Published online Dec 14, 2009. doi: 10.3748/wjg.15.5855
Revised: October 20, 2009
Accepted: October 27, 2009
Published online: December 14, 2009
AIM: To evaluate the role of genetic factors in the pathogenesis of idiopathic infant cholestasis.
METHODS: We performed a case-control study, including 78 infants with idiopathic infant cholestasis and 113 healthy infants as controls. Genomic DNA was extracted from peripheral venous blood leukocytes using phenol chloroform methodology. Polymerase chain reaction was used to amplify the multidrug resistance protein 3 (MDR3) R652G fragment, and products were sequenced using the ABI 3100 Sequencer.
RESULTS: The R652G single nucleotide polymorphism (SNP) was significantly more frequent in healthy infants (allele frequency 8.0%) than in patients (allele frequency 2.60%) (P < 0.05), odds ratio, 0.29; 95% confidence interval, 0.12-0.84. The conjugated bilirubin in patients with the AG genotype was significantly lower than in those with the AA genotype (44.70 ± 6.15 μmol/L vs 95.52 ± 5.93 μmol/L, P < 0.05).
CONCLUSION: MDR3 R652G is negatively correlated with idiopathic infant cholestasis. Children with the R652G SNP in Guangxi of China may have reduced susceptibility to infant intrahepatic cholestasis.
- Citation: Chen XQ, Wang LL, Shan QW, Tang Q, Lian SJ. Multidrug resistance protein 3 R652G may reduce susceptibility to idiopathic infant cholestasis. World J Gastroenterol 2009; 15(46): 5855-5858
- URL: https://www.wjgnet.com/1007-9327/full/v15/i46/5855.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5855
Idiopathic infant cholestasis is of unknown etiology and can occur in either a sporadic or a familial form. Most affected infants have a good prognosis. Approximately 5%-10% have persistent inflammation or fibrosis, and a few develop more severe liver disease, such as cirrhosis. Although the cause of idiopathic infant cholestasis is unknown, genetic factors have been suggested by the existence of some other entities with cholestasis, such as familial intrahepatic cholestasis-1 mutation in progressive familial intrahepatic cholestasis type 1 (PFIC-1), bile salt export pump deficiency in PFIC-2[1-3], multidrug resistance protein 3 (MDR3) deficiency in PFIC-3[4-6].
Studies have investigated the involvement of MDR3 variants in the development of disease, and we were interested to note that the MDR3 R652G single nucleotide polymorphism (SNP) has a high allele frequency in generally healthy people[7]. Our aim was to study the MDR3 R652G SNP distribution frequency in idiopathic infant cholestasis and healthy infants and to determine whether there were any differences between them and, if so, their relevance.
Seventy eight infants were diagnosed with idiopathic infant cholestasis in the case group and there were 113 healthy infants in the control group. The patients were included in the study on the basis of the following criteria: (1) a history of chronic cholestasis onset in the neonatal/infant period with unknown origin; (2) presence of hepatomegaly or hepatosplenomegaly; (3) persistent marked serum alanine aminotransferase (ALT) activity; (4) elevated total bile acid concentration and total bilirubin (TB), mainly marked conjugated bilirubin (CB); (5) absence of viral infections (such as hepatitis A virus, hepatitis B virus, hepatitis C virus, cytomegalovirus).
For patients and healthy infants, the following biochemical parameters were recorded: serum ALT and aspartate aminotransferase (AST); γ-glutamyltransferase (γ-GT) and serum TB and CB.
We received informed consent from all the subjects’ guardians to take part in the study, and the protocol was approved by the local ethical committee of the Hospital.
