Published online Dec 28, 2009. doi: 10.3748/wjg.15.6096
Revised: October 5, 2009
Accepted: October 12, 2009
Published online: December 28, 2009
AIM: To compare a first diagnostic procedure of transbronchial needle aspiration (TBNA) with selection of endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) or TBNA for mediastinal lymphadenopathy.
METHODS: Sixty-eight consecutive patients with mediastinal lymphadenopathy on computed tomography (CT), who required cytopathological diagnosis, were recruited. The first 34 underwent a sequential approach in which TBNA was performed first, followed by EUS-FNA if TBNA was unrevealing. The next 34 underwent a selective approach where either TBNA or EUS-FNA was selected as the first procedure based on the CT findings.
RESULTS: The diagnostic yield of TBNA as the first diagnostic procedure in the sequential approach was 62%. In the selective approach, the diagnostic yield of the first procedure was 71%. There was no significant difference in the overall diagnostic yield, but there were significantly fewer combined procedures with the selective approach.
CONCLUSION: Selecting either EUS-FNA or TBNA as the first diagnostic procedure achieved a comparable diagnostic yield with significantly fewer procedures than performing TBNA first in all patients.
- Citation: Khoo KL, Ho KY, Khor CJL, Nilsson B, Lim TK. First endoscopic procedure for diagnosis and staging of mediastinal lymphadenopathy. World J Gastroenterol 2009; 15(48): 6096-6101
- URL: https://www.wjgnet.com/1007-9327/full/v15/i48/6096.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.6096
Lung cancer is the commonest cause of mediastinal lymphadenopathy. For non-small cell lung cancer (NSCLC), which accounts for about 80% of lung cancers, mediastinal lymph node enlargement occurs in up to 38% of cases at diagnosis[1]. As surgical resection of NSCLC offers the best chance of cure in patients without distant metastases, the pathological confirmation of cancer spread to enlarged mediastinal lymph nodes is crucial to staging because this excludes curative surgical resection.
In the approach to suspected lung cancer without distant metastases, the lung mass is the initial target for cytopathological diagnosis. Following a diagnosis of NSCLC, mediastinal staging is the next step. In patients with mediastinal lymphadenopathy however, the mediastinum may be targeted first, even when a lung mass is present. This might achieve simultaneous diagnosis and mediastinal staging of lung cancer with a single procedure.
The esophagus and tracheobronchial tree offer endoluminal access to mediastinal lymph nodes, therefore endoscopic techniques such as endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) and transbronchial needle aspiration (TBNA) offer minimally invasive approaches for diagnosis of mediastinal lymphadenopathy.
Although EUS-FNA has a higher accuracy than TBNA, the transbronchial approach is preferred for anterior and right paratracheal lymph nodes. Real-time endobronchial ultrasound-guided TBNA (EBUS-TBNA) is now available but requires expensive specialized equipment and operator training. TBNA does not require specialized equipment and can be performed during the initial diagnostic bronchoscopy[2-4]. When we evaluated patients with mediastinal lymphadenopathy with bronchoscopy and TBNA, the diagnostic yield for mediastinal lymphadenopathy was 65%[3].
We have also used EUS-FNA for cases in which TBNA was unrevealing or non-diagnostic, given its higher accuracy[4]. However, this resulted in subjecting these patients to two diagnostic procedures even though both procedures could be performed in the same outpatient session[4,5].
We then hypothesized that bronchoscopy with TBNA need not be performed as the first procedure in all cases of mediastinal lymphadenopathy, and that by selecting the appropriate endoscopic procedure based on anatomical access, a higher diagnostic yield could be obtained after the first procedure. This could also result in subjecting the patient to fewer diagnostic procedures. Therefore, in this study, we compared an approach utilizing TBNA as the first diagnostic procedure with one utilizing selection of either EUS-FNA or TBNA.
Between December 2003 and June 2006, consecutive patients with mediastinal lymphadenopathy on thoracic computed tomography (CT) who presented to, or were referred to our respiratory division for cytopathological diagnosis were recruited for the study. Mediastinal lymphadenopathy was defined as a node larger than 1 cm in its short axis. The institutional review board of our hospital approved the study and informed consent was obtained for all the procedures.
