Published online Nov 14, 2009. doi: 10.3748/wjg.15.5346
Revised: September 20, 2009
Accepted: September 27, 2009
Published online: November 14, 2009
AIM: To analyze the factors influencing radical (R0) resection rate and surgical outcome for malignant tumor of the pancreatic body and tail.
METHODS: The clinical and operative data and follow-up results of 214 pancreatic body and tail cancer patients were analyzed retrospectively.
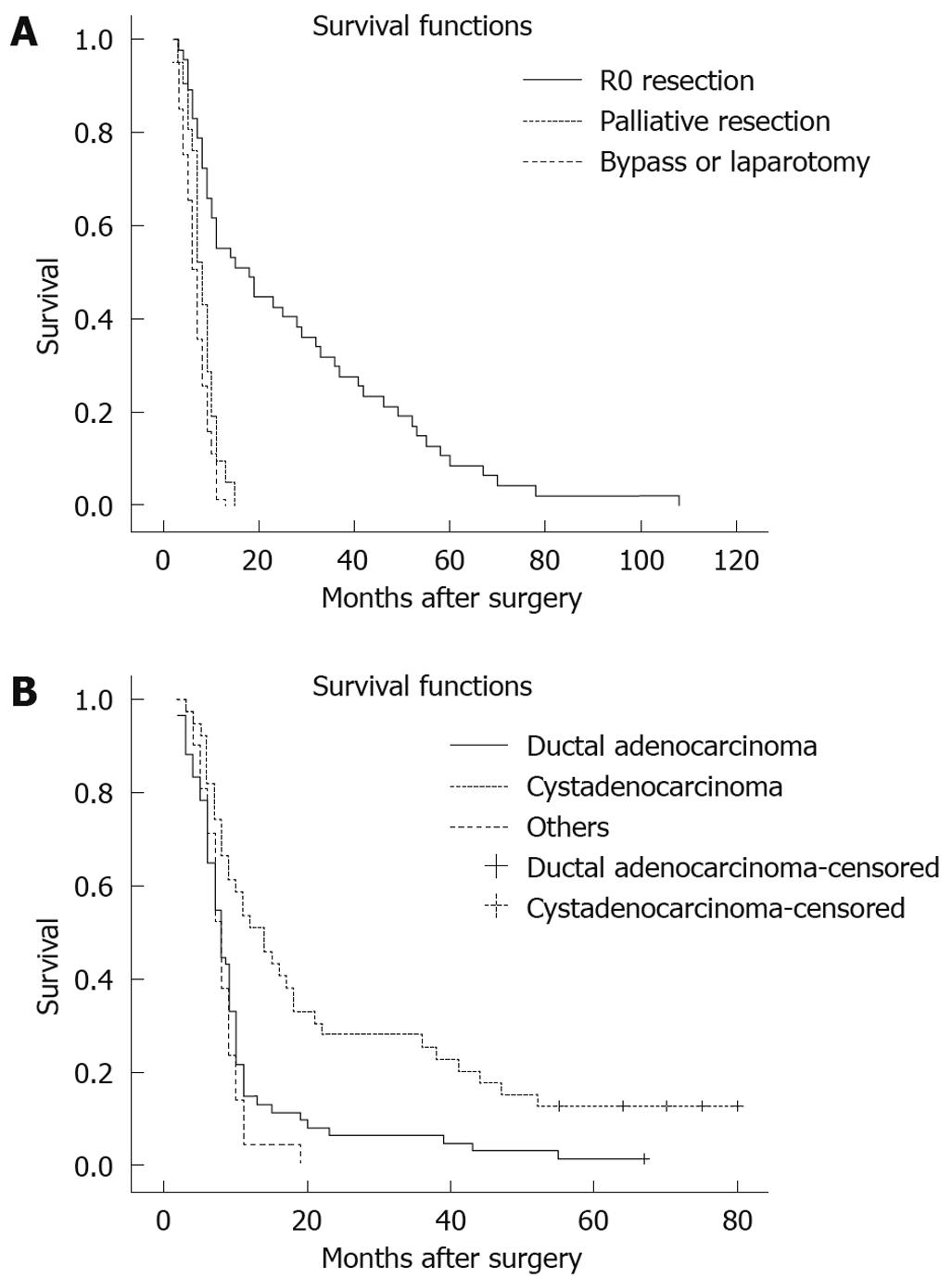
RESULTS: One hundred and twenty/214 pancreatic body and tail cancer patients underwent surgical treatment; the overall resection rate was 59.2% (71/120), and the R0 resection rate was 40.8% (49/120). Compared with non-R0 treatment, the patients receiving an R0 resection had smaller size tumor (P < 0.01), cystadenocarcinoma (P < 0.01), less lymph node metastasis (P < 0.01), less peri-pancreatic organ involvement (P < 0.01) and earlier stage disease (P < 0.01). The overall 1-, 3- and 5-year survival rates for pancreatic body and tail cancer patients were 12.7% (25/197), 7.6% (15/197) and 2.5% (5/197), respectively, and ductal adenocarcinoma patients had worse survival rates [15.0% (9/60), 6.7% (4/60) and 1.7% (1/60), respectively] than cystadenocarcinoma patients [53.8% (21/39), 28.2% (11/39) and 10.3% (4/39)] (P < 0.01). Moreover, the 1-, 3- and 5-year overall survival rates in patients with R0 resection were 55.3% (26/47), 31.9% (15/47) and 10.6% (5/47), respectively, significantly better than those in patients with palliative resection [9.5% (2/21), 0 and 0] and in patients with bypass or laparotomy [1.2% (1/81), 0 and 0] (P < 0.01).
CONCLUSION: Early diagnosis is crucial for increasing the radical resection rate, and radical resection plays an important role in improving survival for pancreatic body and tail cancer patients.
- Citation: Han SL, Zhang WJ, Zheng XF, Shen X, Zeng QQ, Ke QH. Radical resection and outcome for malignant tumors of the pancreatic body and tail. World J Gastroenterol 2009; 15(42): 5346-5351
- URL: https://www.wjgnet.com/1007-9327/full/v15/i42/5346.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5346
| Clinicopathological variables | Radicality | ||
| R0 resection (n = 49) | R1 + R2 resection (n = 22) | Bypass or laparotomy (n = 49) | |
| Tumor size (cm) | 4.8 ± 1.3b | 7.9 ± 2.2 | 11.0 ± 3.8 |
| Histopathological type | |||
| Ductal adenocarcinoma (n = 60) | 15 (25.0)b | 10 (16.7) | 35 (58.3) |
| Cystadenocarcinomas (n = 39) | 25 (64.1) | 7 (17.9) | 7 (17.9) |
| Others (n = 21) | 7 (33.3) | 6 (28.6) | 8 (38.1) |
| Lymph node metastasis | 16 (32.7)b | 19 (86.4) | 43 (87.8) |