Genomic DNA was extracted from peripheral venous blood leukocytes using standard phenol chloroform procedures. The DNA concentration was quantified by spectrophotometry. Primers for MDR3 R652G were forward (5'-CATCCATTTGGAGACACACACAC-3') and reverse (5'-GTAGCAGTCATCTGTGCCTGAAA-3')[7]. The primary PCR product fragments were 348 bp. PCRs for generating the R652G fragments were generally performed in a reaction volume of 50 μL with 100 ng of genomic DNA, 1.5 U of Taq polymerase (Fermentas), 10 × PCR buffer (Fermentas), 1.5 mmol/L of MgCl2 (Fermentas), 200 μmol/L deoxynucleoside-5-triphosphate (Takara) and 20 μmol of each primer. PCR conditions included an initial denaturation step at 95°C for 5 min, followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 65°C for 30 s and extension at 72°C for 1 min. The PCR reaction was terminated after an extension step at 72°C for 10 min. The PCR products were analyzed by 2% agarose gel with 0.5 μg/mL ethidium bromide and quantitated approximately with a DNA marker (Takara, DaLiang). The PCR products sequence analysis was run in the ABI 3100 automated DNA sequencer. Sequences were compared with the sequence of the MDR3 in GenBank (NG_007118.1, GI: 169234676).
Data are given as mean ± SD. Comparison of the frequency of the R652G SNP was made between patients and controls, and the analysis of association with the phenotype was performed by χ2 test or Fisher’s exact test when appropriate. A P-value < 0.05 was considered statistically significant. The data was analyzed using SPSS 13.0. The odds ratio (OR) was calculated with the corresponding 95% confidence intervals (95% CI). Allele frequency was tested for the Hardy-Weinberg equilibrium.
The study comprised 78 infants with idiopathic infant cholestasis and 113 controls. Details of the study groups are shown in Table 1. The mean total bilirubin was 194.43 ± 13.33 μmol/L (normal ≤ 20 μmol/L). The mean CB was 105.75 ± 6.44 μmol/L. The mean serum transaminase levels were 273.86 ± 27.90 and 123.80 ± 11.63 U/L for AST and ALT, respectively (normal ≤ 40 U/L), and mean serum γ-GT was 308.60 ± 46.30 U/L (normal ≤ 50 U/L). The levels were all normal in the control group. All the patients had hepatomegaly, and 62 of the 78 cases had splenomegaly.
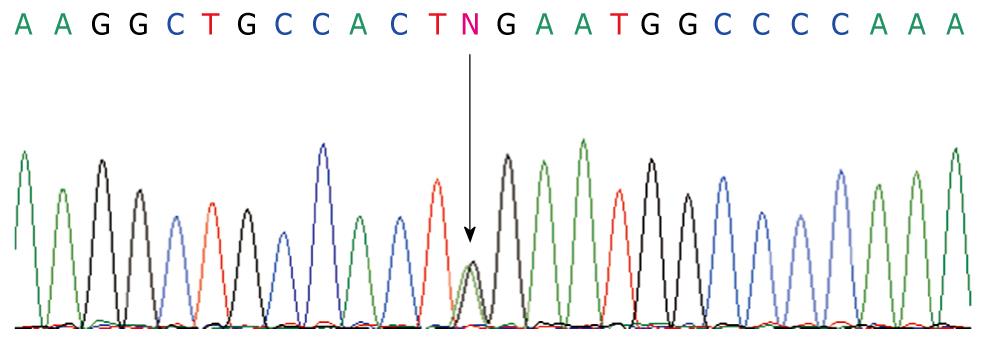
PCR products sequence analysis showed a heterozygous substitution A>G (Figure 1) in codon 652 which creates an amino acid substitution in codon R652G in exon 16. Distribution of the genetic polymorphisms in patients and controls is shown in Table 2. Test results showed that the R652G genotype distribution in the 2 groups was in line with the Hardy-Weinberg equilibrium, indicating that the selected sample was representative of the population. The patient AG genotype frequency was significantly lower than in the control group (P < 0.05). The AG genotype was negatively correlated with idiopathic infant cholestasis (OR, 0.29, 95% CI: 0.12-0.84) showing that the AG genotype has a protective effect in the normal population (Table 2).
