Published online Nov 28, 2009. doi: 10.3748/wjg.15.5604
Revised: September 20, 2009
Accepted: September 28, 2009
Published online: November 28, 2009
AIM: To investigate the role of sex hormones in the early postoperative complications of gastrointestinal diseases.
METHODS: A total of 65 patients who underwent operations for gastric and colorectal diseases (mainly malignant diseases) were included in the study. Peripheral venous blood samples were collected at different times for analysis of estradiol, testosterone and progesterone. The only study endpoint was analysis of postoperative complications.
RESULTS: Patients of both sexes were uniform but postoperative complication rate was significantly higher in female patients (P = 0.027). There was no significant association of estradiol and progesterone with postoperative complications. Testosterone levels in complicated patients were significantly lower than in uncomplicated patients (P < 0.05). Area under the receiver operating characteristic curve showed that a lower value of testosterone was a predictor for higher complication rate (P < 0.05), and a lower value of testosterone at later times after surgery was a better predictor of complications.
CONCLUSION: Patients with low testosterone level were prone to higher postoperative complications, which was evident in both sexes. However, further studies are necessary to support this result.
- Citation: Sah BK, Chen MM, Peng YB, Feng XJ, Yan M, Liu BY, Fan QS, Zhu ZG. Does testosterone prevent early postoperative complications after gastrointestinal surgery? World J Gastroenterol 2009; 15(44): 5604-5609
- URL: https://www.wjgnet.com/1007-9327/full/v15/i44/5604.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5604
There have been numerous reports of disparity in pathophysiology of different types of disease between male and female subjects, however, the reports have been quite diverse. Female sex is an independent predictor of mortality in patients with enterococcal bloodstream infections[1]. Women, in addition, might have a higher mortality among patients with necrotizing soft tissue infection[2]. Although most studies have suggested increased susceptibility to infectious complications among men, generally they have demonstrated a higher mortality rate for women from infections and sepsis[3-6], but again, this is not universal[7-9]. On the other hand, male sex is an independent risk factor for the development of nosocomial bloodstream infection[10], and is associated with in-hospital mortality in septic surgical patients[11]. Male sex is associated with anastomotic leakage in colorectal surgery[12], as well as in rectal surgery[13-15].
Female sex is an independent risk factor for early postoperative complications of gastric cancer surgery[16,17]. Early postoperative complications of gastric cancer surgery are significantly more common in female patients, especially mortality and infection rate. Similarly, the duration of postoperative stay is significantly longer in female patients and they have more severe complications than male patients[17].
We observed a significantly higher complication rate in female than male patients, therefore, we assumed that sex hormones may have influenced this disparity. Therefore, we conducted a prospective study to investigate the possible role of sex hormones in early postoperative complications.
A total of 65 patients who underwent operations for gastric and colorectal diseases (mainly malignant diseases) from 2008 to 2009 were included in the study. We applied TNM classification and Duke’s classification for gastric cancer and colorectal cancer, respectively. In gastric cancer patients, the majority was diagnosed with loco-regional advanced stage, and few patients were diagnosed as late stage (Table 1). There was one case of gastric polyp disease, one of malignant gastrointestinal stromal tumor, and one of mesenteric metastasis. The median age of the patients was 61 years (range: 22-82 years). All the operations were performed by senior consultants of a single department at Rui Jin Hospital, School of Medicine, Shanghai Jiao Tong University.
| Details | Frequency |
| Age group (yr) | |
| ≤ 60 | 32 |
| 61–70 | 19 |
| ≥ 71 | 14 |
| Sex | |
| Male | 42 |
| Female | 23 |
| No. of procedures | |
| Gastrectomy | 49 |
| Colorectal resection1 | 9 |
| Gastro-jejunal anastomosis | 2 |
| Exploratory laparotomy | 5 |
| TNM classification (Gastric cancer) | |
| IA | 8 |
| IB | 6 |
| II | 7 |
| IIIA | 10 |
| IIIB | 4 |
| IV | 12 |
| Dukes classification (Colorectal) | |
| A | 7 |
| B | 0 |
| C | 1 |
All the patients with early and resectable advanced gastric cancer (without significant distant metastases) underwent gastrectomy with D2 lymphadenectomy. Thirty partial and 17 total gastrectomies were performed for gastric cancer. One patient underwent total gastrectomy for gastric polyp disease and partial gastrectomy was performed for one malignant gastrointestinal stromal tumor. Anterior resection was performed on three patients, and one patient underwent abdominoperineal resection for rectal cancer. Three patients had partial colectomy and one underwent subtotal colectomy for colon cancer. One patient case of mesenteric metastasis was resected along with partial enterectomy.
