial effect in the pancreatitis-associated tissue injury, whereas the same concentration of NBD peptide caused a significant reduction of the tissue damage (Table 1). More beneficial effect can be seen in Tat-NBD (WT) group. The Spormann score was lowered to 5.04 ± 0.41 at 6 h and 5.45 ± 0.34 at 12 h, which was mainly due to a decrease in degree and amount of fatty tissue and parenchymal cell necrosis (Figure 1B and D). The histological scores in different groups are shown in Table 1 and Figure 2.
We used LPS (10 mg/L) to stimulate AR42J and explore whether the peptide could play a direct role in pancreatic acinus cells. Cells were cultured as previously stated.
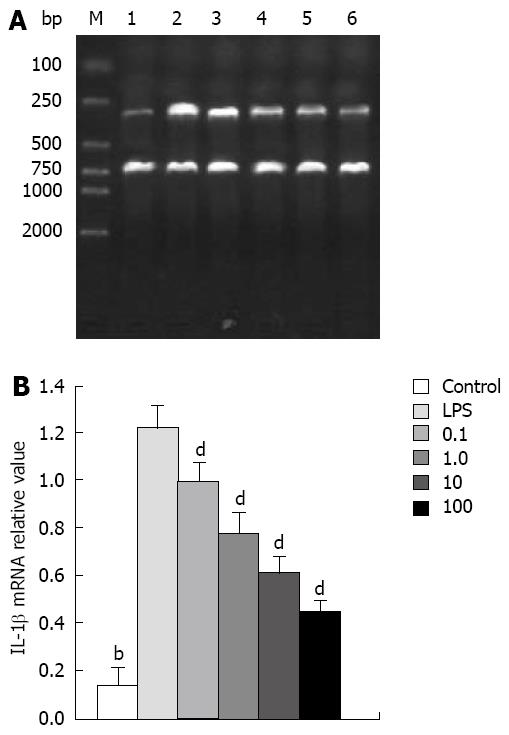
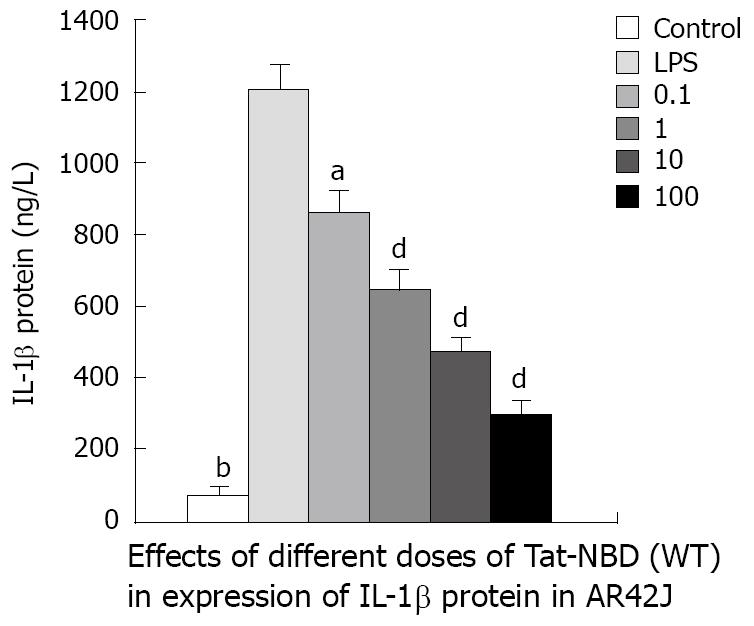
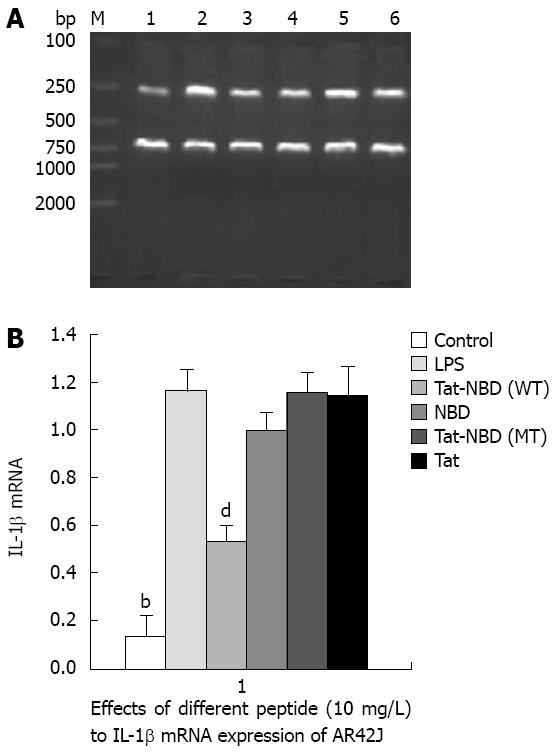
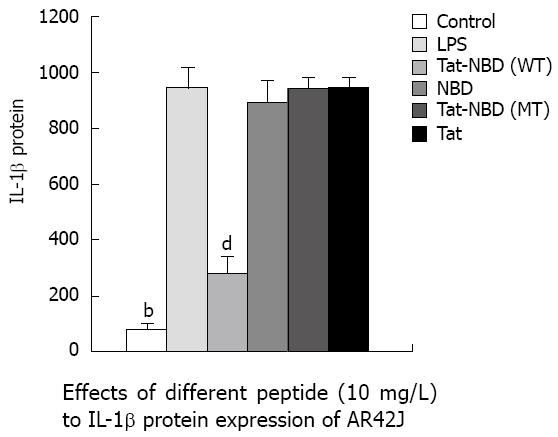
IL-1β: LPS (10 mg/L) resulted in an approximately 10-fold increase of IL-1β mRNA, whereas significant inhibitory effects were observed when cells were incubated with Tat-NBD (WT) at a dose of 0.1 mg/L (Figure 3). The Tat-NBD (WT) peptide decreased IL-1β mRNA expression in a dose-dependent manner and its peak role appeared at a dose of 100 mg/L (Figure 4). We next determined whether Tat-NBD (WT) peptide attenuated IL-1β protein, which was activated by LPS. Culture medium was analyzed by ELISA. Results confirmed that IL-1β protein was inhibited