| Peri-organ involvement1 | 26 (53.1)b | 18 (81.8) | 40 (81.6) |
| TNM staging | |||
| Stage I + II (n = 17) | 17 (100.0)b | 0 | 0 |
| Stage III (n = 16) | 14 (87.5) | 2 (12.5) | 0 (0) |
| Stage IVA (n = 34) | 18 (52.9)2 | 7 (20.6) | 9 (26.5) |
| Stage IVB (n = 53) | 0 | 13 (24.5) | 40 (75.5) |
| Histopathological classification | Cases |
| Ductal adenocarcinoma | 60 |
| Invaded and metastatic tumor | 7 |
| Pancreas invaded from gastrointestinal stromal tumor | 6 |
| Pancreatic metastasis from renal cell carcinoma | 1 |
| Cystadenocarcinomas | 39 |
| Serous cystadenocarcinomas | 30 |
| Mucinous cystadenocarcinomas | 9 |
| Endocrine malignant tumor | 14 |
| Insulinoma | 3 |
| Gastrinoma | 11 |
| Surgical radicality | Cases with follow-up | 1-yr survival | 3-yr survival | 5-yr survival |
| R0 resection (n = 49) | 47 | 26 (55.3)b | 15 (31.9)b | 5 (10.6)b |
| R1 + R2 (palliative) resection (n = 22) | 21 | 2 (9.5)b | 0 | 0 |
| Bypass or laparotomy (n = 49) | 48 | 6 (12.5)b | 0 | 0 |
| Non-surgical treatment (n = 94) | 81 | 1 (1.2)b | 0 | 0 |
| Total (n = 214) | 197 | 25 (12.7) | 15 (7.6) | 5 (2.5) |
Pancreatic carcinoma is one of the most fatal malignant diseases and ranks fifth in cancer mortality worldwide[1,2]. Survival after resection remains disappointing, with 5-year survival rates ranging from 10% to 29%[3-8]. Advances in diagnostic and operative techniques and in perioperative care have increased the resectability of pancreatic cancer, and have decreased rates of operative morbidity and mortality. The definition of a resectable tumor has become more clearly defined anatomically based on the availability of high-quality computed tomography (CT) scans[7-10]. Such imaging now permits a precise, preoperative, noninvasive assessment of tumor respectability and adds an important level of objectivity to the staging of patients for entry into clinical trials. Importantly, the role of laparotomy is now largely restricted to patents judged “resectable” on preoperative imaging[9,10]. For the 80%-90% of patients with pancreatic adenocarcinoma who have unresectable disease, biliary obstruction, when present, can be palliated using minimally invasive endoscopic techniques.
In patients with a malignant neoplasm in the body-tail of the pancreas, splenectomy has a negative influence on long-term survival after resection[11,12]. The incidence of diabetes after spleen-preserving distal pancreatectomy for chronic pancreatitis is less than after en-bloc splenectomy[13-15]. Spleen salvage eliminates the risk of overwhelming infections. In the past decade, advances in surgical techniques have reduced the operative mortality rate of pancreatic resections to below 5% in high-volume centers, yet morbidity rates have remained essentially unchanged, ranging from 30% to 40%[11-16].