| Groups | n | Genotypes | Allelefrequency (%) | P-value | ||
| A/A | A/G | G/G | ||||
| Patients | 78 | 74 (94.9) | 4 (5.1) | 0 | 2.6 | < 0.051 |
| Controls | 113 | 95 (84.1) | 18 (15.9) | 0 | 8.0 | |
The patient group was divided into 2 sub-groups according to genotype and serum biochemical parameters were compared. The CB of the AG genotype (44.70 ± 6.15 μmol/L) was significantly lower compared with that of the AA genotype (95.52 ± 5.93 μmol/L) (P < 0.05). Other serum markers in the sub-groups with the 2 genotypes were not significantly different (P > 0.05) (Table 3).
| Patients A/A | Patients A/G | |
| TB (μmol/L) | 198.94 ± 14.85 | 91.65 ± 15.70 |
| CB (μmol/L) | 95.52 ± 5.93 | 44.70 ± 6.15a |
| ALT (U/L) | 131.54 ± 11.91 | 92.50 ± 10.01 |
| AST (U/L) | 281.29 ± 28.26 | 129.25 ± 12.43 |
| γ-GT (U/L) | 317.68 ± 47.64 | 77.00 ± 18.78 |
Our study is the first report of the MDR3 R652G SNP in children in China. Our data showed that the R652G SNP had a significantly higher proportional distribution in normal infants than in idiopathic infant cholestasis patients. Moreover, in the cholestasis group, the CB of AG genotype patients had a lower mean value than in the AA genotype patients. It is known that CB is a very important index that can reflect the extent of intrahepatic cholestasis. For this reason, we can infer that the R652G variant has a specific protective effect in the normal population.
In previous studies, in the analysis of MDR3 gene sequence variants in different countries, the R652G SNP was the only protein-altering variant with high allele frequency in all groups. It was 7.3% in Caucasians, 1.4% in Japanese and 2.3% in Koreans[7]. In another study, the variant was 7.3% in healthy Caucasians, 8.6% in primary biliary cirrhosis patients and 12.8% primary sclerosing cholangitis patients[8]. R652G is a MDR3 gene mutation that results in non-synonymous amino acid substitutions but is not associated with disease. In Switzerland, a study showed that the MDR3 R652G variant was 7% in healthy Caucasians, 9% in drug-induced cholestatic patients and 4% in hepatocellular/mixed liver injury[9]. Pauli-Magnus et al[10] reported the R652G variant in 10% of intrahepatic cholestasis pregnancy cases and 16.3% in healthy controls.
The previous studies indicated that the R652G variant was prevalent in the general population. Furthermore, studies of MDR3 R652G in a variety of diseases showed no evidence that the R652G variant was related to disease. In our study, the R652G variant was 15.9% in healthy children, with a higher frequency than in infant cholestasis patients. Therefore, we believe that the AG genotype has a protective effect in infant cholestasis, and the AG genotype is likely to reduce the risk of children suffering from cholestasis. In this study, the proportion of the AG gene type in the normal population was higher than in previous reports from other regions, possibly because of the existence of racial and geographic differences.
There is also another phenomenon whereby R652G may have a protective effect and reduce the risk of suffering from disease. Jacquemin et al[11] reported one patient with intrahepatic cholestasis of pregnancy carrying a R652G substitution, and whose biliary phospholipids were lower than other patients. Another study showed the A/G allele frequency was 6% in intrahepatic cholestasis of pregnancy patients and 17% in healthy controls in Sweden. The AA genotype subjects were more likely to suffer from cholestasis[12]. Our result also confirmed this conclusion.
A recent study of gallstones in sibling pairs and controls showed that R652G variant frequency was 18% in patients and 25% in controls. In the control sub-groups, subjects with the GG genotype had significantly lower cholesterol compared with those with the AA genotype. It is known that cholesterol is the raw material for synthesis of bile acids[13]. This gives an indication of the direction of future research.
It is worth noting that this is an interesting new result. Most of the MDR3 gene variants have reported an association with disease causation, such as PFIC and intrahepatic cholestasis of pregnancy[14,15]. In contrast, the R652G variant seems to have a protective effect. It has been assumed that the missense mutation R652G is a neutral polymorphism. Sequence comparisons of MDR3 genes identified that the glycine residue is conserved in other species such as rat and hamster. More research will be required to confirm that in the future.