The only study endpoint was analysis of postoperative complications. Complications were recorded in accordance with the definitions reported by Copeland et al[18]. Other complications were recorded separately.
This study was approved by the Hospital Institutional Review Boards for human subject research. All samples were obtained with informed consent. Patients on hormonal therapy or chemotherapy were excluded. Similarly, the patients with concomitant immunological disease were excluded. Peripheral venous blood samples were collected from 65 patients before operation (baseline measurements) and on postoperative day (POD)1, POD3, POD5, and POD7 for extensive analysis of their variation at different times. Samples were collected into Venoject tubes containing EDTA.
Assays for all parameters were performed in the laboratory of the Rui Jin Hospital. Venous blood was centrifuged at a speed 2500 r/min for 10 min, and serum was then preserved at -80°C, until further investigation. Estradiol, total testosterone and progesterone were measured using a Dxl 800 analyzer (Beckman-Coulter, Fullerton, CA, USA).
The statistical analysis was performed with SPSS for Windows version 13.0 (SPSS, Inc, Chicago, IL, USA). Test of normality for all related variables were checked by the Shapiro-Wilk method, and a nonparametric statistical method (Mann-Whitney U test) was applied to the variables without a normal distribution. The means were compared by Student’s t test in normally distributed cases. Differences in proportion were compared by Pearson’s χ2 test. P < 0.05 was considered statistically significant.
Fifteen patients with gastric disease and with non-gastric disease were compromised by different complications. There were several patients who had pyrexia of unknown origin with a high white blood cell count, but who did not have any diagnosis, mainly because of a lack of further investigation. The definition of postoperative complication introduced by Copeland et al[18] does not cover many complications that are common after gastrointestinal surgery, therefore, complications which were confirmed clinically were also included. Details of all complications are summarized in Table 2. The sum of the individual complications was not equal to the total number of complications, and multiple complications