The objective of this study was to analyze factors contributing to radical resection rate and outcome following radical resection for malignant tumors of the pancreatic body and tail.
Two hundred and fourteen patients with malignant tumors of the pancreatic body and tail underwent radical pancreatectomy at the First Medical College of Wenzhou Medical College between January 2000 and December 2006, and at the First Affiliated Hospital of Zhejiang University between January 1990 and March 2002 and were eligible for study. The demographic and clinical courses of each patient were collected, including age, sex, and indication for radical pancreatectomy, concomitant splenectomy, symptomatology, diagnostic methods, operative management, pathology report, postoperative morbidity and mortality.
Of the 214 patients, 125 were men and 89 were women, with a mean age of 59.7 years (ranging from 15 to 81 years). Tumors were staged according to American Joint Committee on Cancer staging[17], 11 patients (5.1%)were categorized into stage I, 6 patients (2.8%) into stage II, 16 patients (7.5%) into stage III, 62 patients (29.0%) into stage IVA and 119 patients (55.6%) into stage IVB. The preoperative diagnosis of pancreatic carcinoma was made using abdominal ultrasonography (US), CT, endoscopic retrograde cholangiopancreatography, magnetic resonance cholangiopancreatography and detection of serum tumor markers such as carcinoembryonic antigen, carbohydrate antigen 19-9.
The main surgical techniques used for transection and closure of the pancreatic remnant included: (1) Anastomosis: the pancreaticojejunal end-to-end anastomosis was carried out followed Peng’s invagination method[18]; (2) Closure by suture: the pancreas was transected with a knife, followed by identification of the main pancreatic duct and closure of the duct using single stitches of 2-0 silk suture. The parenchyma was then closed using single stitches of 2-0 silk suture; (3) In some cases, the suture line was reinforced by laying a fibrinogen/thrombin-coated collagen patch (Fibrin Sealant®, Guangzhou Bioseal Technology Co. Lt, China) onto the transected end; (4) In our university hospitals, radical resection, especially pancreatoduodenectomy, is performed only by experienced surgeons who are the professors or directors of general surgery. Recently, most operations for pancreatic tumors have been performed by experienced surgeons in pancreatic disease.