In conclusion, the MDR3 R652G SNP is negatively correlated with cholestasis (OR, 0.29, 95% CI, 0.12-0.84). Children in Guangxi of China with the R652G variant may have reduced susceptibility to infant intrahepatic cholestasis.
Idiopathic infant intrahepatic cholestasis is a common clinical disease that can occur in either a sporadic or a familial form. The incidence of idiopathic infant intrahepatic cholestasis is very high in China. If not treated early, approximately 5%-10% of patients can have persistent inflammation or fibrosis, and a few develop more severe liver disease, such as cirrhosis.
All studies in recent years on the multidrug resistance protein 3 (MDR3) variants have involved the development of disease. However, an interesting phenomenon is that there was no positive evidence for the MDR3 R652G variant being involved in the pathogenesis of intrahepatic cholestasis. This study investigated the MDR3 R652G distributed allele frequency in idiopathic infant cholestasis and healthy infants to determine whether there were any difference and, if so, their relevance.
Most studies have concentrated on MDR3 mutations in the pathogenesis of progressive familial intrahepatic cholestasis type 3, intrahepatic cholestasis of pregnancy and cholelithiasis. There have been few studies investigating MDR3 and idiopathic infant intrahepatic cholestasis. In order to evaluate the role of the MDR3 R652G variant in the pathogenesis of idiopathic infant cholestasis, the authors analyzed the MDR3 R652G polymorphism in a case-control study in Guangxi Chinese infants. The authors found that the R652G variant was significantly more frequent in healthy infants than in patients. The results showed that the R652G variant has a protective effect in healthy infants and reduces the possibility of suffering from idiopathic infant cholestasis. The result was not the same as a previous study. The reason may be a result of ethnic population differences and the variability in geographical location.
The study results suggest that the MDR3 R652G variant has a protective effect in healthy infants. This will give further information for comparing geographical regions, and it is very important to establish particular characteristics of MDR3 R652G that can be useful in the differential diagnosis of idiopathic infant cholestasis, and furthermore, may establish the influence of such an single nucleotide polymorphism (SNP) in prognosis.
MDR3 R652G: MDR3 R652G is a SNP of the MDR3 in the 652 coding site. The MDR3 is encoded by the ABCB4 gene, and the 652 coding site is located in ABCB4 exon 16. The R652G is a gene mutation where the adenine (A) mutates into guanine (G) which causes AGA>GGA resulting in arginine substitution by glycine (R652G).
The paper gives a significant contribution to basic science knowledge, as well as clinical practice. Publishing this manuscript will allow further comparison with data from other regions and help to clarify the pathogenesis of idiopathic infant cholestasis.
Peer reviewer: Tamara Alempijevic, MD, PhD, Assistant Professor, Clinic for Gastroenterology and Hepatology, Clinical Centre of Serbia, 2 Dr Koste Todorovica St., Belgrade 11000, Serbia
S- Editor Wang JL L- Editor Cant MR E- Editor Zheng XM
| 1. | Strautnieks SS, Kagalwalla AF, Tanner MS, Knisely AS, Bull L, Freimer N, Kocoshis SA, Gardiner RM, Thompson RJ. Identification of a locus for progressive familial intrahepatic cholestasis PFIC2 on chromosome 2q24. Am J Hum Genet. 1997;61:630-633. |