by Tat-NBD (WT) at different doses, which also presented in a dose-dependent manner. Finally, we investigated whether the control peptide of NBD, Tat or Tat-NBD (MT) could block LPS-induced IL-1β mRNA or protein increasing in AR42J cells. Cells were preincubated for 2 h at a concentration of 10 mg/L of Tat-NBD (WT) or control prior to stimulation with LPS. Cells were harvested 120 min after addition of LPS. RT-PCR and ELISA were performed as stated. Only Tat-NBD (WT), not the control peptides, inhibited IL-1β expression (Figures 5 and 6).
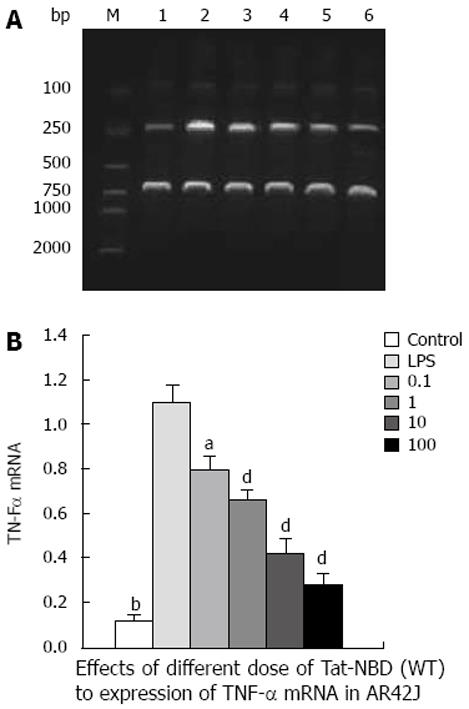
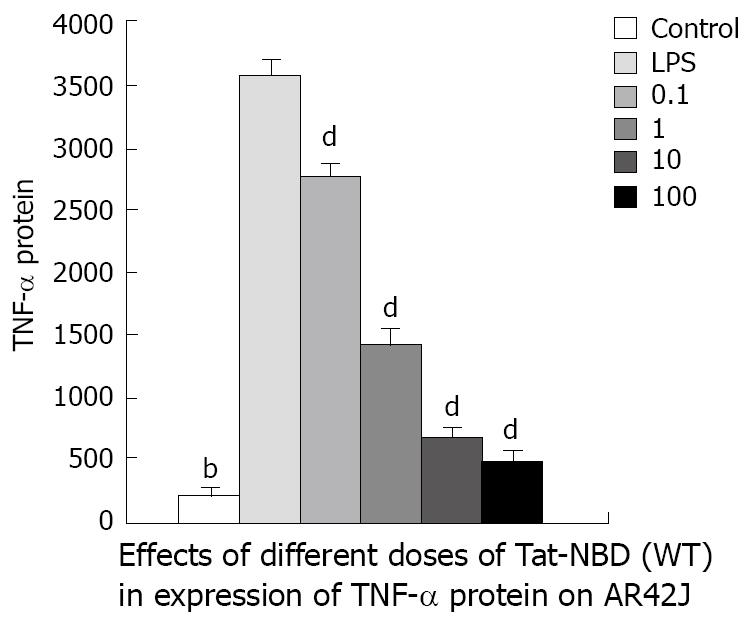
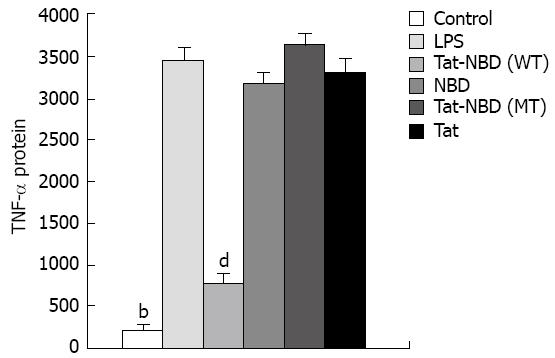
TNF-α: LPS (10 mg/L) resulted in an approximately 10-fold increase of TNF-α mRNA, whereas significant inhibitory effects were observed when cells were incubated with Tat-NBD (WT) at a dose of 0.1 mg/L (Figure 7). The data indicate that TAT-NBD (WT) decreased TNF-α protein in a dose-dependent fashion over a range of 0.1-100 mg/L (Figure 7). We determined whether Tat-NBD (WT) peptide attenuated TNF-α protein, which was activated by LPS. Culture medium was analyzed by ELISA. Result confirmed that TNF-α protein was inhibited by Tat-NBD (WT) at different doses, which also presented in a dose-dependent manner (Figure 8). Finally, we investigated whether the control peptide of NBD, Tat or Tat-NBD (MT) could