In this study, R0 resection means negative resection margins and no residual tumor. If a frozen section taken of the cut pancreatic and bile duct margins was positive, more tissue was taken.
In this study, all patients received regular or irregular adjuvant therapy (immuno-chemotherapy, radiotherapy and/or Chinese traditional drugs) after surgery, but no patients received neoadjuvant chemotherapy or radiation therapy before surgery.
In this study, pancreatic leakage was defined as: (1) discharge from the postpancreatic drain ≥ 50 mL/d after postoperative day 3, and (2) an amylase level in drainage fluid exceeding three times that of the serum concentration. Postoperative mortality was defined as death occurring in the first 30 postoperative days or before discharge from the hospital. Other complications were categorized and defined as any of the following: intra-abdominal bleeding (requiring transfusion or operative intervention); gastrointestinal bleeding (requiring transfusion or endoscopic or operative intervention); intra-abdominal abscess (fluid requiring drainage and with positive bacterial culture); wound infection (purulent drainage requiring open packing); bile leak (bilious drainage from intraoperatively placed drains or bile collection requiring drainage); wound dehiscence (partial or total disruption of the fascial or all the layers of the incision).
Follow-up information was obtained through office visits and telephone contact with the patients until the time of the patients’ deaths or at the end of this study. In order to confirm the dates of deaths, if any, the data were verified at the regional station of the public records department for telecommunication and computer science. Local recurrence was defined as tumor relapse within the region or presence of a pancreatic stump. Distant metastases (or dissemination) were tumor lesions in other organs (outside the pancreas under treatment) such as liver and lung or remote lymph nodes, e.g. paraaortic lymph node. Upon their discharge from hospital, the patients were seen at least four times a year, i.e. every 3 mo, within the first 5 years, and every half a year thereafter. Physical examinations, basic routine X-ray examinations and abdominal US were performed. CT of the abdomen (twice a year), and if necessary the chest or head was added.
The statistical analysis was performed with SPSS software (version 13.0; SPSS Inc, Chicago, Ill). All results are expressed as mean ± SD. Univariate analyses of categorical variables were performed using χ2 tests, and the multivariate analysis was performed using a nonconditional logistic regression model expressed in odds ratios. To test the independence of the risk factors, the significant variables (P < 0.05) in the univariate analysis were entered into a multivariate logistic regression model with likelihood ratio forward selection with a criterion of P < 0.05.
Ninety-four of 214 patients with malignant tumor of pancreatic body and tail accepted non-surgical treatment, and 120 patients underwent surgery. The overall resection rate was 59.2% (71/120), and the R0 resection rate was 40.8% (49/120). R0 resections were those where the tumors were resected with clear surgical margins, as shown by intraoperative frozen sections and confirmed by definitive histopathological examination. Twenty-two patients underwent palliative resection (R1 or R2), 49 underwent bypass or laparotomy.
Compared with patients who underwent palliative resection (R1 + R2) (7.9 ± 2.2 cm) or bypass/laparotomy (11 ± 3.8 cm), the tumor size (4.8 ± 1.3 cm) in patients who underwent radical resection was significantly smaller (P < 0.01). Similar results were found in patients with pancreatic cystadenocarcinoma [25.0% (15/60) vs 64.1% (25/39) and 33.3% (7/21), P < 0.01], less lymph node metastasis [32.7% (16/49) vs 86.4% (19/22) and 87.8% (43/49), P < 0.01] or less peri-organ involvement [53.1% (26/49) vs 81.8% (18/22) and 81.6% (40/49), P < 0.01]. Moreover, the radical resection rates in stage I + II and stage III were 100.0% (17/17) and 87.5% (14/16), which were much higher than those in stage IVA (52.9%, 18/34) and stage IVB (0) (P < 0.01) (Table 1).