were possible in a single patient. Patients of both sexes were uniform and there was no significant difference in age (P = 0.356), physiological score (P = 0.616) and operative severity score (P = 0.304). However, there was a significant difference in complication rate between male and female patients (P = 0.027). The complication rate for male and female patients was 21.4% and 47.8%, respectively. We analyzed the role of sex hormones in postoperative complications. Normal ranges of different sex hormones and demographics of patients according to sex are summarized in Tables 3 and 4, respectively.
| Complications | Frequency | |
| Male | Female | |
| Overall | 9 | 11 |
| Intra-abdominal hemorrhage | 1 | 0 |
| Anastomotic leak | 1 | 1 |
| Abdominal infection | 2 | 1 |
| Pyrexia of unknown origin | 7 | 9 |
| Renal failure | 1 | 0 |
| Respiratory failure | 0 | 1 |
| Cardiac failure | 0 | 1 |
| Hypotension | 0 | 1 |
| MODS | 0 | 1 |
| Gastroplegia or enteroplegia | 0 | 2 |
| Anastomosis site bleeding | 1 | 1 |
| Pleural effusion | 0 | 2 |
| Seroperitoneum | 2 | 1 |
| Death | 0 | 1 |
| Male | Female (postmenopause) | |
| Estradiol (pg/mL) | 20-75 | 20-88 |
| Progesterone (ng/mL) | 0.1-0.84 | 0.08-0.78 |
| Testosterone (ng/mL) | 2.62-15.9 | 0.1-0.8 |
| Male (n = 42) | Female (n = 23) | P value | |
| Age (yr) | 60.9 | 58.1 | 0.356 |
| Physiological score | 14 | 17 | 0.203 |
| Operative severity score | 16 | 18 | 0.365 |
| Complications (%) | 21.4 | 47.8 | 0.027 |
| Baseline estradiol (pg/mL) | 23 | 12 | 0.320 |
| Baseline progesterone (ng/mL) | 0.46 | 0.37 | 0.450 |
| Baseline testosterone (ng/mL) | 3.26 | 0.23 | 0.000 |
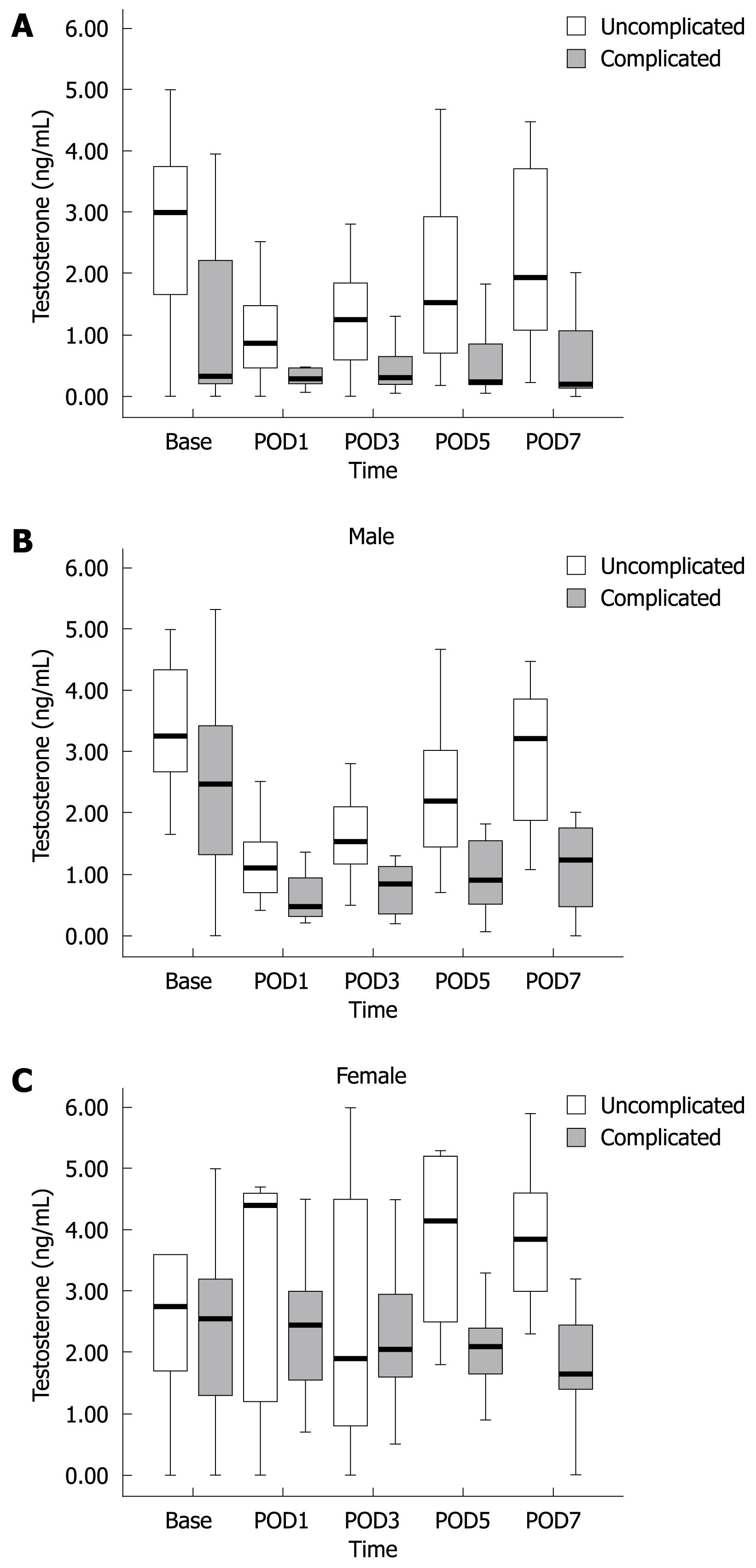
There was no significant association of estradiol and progesterone with postoperative complications. Besides, the normal range of these hormones varies greatly at different times in a month in female patients, and there were not sufficient patients to stratify according to age or time period in women. Given the significant association between testosterone and postoperative complications, we focused additional analysis on this relationship. Testosterone decreased significantly after surgery in both sexes. Testosterone level in complicated patients was significantly lower (P < 0.05) than in uncomplicated patients (Figure 1A).