During the first 16 mo of the study period, we employed a sequential approach for which bronchoscopy with TBNA was performed as the first diagnostic procedure, with or without other conventional bronchoscopic techniques. If TBNA was unrevealing on rapid on-site cytopathological evaluation (ROSE), EUS-FNA was performed immediately after TBNA, during the same session. Details of this approach and the results of the first 20 patients have been described when we explored the one-stop approach to mediastinal lymphadenopathy[4,5].
From April 2005, we employed a selective approach for which either EUS-FNA or TBNA was performed as the first diagnostic procedure. This was selected based on the predominant location of the lymphadenopathy on CT. If either the esophageal or transbronchial approach could access the nodes, the pulmonologist was left to decide which procedure he deemed most appropriate. In general, TBNA was selected mainly for patients with right paratracheal lymphadenopathy, whereas EUS-FNA was the preferred option for left paratracheal lymphadenopathy. Subcarinal lymph nodes could be approached by either procedure. If TBNA was selected as the first diagnostic procedure, EUS-FNA remained a subsequent option.
Bronchoscopy was performed by experienced pulmonologists using standard flexible videobronchoscopes (Olympus Optical Co. Ltd., Tokyo, Japan). Premedication with pethidine and atropine and sedation with midazolam were optional, while all patients received topical anesthesia with xylocaine. TBNA was performed blind with a Wang 22-gauge (MW 222) cytology needle (Bard Endoscopic Technologies, Billerica, MA, USA) at sites of mediastinal lymph node enlargement based on review of the CT scan. TBNA was performed before other conventional bronchoscopic procedures to avoid contamination.
EUS-FNA was performed as previously described using the curved linear array echoendoscope (GF-UC30P; Olympus) by experienced gastroenterologists[6]. Patients received topical anesthesia with xylocaine and sedation with a combination of midazolam and pethidine.
ROSE was employed to determine the adequacy of the needle aspirates. The aspirated material was blown onto a slide using the direct smear technique[7]. The smears were either air-dried and stained with Diff-Quik (American Scientific Products, McGraw Park, IL, USA) or fixed immediately in 95% ethanol and stained with Papanicolaou stain. Solid particles were fixed in formalin, routinely processed, and made into cell blocks for histological examination. The air-dried smears for Diff-Quik staining were reviewed immediately by an experienced cytotechnician. Endoscopists were then advised as to the need for additional needle aspirates (up to a maximum of six passes).
The final cytopathological diagnoses were made based upon analysis of the aspirated material by experienced cytopathologists. The diagnostic yield of TBNA was the number of patients in whom a definite diagnosis was made by TBNA over the total number of patients subjected to TBNA. The diagnostic yield after the first procedure was the number of patients in whom a definite diagnosis was made after the first procedure over the total number of patients. The overall diagnostic yield for each approach was the number of patients in whom a definitive diagnosis was made by needle aspiration over the total number of patients. When a diagnosis could not be made by either procedure, the final diagnostic categories were determined by review of further tests and clinical assessments.
Descriptive statistics are presented as mean ± SD. Discrete variables were analyzed with χ2 test and P < 0.05 was defined as statistically significant.
Sixty-eight consecutive patients with mediastinal lymphadenopathy on CT were recruited during the study period. The main indication for CT was suspected malignancy (n = 58). Other indications included suspected pulmonary embolism (n = 4), pyrexia of unknown origin (n = 2), suspected aortic dissection (n = 1), investigation of weight loss (n = 1), suspected sarcoidosis (n = 1), and follow-up of non-Hodgkin’s lymphoma (n = 1).