Definitive histology of the resected lesions revealed a cystic tumor in a solid malignancy in 67 patients (60 ductal adenocarcinoma, six circumscribed infiltration of a gastrointestinal stromal tumor, one pancreatic metastasis from renal cell carcinoma), 39 cystadenocarcinomas (30 serous cystadenocarcinomas, nine mucinous cystadenocarcinomas), and 14 malignant endocrine tumors (Table 2).
Forty-nine of 120 patients (40.8%) underwent R0 radical operation, including 22 with combined distal pancreatectomy and splenectomy, and 27 with spleen-preserving pancreatectomy. Postoperative complications occurred in 18 patients (15.0%) with pancreatic fistula (eight patients, 6.7%) being the most common, followed by intra-abdominal bleeding (4, 3.3%), gastrointestinal bleeding (2, 1.7%), incisional infection (3, 2.5%), and intestinal fistula (1, 0.8%). There was no operative mortality (defined as any death occurring within 1 mo after surgical procedure). Moreover, no detrimental effects of postoperative complications on oncologic efficacy of R0 pancreatectomy were found in this study.
Long term follow-up was performed using a standardized protocol. The median follow-up time was 23.1 mo, ranging from 4 to 83 mo. Seventeen patients with pancreatic carcinoma failed to follow-up and the overall follow-up rate was 92.1%. The 1-, 3- and 5-year overall survival rates in this group were 15.7% (31/197), 7.6% (15/197) and 2.5% (5/197), respectively. Moreover, the 1-, 3- and 5-year overall survival rates in patients with R0 resection were 55.3% (26/47), 31.9% (15/47) and 10.6% (5/47), respectively, which were significantly better than those in patients with palliative resection [9.5% (2/21), 0 and 0] or those with bypass/laparotomy [1.2% (1/81), 0 and 0] (P < 0.01, Table 3, Figure 1A). Among 49 patients with R0 resection, there was no significant difference in survival between the combined distal pancreatectomy and splenectomy group (n = 22) and spleen-sparing pancreatectomy group (n = 27) (P > 0.05) (Table 4). Furthermore, the 1-, 3- and 5-year overall survival rates [15.0% (9/60), 6.7% (4/60) and 1.7% (1/60), respectively] for pancreatic adenocarcinoma patients were worse than those [53.8% (21/39), 28.2% (11/39) and 10.3% (4/39)] for pancreatic cystadenocarcinomas (Figure 1B, P < 0.01). In addition, the survival of patients with pancreatic adenocarcinoma of the body and tail was somewhat improved from the mid 1990s, but this improvement was not significant.
It has been reported that less than 5% of all patients diagnosed with pancreatic carcinoma can expect to live for more than 5 years[1,2,19-23]. A radical pancreatic resection (R0) is an effective and safe method of treating various benign and malignant diseases of the pancreas. However, only 10%-20% of these individuals are candidates for surgical resection, which remains the only available chance for cure of this lethal disease[6,7,24]. Unfortunately delayed diagnosis, ineffective chemotherapy, lower radical resection rate, radiation resistance, and an intrinsic biologic aggressiveness of tumors all contribute to the poor prognosis associated with pancreatic cancer[1,2,5].