block LPS-induced TNF-α mRNA or protein increasing in AR42J cells. Cells were preincubated for 2 h at a concentration of 10 mg/L of Tat-NBD (WT) or control prior to stimulation with LPS. Cells were harvested 120 min after addition of LPS. RT-PCR and ELISA were performed as stated. Only Tat-NBD (WT), not the control peptides, inhibited TNF-α expression (Figures 9 and 10).
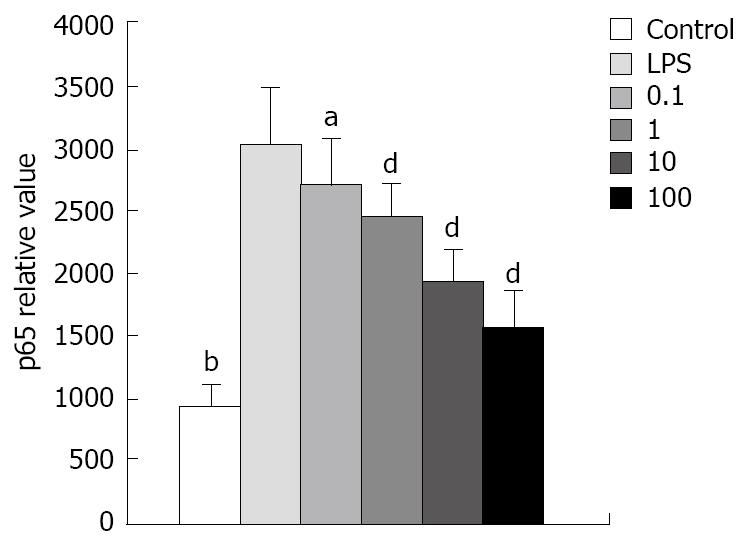
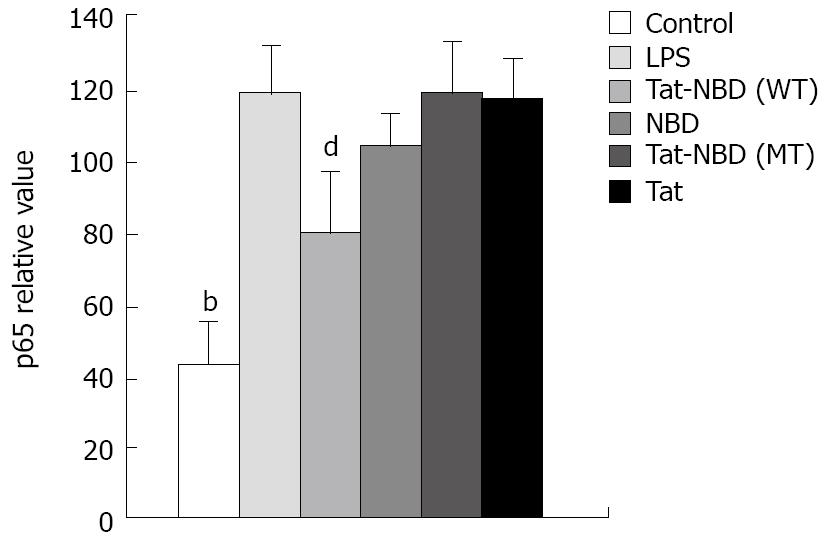
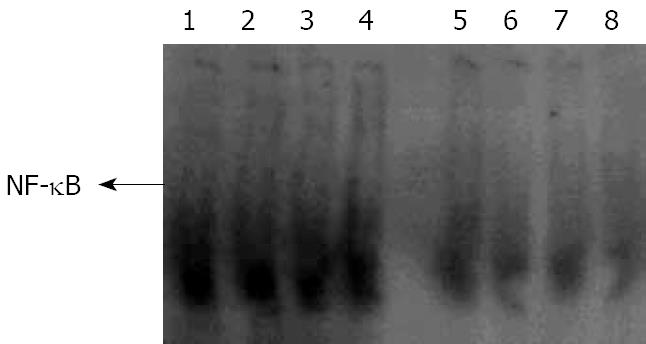
NF-κB change in AR42J: To know the role of p65 in the cytokine change in AR42J cells stimulated by LPS and whether the peptide interfered with p65 expression and nucleus transposition, we stained the AR42J cells by IH method. First, we performed experiments to demonstrate that AR42J cells can successfully express p65 under silence condition. Figure 11 shows the increasing histochemical staining of AR42J cells for p65 after stimulation with 0.1 mg/L of LPS. The data indicated that LPS increased p65 in a dose-dependent fashion over a range of 0.1-100 mg/L. We studied the effects of different doses of Tat-NBD (WT) in p65 expression. Cells were preincubated for 2 h at concentrations ranging from 0.1 mg/L to 100 mg/L. After pretreatment of Tat-NBD (WT), weakened immmunocytochemical staining of p65 in AR42J cells was observed, especially in larger dosage. Finally, we investigated