The baseline characteristics and diagnostic categories of the sequential group (n = 34) and the selective group (n = 34) were similar (Table 1).
| Variables | Sequential approach | Selective approach | P value |
| No. of patients | 34 | 34 | |
| Male/female | 24/10 | 25/9 | |
| Age (mean ± SD, yr) | 64.7 ± 11.2 | 65.1 ± 12.7 | |
| Mass on CT | 24 | 23 | |
| Right-sided/left-sided, | 13/11 | 12/11 | |
| No. of patients undergoing | |||
| TBNA | 34 (100) | 22 (65) | < 0.001 |
| TBNA and EUS-FNA | 11 (32) | 2 (6) | < 0.05 |
| Diagnostic yield (%) | |||
| First procedure | 62 | 71 | 0.6 |
| TBNA | 62 | 73 | 0.6 |
| Overall | 79 | 73 | 0.8 |
| Diagnostic categories | |||
| Malignancy | 26 | 28 | |
| NSCLC/SCLC | 22/3 | 21/7 | |
| Benign tumor | 8 | 6 | |
| Sarcoid/tuberculosis | 2/2 | 1/1 |
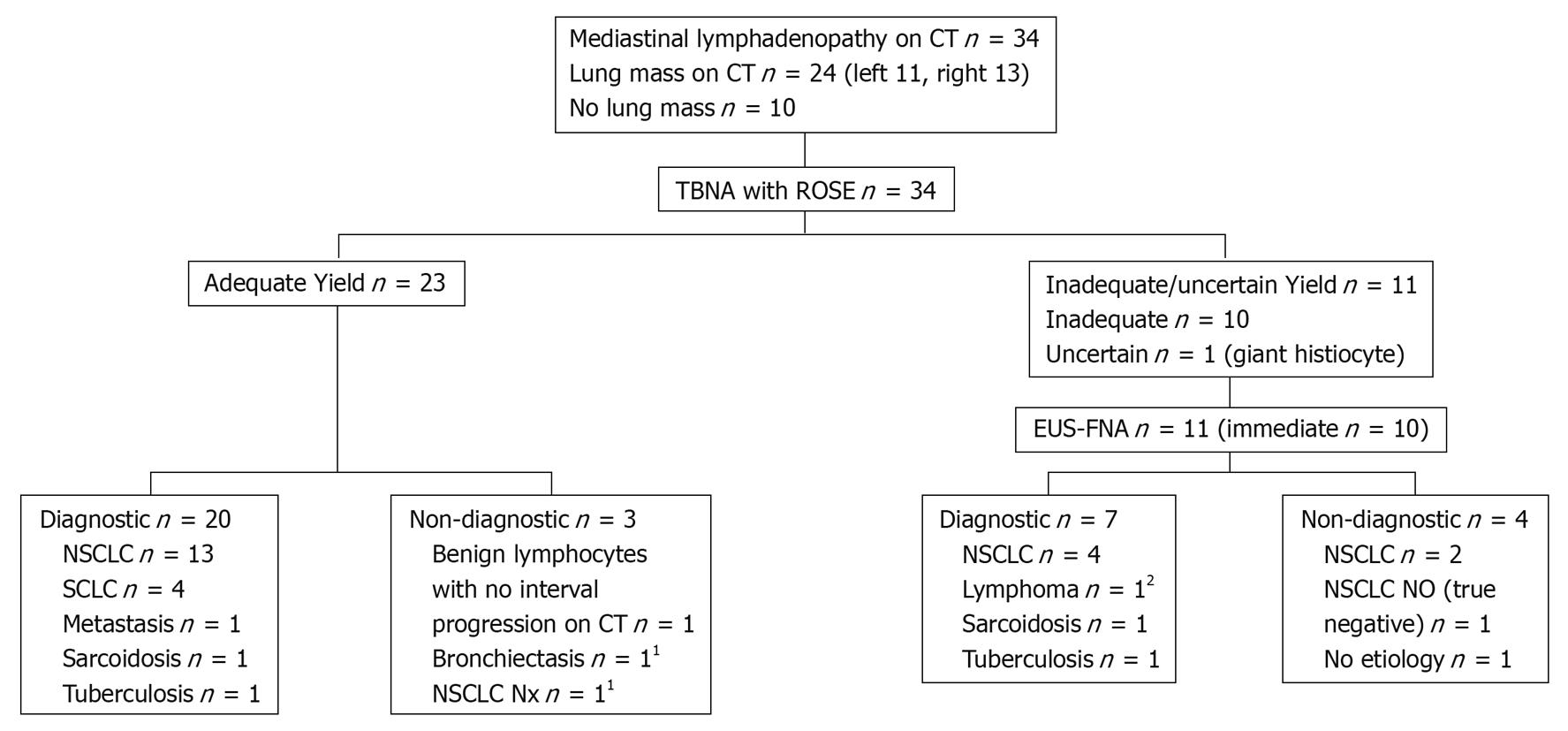
Results of the sequential approach are shown in Figure 1. TBNA was performed at the following mediastinal sites according to regional lymph node map definitions as described by Mountain et al[8]: 4R in 10 patients, 7 in 24 patients, and 4L in 7 patients. The TBNA obtained adequate specimens in 23 of the 34 patients. In the remaining 11 patients, TBNA with ROSE showed the specimens to be inadequate or unrevealing, thus, EUS-FNA was performed immediately after bronchoscopy, at lymph node stations 7 (seven patients), 4L (10 patients) and 4R (one patient). Some patients had TBNA or EUS-FNA performed at more than one lymph node station. When the final cytopathological results were analyzed, TBNA with ROSE was falsely negative in one patient. In another patient, TBNA with ROSE showed a giant histiocyte and a decision was made to proceed with EUS-FNA. The final cytopathological diagnosis for both specimens returned as granulomatous inflammation. Results of the first 20 patients with this approach have been described previously[4].