Early detection and diagnosis are the key points to improve the outcome of pancreatic carcinoma, however almost 70% of patients with pancreatic carcinomas have unresectable disease at the time of initial diagnosis and are unable to undergo curative resection[1,5,6,25]. It has been reported that the resection rate is 20%-42.6% for pancreatic carcinoma, and the 5-year survival is 8.5%-10.6% after radical resection[1-4]. As for pancreatic carcinoma of the body and tail, the resection rate is much lower, only 10%-22%, and the prognosis is much poor because the tumor in this portion of the gland tends to invade surrounding organs and vascular structures[5,6]. Wu et al[5] compared clinical manifestations, pathological behavior and postoperative survival between malignant tumor of the pancreatic body and tail (n = 106) and malignant pancreatic head cancer (n = 451). The authors found postoperative median survival for resection of non-metastatic pancreatic body and tail cancer was significantly longer than similar resections in patients with metastatic disease. These results were no different than in those patients who had no resection. The overall and R0 resection rates in this study were 59.2% and 40.8%, respectively, and the 1-, 3- and 5-year overall survival rates in patients with R0 resection were 55.3%, 31.9% and 10.6%, respectively, which were better than those found in patients who had palliative resection or patients with bypass/laparotomy (P < 0.01). In addition, ductal adenocarcinoma patients had worse survival times than patients with pancreatic cystadenocarcinoma and other malignant tumors (P < 0.01). Liu et al[6] reported that factors influencing resection rate of pancreatic carcinoma included lymph node metastasis, tumor size and peri-pancreatic invasion, and the median survival times of radical resection, palliative resection and laparotomy for tumors of the body and tail of the pancreas were 18, 8 and 3.5 mo, respectively. Lim et al[26] collected a group of 396 patients aged > 65 years who were diagnosed with nonmetastatic pancreatic adenocarcinoma and found median survival was 17.6 mo, with 1- and 3-year survival rates of 60.1% and 34.3%, respectively. In this study, our findings revealed that compared with tumors which underwent palliative and bypass/laparotomy, the patients receiving R0 resections had smaller sized tumors, (P < 0.01), cystadenocarcinoma histopathology (P < 0.01), less lymph node metastasis (P < 0.01), less peri-pancreatic organ involvement (P < 0.01), and earlier stage (P < 0.01). These results indicate that small size, cystadenocarcinoma histopathology, lymph node metastases, and peri-pancreatic organ involvement were factors influencing the R0 resection rate.
Long-term survival after pancreatoduodenectomy for pancreatic carcinoma is far from excellent. The 5-year survival rate nears 10% and patients surviving more than 5 years are exceptional. It has been reported that the strongest predictors of survival are adjuvant combined chemoradiotherapy, small tumors (< 2 cm in diameter), negative lymph nodes, well-differentiated histology, undergoing surgery in a teaching hospital and high socioeconomic status[16,19,27]. A multivariate analysis of the 443 patients with periampullary adenocarcinoma from Yeo et al[28], indicated that the most powerful independent predictors favoring long-term survival included a pathologic diagnosis of duodenal adenocarcinoma, tumor diameter < 3 cm, negative resection margins, absence of lymph node metastases, well-differentiated histology, and no reoperation. Schwarz et al[11] studied the outcomes in a group of patients (326 patients, 37 underwent splenectomy) with adenocarcinoma after distal and total pancreatectomy with or without splenectomy and concluded that splenectomy was a statistically significant unfavorable prognostic factor in survival, but not in postoperative morbidity. Shoup et al[4], in a cohort with benign and low-grade malignant diseases (125 patients), reported that spleen preserving distal pancreatectomy is associated with lower infectious complications rate and reduced hospital stay, compared with distal pancreatectomy with splenectomy (P = 0.01 and P < 0.01, respectively). The median survival following resection was 15.9 mo compared to 5.8 mo in patients who were not resected (P < 0.0001). Actual 5- and 10-year survival rates were 22% and 18%, respectively, following extended resection, 8% and 8% following standard resection, and 0% and 0% if no resection was attempted because of locally unresectable disease. Patients undergoing extended resection for adenocarcinoma of the pancreatic body or tail have long-term survival rates similar to those for patients undergoing standard resection; they also have markedly improved long-term survival compared to those who are not considered resectable because of locally advanced disease.