Perioperative testosterone level was associated significantly with postoperative complication rate, and the result was similar for either overall complication rate or complications of gastric disease surgery alone (Table 5). Similarly, the difference was also significant after stratification of all complications according to sex. However, after stratification, we found there was no significant difference of testosterone level between complicated and uncomplicated patients during the first 3 d after surgery, in both sexes (Table 6). The median value of testosterone in complicated patients was approximately half of that in uncomplicated patients. The significance of the difference increased in later days after surgery, especially on POD5 and POD7. The result was similar in both sexes (Figure 1B and C).
| Complication | Baseline | POD1 | POD3 | POD5 | POD7 |
| Overall | 0.008 | 0.002 | 0.007 | 0.001 | 0.000 |
| Gastric disease | 0.025 | 0.008 | 0.026 | 0.005 | 0.005 |
| Time | Complications (male) | Complications (female) | ||||
| Absent | Present | P value | Absent | Present | P value | |
| Base | 3.66 | 2.74 | 0.104 | 0.23 | 0.29 | 0.689 |
| POD1 | 0.91 | 0.87 | 0.141 | 0.40 | 0.23 | 0.268 |
| POD3 | 1.54 | 0.85 | 0.046 | 0.19 | 0.20 | 0.948 |
| POD5 | 2.20 | 0.91 | 0.040 | 0.41 | 0.21 | 0.038 |
| POD7 | 3.22 | 1.24 | 0.030 | 0.38 | 0.16 | 0.007 |
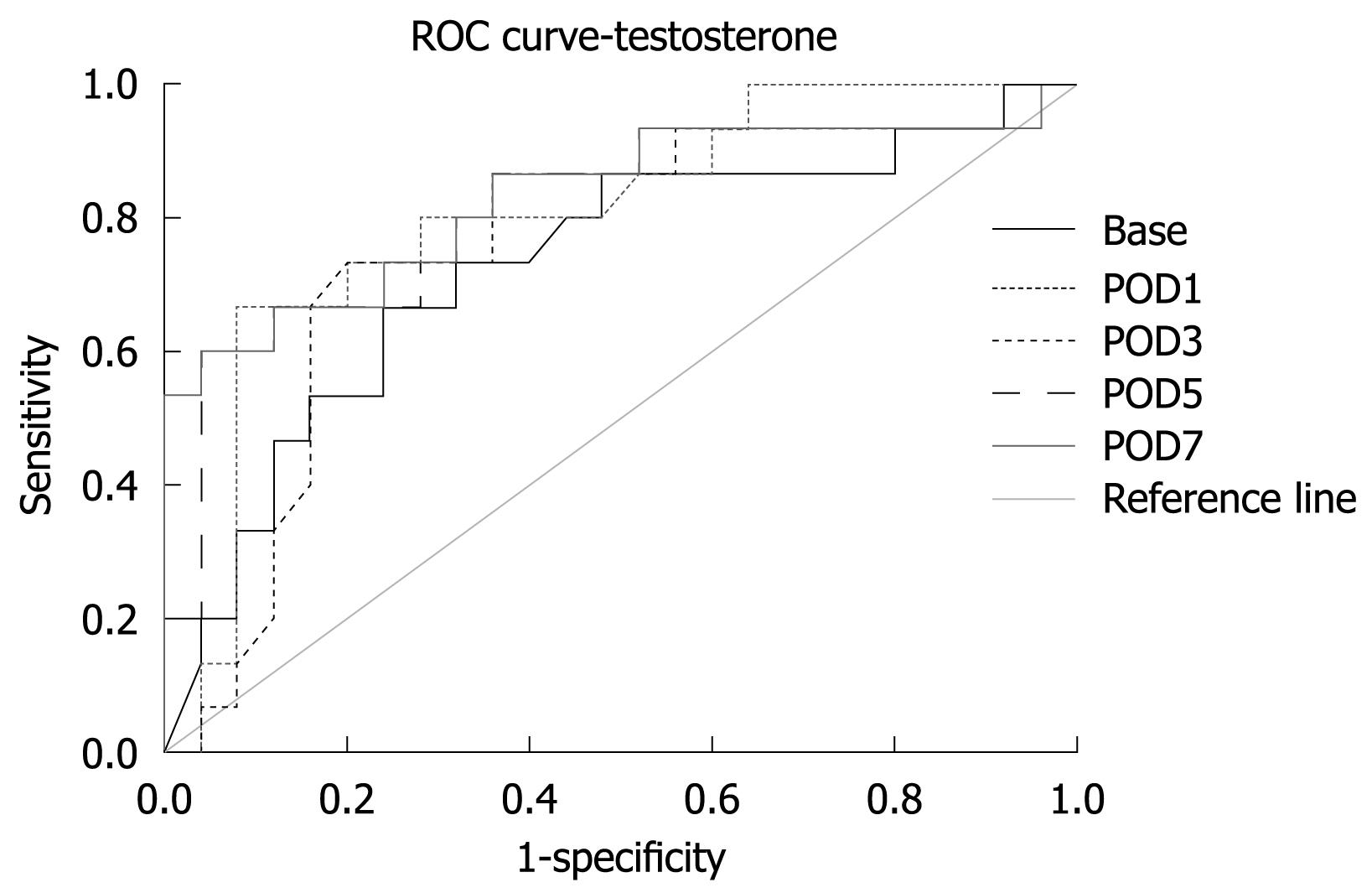
Area under the receiver operating characteristic curve showed that a lower value of testosterone was a predictor for higher complication rate (Table 7, Figure 2). A lower value of testosterone at a later time after surgery was a better predictor of complications (Table 8).
| Time | Area | P value | 95% CI | |
| Lower bound | Upper bound | |||
| BASE | 0.729 | 0.016 | 0.562 | 0.897 |
| POD1 | 0.801 | 0.002 | 0.658 | 0.945 |
| POD3 | 0.756 | 0.007 | 0.593 | 0.919 |
| POD5 | 0.813 | 0.001 | 0.664 | 0.963 |
| POD7 | 0.835 | 0.000 | 0.692 | 0.977 |
| Testosterone level | Sensitivity | Specificity | |
| Base | ≤ 1.95 | 70.0 | 68.9 |
| POD1 | ≤ 0.43 | 60.0 | 84.4 |
| POD3 | ≤ 0.45 | 73.3 | 80.0 |
| POD5 | ≤ 0.24 | 60.0 | 90.0 |
| POD7 | ≤ 0.32 | 66.7 | 92.0 |
Sex has been hypothesized to be a determinant of immunological variability after severe traumatic and surgical stress and, at least in animal models, accounts for differences in outcomes[3,7,19-22]. Some studies have demonstrated that altered levels of sex hormones rather than sex itself are related to postoperative complications. In particular, progesterone in male and testosterone in female