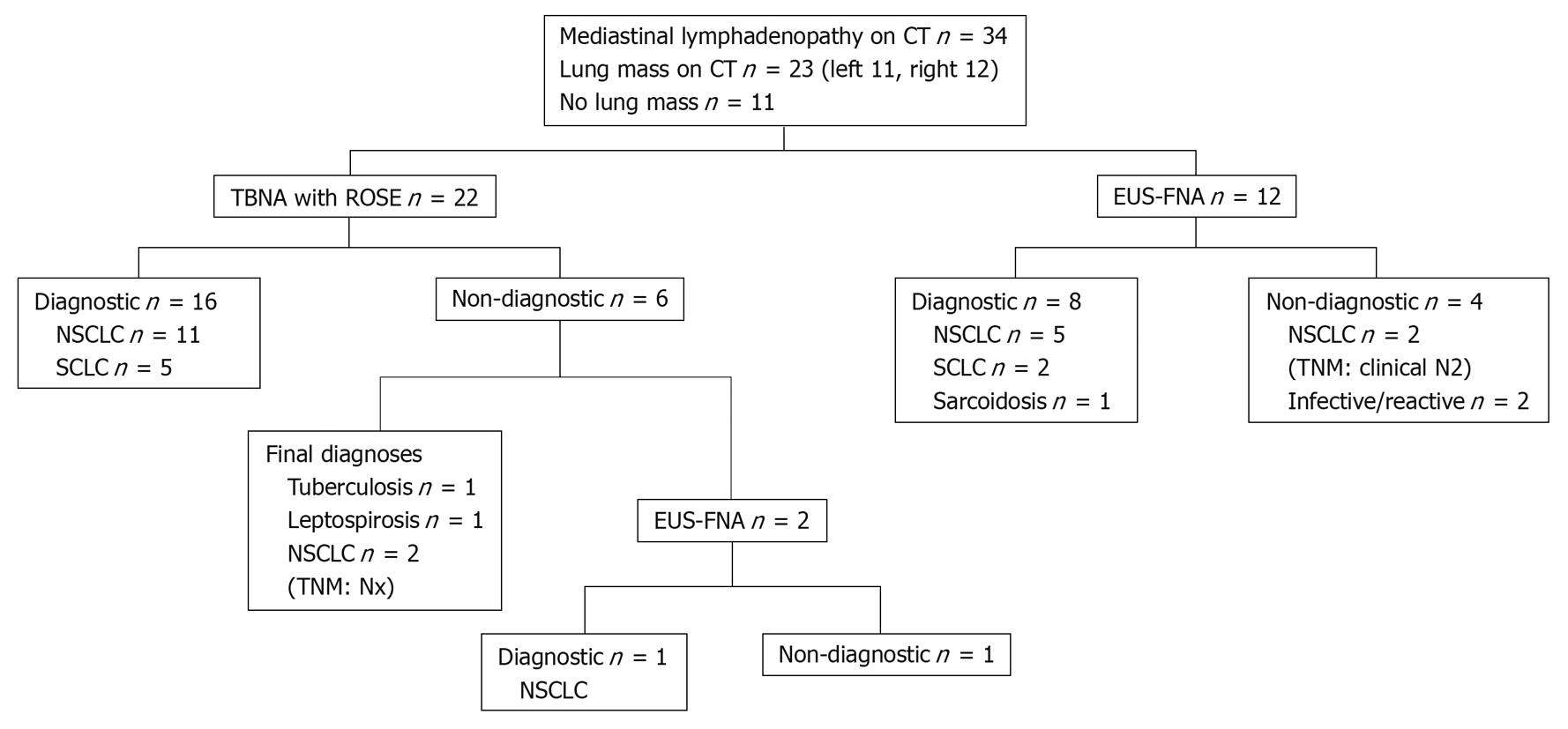
Results of the selective approach are shown in Figure 2. TBNA was performed in 22 patients in the following mediastinal sites: 4R (six patients) and 7 (19 patients). EUS-FNA was performed as a first diagnostic procedure in 12 patients at lymph node stations 7 (12 patients), 4L (five patients) and 2R (one patient, Figure 3). In contrast to the sequential approach for which all 34 patients had TBNA performed first, 35% (12/34) of patients in the selective approach had EUS-FNA performed first, while the remaining 65% (22/34) had TBNA performed first. In the selective approach, TBNA was performed only for right paratracheal and subcarinal stations, whereas EUS was performed predominantly in the left paratracheal and subcarinal stations.
The diagnostic yield of TBNA as the first diagnostic test was 62% in the sequential approach, while the diagnostic yield of the first diagnostic procedure in the selective approach was 71%. The diagnostic yield of EUS-FNA was 67% (8/12). The overall diagnostic yield of the sequential approach was 79% (27/34) and that of the selective approach was 74% (25/34). There was no significant difference in the overall diagnostic yields. Significantly fewer combined diagnostic procedures (6% vs 32%, P < 0.05) were required with the selective approach. The yield of TBNA was higher with the selective approach (73%) as compared to the sequential approach (62%). There were no complications with either TBNA or EUS-FNA, or damage to the bronchoscopes or endoscopes.