whether the control peptide of NBD, Tat or Tat-NBD (MT) could block LPS-induced p65 increasing in AR42J cells. The results showed that only Tat-NBD (WT), not the control peptide inhibited p65 expression. EMSA demonstrated that the NF-κB DNA-binding activity was similar to the IH results (Figures 12 and 13).
It is known that a “cascade” induced by recruitment of inflammatory cells and release of inflammatory mediators is an important pathogenesis in the development of acute pancreatitis[17]. Inflammatory mediators, such as LPS and cytokines, may play a critical role in the development of murine acute pancreatitis. NF-κB formed dimeric complex that controls the expression of a variety of inducible genes involved in inflammation and proliferation[13]. It was reported that a selective inhibitor of NF-κB activation, NBD, demonstrated a significant decrease in pancreatic inflammation and lung hemorrhage associated with acute pancreatitis[11]. Here, rats were pretreated with the peptide at a dose larger than that used by Ethridge et al[12]. The Tat-NBD or NBD peptide resulted in tissue damage, including edema, hyperemia, necrosis, hemorrhage, and infiltration of inflammatory cells, decreased. Neither mutant Tat-NBD nor Tat peptide had preventative effects. It is suggested that treatment with Tat-NBD or NBD may be effective in this kind of pancreatitis model.