subjects has an impact on survival. In both sexes, higher 17β-estradiol levels are associated clearly with shorter survival times[23]. However, we did not find a significant relationship between estradiol and postoperative complications. Unlikely, we observed a difference in progesterone level between complicated and uncomplicated patients. However, these observations were limited by the low number of patients, and variation of normal values of this hormone at different times in female subjects made it difficult to obtain any conclusive result.
The uncertainty regarding the role of sex in influencing outcomes in humans may simply result from the clinical, phenotypic, and genetic diversity among populations, and the difficulty in detecting a difference due to these confounders. However, whether men and women respond differently to trauma or surgical insult, either in terms of the host response or the eventual outcome, is important for clinical care and the design of future research on anti-infective treatments or immunological regulators.
As mentioned above, our study was affected by the limitation of statistical calculation due to the low number of postoperative complications. Therefore, we could not analyze the differences in testosterone level in complicated and uncomplicated cases in different age groups, or its effect on different types of complications separately, because the number of patients and their relevant complications were low in those groups. It was still unknown whether the lower level of testosterone caused postoperative complications, or whether it was just a phenomenon observed in complicated patients. However, it was evident that even the preoperative level of testosterone was lower in patients who had complications after surgery. The testosterone level decreased significantly after surgery in all patients and it started to regain its preoperative level, or even higher, on POD3. However, the testosterone level in complicated patients did not regain its preoperative value, and moreover, it kept on decreasing. Even after controlling for sex by stratifying the group and looking at testosterone levels, the testosterone level decreased significantly in male and female patients with postoperative complications. Therefore, this observation at least confirmed that the testosterone level had some kind of association with postoperative complications. However, without detailed investigation of the effects of other hormones, it was very hard to conclude that only testosterone was responsible for such disparity.