The present study compared a diagnostic approach utilizing TBNA as the first diagnostic procedure with one in which EUS-FNA or TBNA was selected as the first procedure. The selection was based on whether the optimal anatomical approach was transesophageal or transbronchial. We found a higher diagnostic yield after the first diagnostic procedure with the selective approach, and this translated to a significant reduction in the number of diagnostic procedures performed.
The transesophageal and transbronchial routes to the mediastinum are complementary. The transesophageal approach has limited access to the right paratracheal nodes, therefore, the endobronchial route offers better access, as shown by Herth et al[9]. Therefore, the procedure of choice for right paratracheal lymphadenopathy with the selective approach was TBNA, unless CT showed a peri-esophageal location of these nodes that was easily accessed by EUS-FNA (Figure 3). Harrow et al[10] also have shown that the right paratracheal and subcarinal locations are predictors of a positive aspirate with TBNA. With the selective approach, TBNA was limited to these two locations, and the yield of TBNA improved from 62% to 73%.
Although mediastinoscopy remains the diagnostic standard for the mediastinal staging of lung cancer, with a sensitivity of 80%-85%, this invasive surgical procedure requires general anesthesia and has a morbidity and mortality rate of 2% and 0.08%, respectively[11]. In contrast, both TBNA and EUS-FNA are minimally invasive and can be performed in the outpatient setting under local anesthesia and sedation. EUS-FNA permits real-time visualization of needle sampling and has been shown to be highly accurate in the mediastinal staging of lung cancer, as well as in the diagnosis of mediastinal lymphadenopathy of unknown etiology[6,12-18].