Inflammation and acinar cell death are the hallmarks of both human and experimental pancreatitis[18]. It is not clear whether peptides can have such direct anti-inflammatory effects in acinus cells. LPS stimulates cells by interaction with CD-14 in the context of Toll-like receptor, whose activation leads to NF-κB nuclear translocation through degradation of IκB and subsequent release of NF-κB[19]. The AR42J cell line is the only currently available cell line that maintains many characteristics of normal pancreatic acinar cells, such as the synthesis and secretion of digestive enzymes[20]. AR42J cell receptor expression and signal transduction mechanisms parallel those of pancreatic acinar cells. Thus, this cell line has been widely used as an in vitro model to study cellular secretion, growth, proliferation, and apoptosis of the exocrine pancreas[21]. So, we used LPS and AR42J as tools to explore peptide effects in present study. Further studies showed that in the early stages of LPS-stimulated inflammation in AR42J cells, consisting with sharp increasing expression of cytokine mRNA, such as IL-1β and TNF-α, which is confirmed as critical factors related to acinus injury[22]. The level of IL-1β and TNF-α protein also were increased in the culture medium. Our data, obtained from parallel studies, demonstrate that pretreatment with different doses of Tat-NBD peptide had constitutive inhibitory effects in acinus cells, resulting in reduced inflammatory cytokines IL-1β and TNF-α. These results are in agreement with previous reports[71011]. We found that the effects of peptide had a dose-dependent manner ranging from 0.1 to 100 mg/L. The largest anti-inflammation effects were found at a dose of 100 mg/L. We used the same concentration of control peptide, NBD, mutate Tat-NBD or Tat, and compared effects with those of Tat-NBD (WT). The results suggest that no control peptide had a preventive role. It has been confirmed that the Tat protein of human immunodeficiency virus 1 (HIV-1) can enter cells efficiently when added exogenously in the tissue culture[2324]. Also, Tat-mediated uptake may allow the therapeutic delivery of macromolecules previously thought to be impermeable to living cells[23]. Our data show that the sequence of Tat plays an intensive role in NBD sequence, and only the NBD sequence had a therapeutic role in STC-induced rat model as stated previously. The degree of permeability of Tat-NBD and NBD sequences in acinus cells must be confirmed in further studies.
The NBD peptide is a novel six-amino acid cell-permeable peptide that selectively inhibits NF-κB activation in vitro and in vivo by blocking the interaction of the regulatory protein NEMO with the IKK complex. In the present study, we detected AR42J cell NF-κB activity by EMSA at 24 h after LPS stimulation. During the parallel time course, the DNA-binding of NF-κB markedly inhibited Tat-NBD (WT) peptide (Figure 12), especially at the dose of 100 mg/L. Mutant Tat-NBD, NBD or Tat sequence had no significant effect on NF-κB activity. Consistent with the changes of NF-κB activity, the cell expression of p65 was also attenuated by Tat-NBD (WT) peptide in a dose-dependent manner (Figure 13). Like other studies, our results suggests that the Tat-NBD (WT) peptide plays a part in NF-κB activating pathway.
In conclusion, our results support the idea that active NF-κB participates in the pathogenesis of STC-induced acute pancreatitis in rats. Tat-NBD (WT) peptide has anti-inflammatory effects in this model and inhibits the inflammation of acinus simulated by LPS directly. Inhibition of NF-κB activity may be a protective measure in the treatment of acute pancreatitis.
The Tat-NEMO-binding domain (NBD) confirmed that it could attenuate the severity of cerulein-induced pancreatitis in mice which improved the inflammation in the pancreas, reduced hemorrhage in the lungs, and lowered myeloperoxidase activity in both pancreas and lung.
In rat pancreatitis induced by taurocholate, activation of NF-κB in pancreas has been reported. It is not clear yet whether peptide has anti-inflammatory effects in taurocholate-induced severe acute pancreatitis (SAP) model and inhibits the inflammation of acinus stimulated by lipopolysaccharides (LPS).
Tat-NBD peptide is able to inhibit the inflammation of AR42J cells. Further studies are needed to prove its mechanism of action in pancreatic acinar cells.
Tat-NBD (WT) peptide has anti-inflammatory effects in STC-induced pancreatitis model and inhibits the inflammation of acinus simulated by LPS directly. Inhibition of NF-κB activity may be a protective measure in the treatment of acute pancreatitis.