Although there were many limitations and unanswered questions in the present study, at least, our observations reflected that some kind of difference does exist between the sexes, which may be responsible for the disparity in postoperative complication rate. If a difference does indeed exist, it is possible that future studies will have to stratify for sex, infection source, and immunological phenotype. Similarly, future treatment strategy must be tailored for male and female patients separately, and different modes or treatment management will be necessary for the different sexes.
If there is an association between low testosterone level and postoperative complication rate, then serial tests of testosterone levels can easily identify the susceptible patients, and supplementary hormonal replacement therapy might be a novel treatment to prevent postoperative complications in such patients.
In conclusion, there was a significant association between testosterone and overall complications, and also with complications of gastric surgery. Association with testosterone was seen even after controlling for sex by stratifying the group and looking at testosterone levels. Postoperative complications were higher in patients who did not recover normal testosterone levels at POD5 or POD7. However, the results of this small-scale study need confirmation by a large cohort of patients, and prospective studies with hormone supplementation to reevaluate the role of testosterone in postoperative complications.
There have been numerous reports of disparity in pathophysiology of different types of disease between male and female patients, however, the reports have been quite diverse. Female sex is an independent risk factor for early postoperative complications of gastric cancer surgery. Early postoperative complications of gastric cancer surgery are significantly more common in female patients, especially mortality and infection rate. Similarly, the duration of postoperative stay is significantly longer in female patients, and they have more severe complications than male patients do.
The authors found that there was a significant association between testosterone and overall complications, and also with complications of gastric surgery. Association with testosterone was seen even after controlling for sex by stratifying the group and looking at testosterone levels. Postoperative complications were higher in patients who did not recover normal testosterone levels at POD5 or POD7.
By understanding the effect of sex hormones on postoperative complications, the present study might represent a future strategy for therapeutic intervention in the treatment of patients with gastrointestinal disease. If there is an association of low testosterone level with postoperative complication rate, then serial testing of testosterone level can easily identify the susceptible patients, and supplementary hormonal replacement therapy could be a novel treatment to prevent postoperative complications in these patients
Estradiol, testosterone and progesterone are sex hormones that are measured to understand dysfunction in gonadal activity.
This study is unique and interesting. The authors studied the impact of sex hormones, especially testosterone, on the prevalence of complications after gastrointestinal surgery. This study gives us some valuable clues to explain why postoperative complications occur more frequently in female patients, as previously noted. It would also be useful in giving us a basis for clinical research to predict the occurrence of surgical complications.
Peer reviewer: Sung Kim, MD, PhD, Professor, Department of Surgery, Sansung Medical Center, Sungkyunkwan University School of Medicine, 50 Irwon-Dong, Gangnam-Gu, Seoul 135-710, South Korea
S- Editor Wang YR L- Editor Kerr C E- Editor Zheng XM
| 1. | Stroud L, Edwards J, Danzing L, Culver D, Gaynes R. Risk factors for mortality associated with enterococcal bloodstream infections. Infect Control Hosp Epidemiol. 1996;17:576-580. |
| 2. | Elliott DC, Kufera JA, Myers RA. Necrotizing soft tissue infections. Risk factors for mortality and strategies for management. Ann Surg. 1996;224:672-683. |