The development of EBUS-TBNA for mediastinal lymph nodes has lagged behind EUS-FNA by more than a decade[19,20]. As such, the new convex-probe EBUS is still not as widely available as EUS. Wallace et al[21] have suggested that the use of ultrasound-guided needle sampling of mediastinal lymph nodes in patients with suspected lung cancer, whether by EUS or EBUS, is superior to conventional TBNA. By combining EUS-FNA and EBUS-TBNA, they have achieved a near-complete medical mediastinoscopy, thus reinforcing the complementary nature these procedures[22].
Our aim was not to achieve comprehensive staging of the mediastinum in the setting of lung cancer, but rather, to demonstrate that, with appropriate selection of the first endoscopic procedure, a higher diagnostic yield could be obtained. This would mean that EUS-FNA could be selected as the first procedure, rather than routinely subjecting all patients to bronchoscopy. Indeed, a recent meta-analysis has suggested that EUS-FNA is the diagnostic test of choice for mediastinal lymphadenopathy[23]. In addition, the transesophageal route may be better tolerated as compared to the transbronchial route, with less coughing and the absence of obstruction of the needle by cartilaginous rings.
Most studies with EUS-FNA for mediastinal evaluation have been performed in patients only after confirmation of the diagnosis of NSCLC. Singh et al[24], however, have demonstrated that EUS-FNA may be performed as the first diagnostic procedure for suspected lung cancer. In the setting of mediastinal lymphadenopathy in NSCLC, this diagnostic procedure also has enabled simultaneous mediastinal staging. Thus, besides showing that bronchoscopy need not be the first diagnostic procedure in patients with suspected lung cancer, they also have demonstrated that diagnosis and staging of lung cancer need not be performed sequentially or require multiple procedures. This highlights a paradigm shift where mediastinal staging is no longer performed only after confirming the diagnosis of NSCLC.
We believe that, in the diagnostic approach to the mediastinum, the transesophageal and transbronchial routes are complementary rather than competing. Instead of pitting TBNA against EUS-FNA, this study emphasizes that the complementary value of these endoscopic approaches is best exploited by appropriate procedure selection. Thus, when either EUS-FNA or TBNA was selected as the first procedure, the diagnostic yield increased from 62% to 71%, thereby significantly reducing the need for additional procedures. Targeting the mediastinum first to enable simultaneous diagnosis and staging, and optimizing the yield of the first diagnostic procedure may lead to fewer delays in the treatment of lung cancer patients. Devbhandari et al[25] have reported that a negative initial bronchoscopy in suspected lung cancer resulted in significant delays in diagnosis and treatment. In that study, initial bronchoscopy was diagnostic in less than 50% of cases.
The present study had several limitations. Firstly, this was not a randomized trial and the patient population was small. However, they were consecutive patients with similar baseline characteristics and diagnostic categories (Table 1). Secondly, a definitive diagnosis could not be made in all cases because some patients and their referring physicians declined further invasive surgical sampling. However, the aim of this study was to determine the diagnostic yield of the sequential and selective approaches rather than the accuracy of either endoscopic procedure. Thirdly, conventional TBNA was employed rather than EBUS-TBNA. This was because at the time of the study, EBUS-TBNA was not available at our center.
Three practical clinical points are highlighted here. Firstly, the cytopathological diagnosis of mediastinal lymphadenopathy may be achieved in the majority of patients utilizing widely available endoscopic techniques. Secondly, targeting the mediastinum first may establish simultaneously diagnosis as well as mediastinal staging for patients with NSCLC. Finally, appropriate selection of the first diagnostic procedure may optimize the yield and minimize the number of procedures required for the diagnosis and/or staging of mediastinal lymphadenopathy. Thus, with the availability of EUS-FNA, bronchoscopy may no longer be required in selected patients with suspected lung cancer.
Endoscopic techniques are becoming essential high-utility tools in the investigative approach to the mediastinum. With the rapid evolution of newer endoscopic techniques, the physician’s diagnostic armamentarium is likely to expand. The question of which is the most appropriate initial diagnostic procedure for mediastinal lymphadenopathy, given what is available, will become even more important. While awaiting further studies comparing the different emerging endoscopic techniques and combination of techniques, we suggest that the optimal diagnostic approach for mediastinal lymphadenopathy depends on selection of the most appropriate initial diagnostic procedure.