This is an interesting study in which the authors used an alternate approach to demonstrate the influence of inflammatory cytokines involvement in acute pancreatitis.
| 1. | Chen X, Ji B, Han B, Ernst SA, Simeone D, Logsdon CD. NF-kappaB activation in pancreas induces pancreatic and systemic inflammatory response. Gastroenterology. 2002;122:448-457. |
| 2. | Rahman SH, Ammori BJ, Larvin M, McMahon MJ. Increased nitric oxide excretion in patients with severe acute pancreatitis: evidence of an endotoxin mediated inflammatory response? Gut. 2003;52:270-274. |
| 3. | Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986;705-716. |
| 4. | Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7-11. |
| 5. | Granger J, Remick D. Acute pancreatitis: models, markers, and mediators. Shock. 2005;24 Suppl 1:45-51. |
| 6. | Long J, Song N, Liu XP, Guo KJ, Guo RX. Nuclear factor-kappaB activation on the reactive oxygen species in acute necrotizing pancreatitic rats. World J Gastroenterol. 2005;11:4277-4280. |
| 7. | Dai S, Hirayama T, Abbas S, Abu-Amer Y. The IkappaB kinase (IKK) inhibitor, NEMO-binding domain peptide, blocks osteoclastogenesis and bone erosion in inflammatory arthritis. J Biol Chem. 2004;279:37219-37222. |
| 8. | Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280-288. |
| 9. | May MJ, Marienfeld RB, Ghosh S. Characterization of the Ikappa B-kinase NEMO binding domain. J Biol Chem. 2002;277:45992-46000. |
| 10. | Strickland I, Ghosh S. Use of cell permeable NBD peptides for suppression of inflammation. Ann Rheum Dis. 2006;65 Suppl 3:iii75-iii82. |
| 11. | May MJ, D'Acquisto F, Madge LA, Glockner J, Pober JS, Ghosh S. Selective inhibition of NF-kappaB activation by a peptide that blocks the interaction of NEMO with the IkappaB kinase complex. Science. 2000;289:1550-1554. |
| 12. | Ethridge RT, Hashimoto K, Chung DH, Ehlers RA, Rajaraman S, Evers BM. Selective inhibition of NF-kappaB attenuates the severity of cerulein-induced acute pancreatitis. J Am Coll Surg. 2002;195:497-505. |
| 13. | Vaquero E, Gukovsky I, Zaninovic V, Gukovskaya AS, Pandol SJ. Localized pancreatic NF-kappaB activation and inflammatory response in taurocholate-induced pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1197-G1208. |
| 14. | Aho HJ, Koskensalo SM, Nevalainen TJ. Experimental pancreatitis in the rat. Sodium taurocholate-induced acute haemorrhagic pancreatitis. Scand J Gastroenterol. 1980;15:411-416. |
| 15. | Spormann H, Sokolowski A, Letko G. Effect of temporary ischemia upon development and histological patterns of acute pancreatitis in the rat. Pathol Res Pract. 1989;184:507-513. |
| 16. | Molloy PL. Electrophoretic mobility shift assays. Methods Mol Biol. 2000;130:235-246. |
| 17. | Vonlaufen A, Apte MV, Imhof BA, Frossard JL. The role of inflammatory and parenchymal cells in acute pancreatitis. J Pathol. 2007;213:239-248. |
| 18. | Cohen MC, Cohen S. Cytokine function: a study in biologic diversity. Am J Clin Pathol. 1996;105:589-598. |
| 19. | Matsuda N, Nishihira J, Takahashi Y, Kemmotsu O, Hattori Y. Role of macrophage migration inhibitory factor in acute lung injury in mice with acute pancreatitis complicated by endotoxemia. Am J Respir Cell Mol Biol. 2006;35:198-205. |
| 20. | Logsdon CD, Guthrie J, Alves F, Rosewicz S. Glucocorticoids have opposite effects on ornithine decarboxylase and cell growth in pancreatic acinar AR42J cells. Yale J Biol Med. 1992;65:449-456; discussion 465-469. |
| 21. | Song JY, Lim JW, Kim H, Morio T, Kim KH. Oxidative stress induces nuclear loss of DNA repair proteins Ku70 and Ku80 and apoptosis in pancreatic acinar AR42J cells. J Biol Chem. 2003;278:36676-36687. |
| 22. | Brivet FG, Emilie D, Galanaud P. Pro- and anti-inflammatory cytokines during acute severe pancreatitis: an early and sustained response, although unpredictable of death. Parisian Study Group on Acute Pancreatitis. Crit Care Med. 1999;27:749-755. |
| 23. | Fawell S, Seery J, Daikh Y, Moore C, Chen LL, Pepinsky B, Barsoum J. Tat-mediated delivery of heterologous proteins into cells. Proc Natl Acad Sci USA. 1994;91:664-668. |
| 24. | Schwarze SR, Dowdy SF. In vivo protein transduction: intracellular delivery of biologically active proteins, compounds and DNA. Trends Pharmacol Sci. 2000;21. |