| 3. | Wichmann MW, Zellweger R, DeMaso CM, Ayala A, Chaudry IH. Mechanism of immunosuppression in males following trauma-hemorrhage. Critical role of testosterone. Arch Surg. 1996;131:1186-1191; discussion 1191-1192. |
| 4. | Schneider CP, Nickel EA, Samy TS, Schwacha MG, Cioffi WG, Bland KI, Chaudry IH. The aromatase inhibitor, 4-hydroxyandrostenedione, restores immune responses following trauma-hemorrhage in males and decreases mortality from subsequent sepsis. Shock. 2000;14:347-353. |
| 5. | Lephart ED, Baxter CR, Parker CR Jr. Effect of burn trauma on adrenal and testicular steroid hormone production. J Clin Endocrinol Metab. 1987;64:842-848. |
| 6. | Majetschak M, Christensen B, Obertacke U, Waydhas C, Schindler AE, Nast-Kolb D, Schade FU. Sex differences in posttraumatic cytokine release of endotoxin-stimulated whole blood: relationship to the development of severe sepsis. J Trauma. 2000;48:832-839; discussion 839-840. |
| 7. | Angele MK, Wichmann MW, Ayala A, Cioffi WG, Chaudry IH. Testosterone receptor blockade after hemorrhage in males. Restoration of the depressed immune functions and improved survival following subsequent sepsis. Arch Surg. 1997;132:1207-1214. |
| 8. | Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546-1554. |
| 9. | Schröder J, Kahlke V, Book M, Stüber F. Gender differences in sepsis: genetically determined? Shock. 2000;14:307-310; discussion 310-313. |
| 10. | Pittet D, Davis CS, Li N, Wenzel RP. Identifying the hospitalized patient at risk for nosocomial bloodstream infection: a population-based study. Proc Assoc Am Physicians. 1997;109:58-67. |
| 11. | Schröder J, Kahlke V, Staubach KH, Zabel P, Stüber F. Gender differences in human sepsis. Arch Surg. 1998;133:1200-1205. |
| 12. | Branagan G, Finnis D. Prognosis after anastomotic leakage in colorectal surgery. Dis Colon Rectum. 2005;48:1021-1026. |
| 13. | Law WI, Chu KW, Ho JW, Chan CW. Risk factors for anastomotic leakage after low anterior resection with total mesorectal excision. Am J Surg. 2000;179:92-96. |
| 14. | Yeh CY, Changchien CR, Wang JY, Chen JS, Chen HH, Chiang JM, Tang R. Pelvic drainage and other risk factors for leakage after elective anterior resection in rectal cancer patients: a prospective study of 978 patients. Ann Surg. 2005;241:9-13. |
| 15. | Rullier E, Laurent C, Garrelon JL, Michel P, Saric J, Parneix M. Risk factors for anastomotic leakage after resection of rectal cancer. Br J Surg. 1998;85:355-358. |
| 16. | Sah BK, Zhu ZG, Chen MM, Xiang M, Chen J, Yan M, Lin YZ. Effect of surgical work volume on postoperative complication: superiority of specialized center in gastric cancer treatment. Langenbecks Arch Surg. 2009;394:41-47. |
| 17. | Sah BK, Zhu ZG, Wang XY, Yang QM, Chen MM, Xiang M, Chen J, Yan M. Post-operative complications of gastric cancer surgery: female gender at high risk. Eur J Cancer Care (Engl). 2009;18:202-208. |
| 18. | Copeland GP, Jones D, Walters M. POSSUM: a scoring system for surgical audit. Br J Surg. 1991;78:355-360. |
| 19. | Knöferl MW, Angele MK, Diodato MD, Schwacha MG, Ayala A, Cioffi WG, Bland KI, Chaudry IH. Female sex hormones regulate macrophage function after trauma-hemorrhage and prevent increased death rate from subsequent sepsis. Ann Surg. 2002;235:105-112. |
| 20. | Zellweger R, Wichmann MW, Ayala A, Stein S, DeMaso CM, Chaudry IH. Females in proestrus state maintain splenic immune functions and tolerate sepsis better than males. Crit Care Med. 1997;25:106-110. |
| 21. | Homo-Delarche F, Fitzpatrick F, Christeff N, Nunez EA, Bach JF, Dardenne M. Sex steroids, glucocorticoids, stress and autoimmunity. J Steroid Biochem Mol Biol. 1991;40:619-637. |
| 22. | Yokoyama Y, Kuebler JF, Matsutani T, Schwacha MG, Bland KI, Chaudry IH. Mechanism of the salutary effects of 17beta-estradiol following trauma-hemorrhage: direct downregulation of Kupffer cell proinflammatory cytokine production. Cytokine. 2003;21:91-97. |
| 23. | Angstwurm MW, Gaertner R, Schopohl J. Outcome in elderly patients with severe infection is influenced by sex hormones but not gender. Crit Care Med. 2005;33:2786-2793. |