In the absence of distant metastasis, mediastinal staging remains crucial for determining prognosis and therapy of non-small cell lung cancer. An approach to patients with mediastinal lymphadenopathy regardless of whether a lung mass in present, is to target the mediastinum first. This may achieve simultaneous diagnosis and mediastinal staging with a single procedure, in the event of diagnosis of lung cancer, which is the commonest cause of mediastinal lymphadenopathy.
The transbronchial and transesophageal routes allow minimally invasive endoscopic needle sampling of mediastinal lymph nodes. These endoscopic procedures may be done under sedation in contrast to the gold standard mediastinoscopy, which requires general anesthesia.
Minimally invasive mediastinal staging with endoscopic ultrasound-guided fine-needle aspiration and blind or endobronchial ultrasound guided transbronchial-needle aspiration may be a substitute for mediastinoscopy.
Not all patients require all three procedures, therefore, appropriate initial procedure selection may be important in the diagnostic approach to mediastinal lymphadenopathy, because only a single procedure may be diagnostic in the majority of cases.
This is a very interesting topic for the readers of World Journal of Gastroenterology.
Peer reviewers: Marko Duvnjak, MD, PhD, Head of Department of Gastroenterology and Hepatology, University Hospital “Sestre milosrdnice”, Vinogradska cesta 29, 10 000 Zagreb, Croatia; Damian Casadesus Rodriguez, MD, PhD, Calixto Garcia University Hospital, J and University, Vedado, Havana City, Cuba
S- Editor Wang YR L- Editor Kerr C E- Editor Zheng XM
| 1. | Dillemans B, Deneffe G, Verschakelen J, Decramer M. Value of computed tomography and mediastinoscopy in preoperative evaluation of mediastinal nodes in non-small cell lung cancer. A study of 569 patients. Eur J Cardiothorac Surg. 1994;8:37-42. |
| 2. | Harrow EM, Oldenburg FA Jr, Lingenfelter MS, Smith AM Jr. Transbronchial needle aspiration in clinical practice. A five-year experience. Chest. 1989;96:1268-1272. |
| 3. | Khoo KL, Chua GS, Mukhopadhyay A, Lim TK. Transbronchial needle aspiration: initial experience in routine diagnostic bronchoscopy. Respir Med. 2003;97:1200-1204. |
| 4. | Khoo KL, Ho KY, Nilsson B, Lim TK. EUS-guided FNA immediately after unrevealing transbronchial needle aspiration in the evaluation of mediastinal lymphadenopathy: a prospective study. Gastrointest Endosc. 2006;63:215-220. |
| 5. | Fritscher-Ravens A, Davidson BL. Targeting the mediastinum: one-stop shopping at all times? Gastrointest Endosc. 2006;63:221-222. |
| 6. | Wallace MB, Silvestri GA, Sahai AV, Hawes RH, Hoffman BJ, Durkalski V, Hennesey WS, Reed CE. Endoscopic ultrasound-guided fine needle aspiration for staging patients with carcinoma of the lung. Ann Thorac Surg. 2001;72:1861-1867. |
| 7. | Wang KP, Selcuk ZT, Erozan Y. Transbronchial needle aspiration for cytology specimens. Monaldi Arch Chest Dis. 1994;49:265-267. |
| 8. | Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest. 1997;111:1718-1723. |
| 9. | Herth FJ, Lunn W, Eberhardt R, Becker HD, Ernst A. Transbronchial versus transesophageal ultrasound-guided aspiration of enlarged mediastinal lymph nodes. Am J Respir Crit Care Med. 2005;171:1164-1167. |
| 10. | Harrow EM, Abi-Saleh W, Blum J, Harkin T, Gasparini S, Addrizzo-Harris DJ, Arroliga AC, Wight G, Mehta AC. The utility of transbronchial needle aspiration in the staging of bronchogenic carcinoma. Am J Respir Crit Care Med. 2000;161:601-607. |