| 2. | Strautnieks SS, Bull LN, Knisely AS, Kocoshis SA, Dahl N, Arnell H, Sokal E, Dahan K, Childs S, Ling V. A gene encoding a liver-specific ABC transporter is mutated in progressive familial intrahepatic cholestasis. Nat Genet. 1998;20:233-238. |
| 3. | Treepongkaruna S, Gaensan A, Pienvichit P, Luksan O, Knisely AS, Sornmayura P, Jirsa M. Novel ABCB11 mutations in a Thai infant with progressive familial intrahepatic cholestasis. World J Gastroenterol. 2009;15:4339-4342. |
| 4. | Jacquemin E, Hadchouel M. Genetic basis of progressive familial intrahepatic cholestasis. J Hepatol. 1999;31:377-381. |
| 5. | Arnell H, Nemeth A, Annerén G, Dahl N. Progressive familial intrahepatic cholestasis (PFIC): evidence for genetic heterogeneity by exclusion of linkage to chromosome 18q21-q22. Hum Genet. 1997;100:378-381. |
| 6. | de Vree JM, Jacquemin E, Sturm E, Cresteil D, Bosma PJ, Aten J, Deleuze JF, Desrochers M, Burdelski M, Bernard O. Mutations in the MDR3 gene cause progressive familial intrahepatic cholestasis. Proc Natl Acad Sci USA. 1998;95:282-287. |
| 7. | Lang T, Haberl M, Jung D, Drescher A, Schlagenhaufer R, Keil A, Mornhinweg E, Stieger B, Kullak-Ublick GA, Kerb R. Genetic variability, haplotype structures, and ethnic diversity of hepatic transporters MDR3 (ABCB4) and bile salt export pump (ABCB11). Drug Metab Dispos. 2006;34:1582-1599. |
| 8. | Pauli-Magnus C, Kerb R, Fattinger K, Lang T, Anwald B, Kullak-Ublick GA, Beuers U, Meier PJ. BSEP and MDR3 haplotype structure in healthy Caucasians, primary biliary cirrhosis and primary sclerosing cholangitis. Hepatology. 2004;39:779-791. |
| 9. | Lang C, Meier Y, Stieger B, Beuers U, Lang T, Kerb R, Kullak-Ublick GA, Meier PJ, Pauli-Magnus C. Mutations and polymorphisms in the bile salt export pump and the multidrug resistance protein 3 associated with drug-induced liver injury. Pharmacogenet Genomics. 2007;17:47-60. |
| 10. | Pauli-Magnus C, Lang T, Meier Y, Zodan-Marin T, Jung D, Breymann C, Zimmermann R, Kenngott S, Beuers U, Reichel C. Sequence analysis of bile salt export pump (ABCB11) and multidrug resistance p-glycoprotein 3 (ABCB4, MDR3) in patients with intrahepatic cholestasis of pregnancy. Pharmacogenetics. 2004;14:91-102. |
| 11. | Jacquemin E, De Vree JM, Cresteil D, Sokal EM, Sturm E, Dumont M, Scheffer GL, Paul M, Burdelski M, Bosma PJ. The wide spectrum of multidrug resistance 3 deficiency: from neonatal cholestasis to cirrhosis of adulthood. Gastroenterology. 2001;120:1448-1458. |
| 12. | Wasmuth HE, Glantz A, Keppeler H, Simon E, Bartz C, Rath W, Mattsson LA, Marschall HU, Lammert F. Intrahepatic cholestasis of pregnancy: the severe form is associated with common variants of the hepatobiliary phospholipid transporter ABCB4 gene. Gut. 2007;56:265-270. |
| 13. | Acalovschi M, Tirziu S, Chiorean E, Krawczyk M, Grünhage F, Lammert F. Common variants of ABCB4 and ABCB11 and plasma lipid levels: a study in sib pairs with gallstones, and controls. Lipids. 2009;44:521-526. |
| 14. | Floreani A, Carderi I, Paternoster D, Soardo G, Azzaroli F, Esposito W, Montagnani M, Marchesoni D, Variola A, Rosa Rizzotto E. Hepatobiliary phospholipid transporter ABCB4, MDR3 gene variants in a large cohort of Italian women with intrahepatic cholestasis of pregnancy. Dig Liver Dis. 2008;40:366-370. |
| 15. | Espinosa Fernández MG, Navas López VM, Blasco Alonso J, Sierra Salinas C, Barco Gálvez A. [Progressive familial intrahepatic cholestasis type 3. An MDR3 defect]. An Pediatr (Barc). 2008;69:182-184. |