| 11. | Kiser AC, Detterbeck FC. General aspects of surgical treatment. Diagnosis and treatment of lung cancer: an evidence-based guide for the practicing clinician. Philadelphia: WB Saunders 2001; 133-147. |
| 12. | Larsen SS, Krasnik M, Vilmann P, Jacobsen GK, Pedersen JH, Faurschou P, Folke K. Endoscopic ultrasound guided biopsy of mediastinal lesions has a major impact on patient management. Thorax. 2002;57:98-103. |
| 13. | Wiersema MJ, Vazquez-Sequeiros E, Wiersema LM. Evaluation of mediastinal lymphadenopathy with endoscopic US-guided fine-needle aspiration biopsy. Radiology. 2001;219:252-257. |
| 14. | Fritscher-Ravens A, Sriram PV, Bobrowski C, Pforte A, Topalidis T, Krause C, Jaeckle S, Thonke F, Soehendra N. Mediastinal lymphadenopathy in patients with or without previous malignancy: EUS-FNA-based differential cytodiagnosis in 153 patients. Am J Gastroenterol. 2000;95:2278-2284. |
| 15. | Gress FG, Savides TJ, Sandler A, Kesler K, Conces D, Cummings O, Mathur P, Ikenberry S, Bilderback S, Hawes R. Endoscopic ultrasonography, fine-needle aspiration biopsy guided by endoscopic ultrasonography, and computed tomography in the preoperative staging of non-small-cell lung cancer: a comparison study. Ann Intern Med. 1997;127:604-612. |
| 16. | Silvestri GA, Hoffman BJ, Bhutani MS, Hawes RH, Coppage L, Sanders-Cliette A, Reed CE. Endoscopic ultrasound with fine-needle aspiration in the diagnosis and staging of lung cancer. Ann Thorac Surg. 1996;61:1441-1445; discussion 1445-1446. |
| 17. | Fritscher-Ravens A, Davidson BL, Hauber HP, Bohuslavizki KH, Bobrowski C, Lund C, Knöfel WT, Soehendra N, Brandt L, Pepe MS. Endoscopic ultrasound, positron emission tomography, and computerized tomography for lung cancer. Am J Respir Crit Care Med. 2003;168:1293-1297. |
| 18. | Caddy G, Conron M, Wright G, Desmond P, Hart D, Chen RY. The accuracy of EUS-FNA in assessing mediastinal lymphadenopathy and staging patients with NSCLC. Eur Respir J. 2005;25:410-415. |
| 19. | Giovannini M, Seitz JF, Monges G, Perrier H, Rabbia I. Fine-needle aspiration cytology guided by endoscopic ultrasonography: results in 141 patients. Endoscopy. 1995;27:171-177. |
| 20. | Yasufuku K, Chiyo M, Sekine Y, Chhajed PN, Shibuya K, Iizasa T, Fujisawa T. Real-time endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. Chest. 2004;126:122-128. |
| 21. | Wallace MB, Pascual JM, Raimondo M, Woodward TA, McComb BL, Crook JE, Johnson MM, Al-Haddad MA, Gross SA, Pungpapong S. Minimally invasive endoscopic staging of suspected lung cancer. JAMA. 2008;299:540-546. |
| 22. | Vilmann P, Krasnik M, Larsen SS, Jacobsen GK, Clementsen P. Transesophageal endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) biopsy: a combined approach in the evaluation of mediastinal lesions. Endoscopy. 2005;37:833-839. |
| 23. | Puli SR, Batapati Krishna Reddy J, Bechtold ML, Ibdah JA, Antillon D, Singh S, Olyaee M, Antillon MR. Endoscopic ultrasound: it's accuracy in evaluating mediastinal lymphadenopathy? A meta-analysis and systematic review. World J Gastroenterol. 2008;14:3028-3037. |
| 24. | Singh P, Camazine B, Jadhav Y, Gupta R, Mukhopadhyay P, Khan A, Reddy R, Zheng Q, Smith DD, Khode R. Endoscopic ultrasound as a first test for diagnosis and staging of lung cancer: a prospective study. Am J Respir Crit Care Med. 2007;175:345-354. |
| 25. | Devbhandari MP, Quennell P, Krysiak P, Shah R, Jones MT. Implications of a negative bronchoscopy on waiting times to treatment for lung cancer patients: results of a prospective tracking study. Eur J Cardiothorac Surg. 2008;34:479-483. |