Elective distal pancreatectomy is safer than pancreaticoduodenectomy but carries a high morbidity rate[5,6]. In the past decade splenectomy was associated with an increased septic complication rate[27]. Furthermore, several authors[12,24] suggested spleen preserving distal pancreatectomy in order to reduce postoperative septic complications. The technique of spleen preserving distal pancreatectomy and its absolute and relative contraindications have been described elsewhere[12,29]. In this study, the postoperative complication rate was 15.0%, with pancreatic fistula (6.7%) being the most common, followed by intra-abdominal bleeding (3.3%), gastrointestinal bleeding (1.7%), incisional infection (2.5%), and intestinal fistula (0.8%). These are similar to previous reports[1,5,6,23]. Few retrospective studies have analyzed the influence of splenectomy in the postoperative course after distal pancreatectomy, while one study has analyzed this relationship after total pancreatectomy[29]. For example, Schwarz et al[11] reported that the median actuarial survival for pancreatic adenocarcinoma was 12.2 mo with splenectomy vs 17.8 mo without splenectomy (P < 0.005), and splenectomy (P = 0.02) as well as pathologic lymph node status (P = 0.0002), tumor diameter (P = 0.0004), and tumor differentiation (P = 0.007) were prognostic factors. In this study, there was no significant difference in survival between the combined distal pancreatectomy and splenectomy group and spleen-preserving pancreatectomy group.
In conclusion, early diagnosis is crucial for increasing the radical resection rate and radical resection plays an important role in the improvement of prognosis for patients with malignant tumors of pancreatic body and tail.
Pancreatic carcinoma is one of the most fatal malignant diseases and ranks fifth in cancer mortality worldwide. Survival after resection remains disappointing, with 5-year survival rates ranging from 10% to 29%. Advances in diagnostic and operative techniques and in perioperative care have increased the resectability of pancreatic cancer and have decreased rates of operative morbidity and mortality. The definition of a resectable tumor has become more clearly defined anatomically based on the availability of high-quality computed tomography scans. Such imaging now permits a precise, preoperative, noninvasive assessment of tumor resectability and adds an important level of objectivity to the staging of patients for entry into clinical trials. Importantly, the role of laparotomy is now largely restricted to patents judged “resectable” on preoperative imaging. The objective of this study was to analyze factors contributing to radical resection rate and outcome following radical resection of malignant tumors of the pancreatic body and tail.
Ways of improving the early diagnosis and radical resection are the hotspots in management of malignant tumors of the pancreatic body and tail.
This study tries to find out the factors influencing early diagnosis and radical resection, by analysis of the factors which have effects on the resection rate of patients with malignant tumors of the pancreatic body and tail.
By using those factors that influence early diagnosis and radical resection, we can evaluate objectively the “resectability” of each case with malignant tumors of the pancreatic body and tail.
This is a retrospective study on surgical treatment of malignant tumors of the pancreatic body and tail.
The work is a retrospective analysis of the radical resection rate in 240 patients suffering from pancreatic cancer in two university hospitals from 1990 to 2006. The authors correctly point out that early diagnosis and curative resection is key to a positive outcome. The paper is well written and the data are adequately discussed.
Peer reviewer: Dr. Georg Alexander Roth, General Anesthesia and Critical Care, Medical University of Vienna, Vienna, A-1090, Austria
S- Editor Wang YR L- Editor O’Neill M E- Editor Zheng XM
| 1. | Zhang QH, Ni QX. [Clinical analysis of 2340 cases of pancreatic cancer]. Zhonghua Yixue Zazhi. 2004;84:214-218. |
| 2. | Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, Hruban RH, Ord SE, Sauter PK, Coleman J. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248-257; discussion 257-260. |
| 3. | Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, Sauter PK, Coleman J, Hruban RH, Lillemoe KD. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567-579. |
| 4. | Shoup M, Conlon KC, Klimstra D, Brennan MF. Is extended resection for adenocarcinoma of the body or tail of the pancreas justified? J Gastrointest Surg. 2003;7:946-952; discussion 952. |
| 5. | Wu TC, Shao YF, Shan Y, Wu JX, Zhao P. [Surgical effect of malignant tumor of body and tail of the pancreas: compare with pancreatic head cancer]. Zhonghua Waike Zazhi. 2007;45:30-33. |
| 6. | Liu Q, Zhao P, Wang CF, Cai JQ, Shao YF, Bai XF. [Surgical therapy of tumor of body and tail of pancreas: report of 117 cases]. Zhonghua Waike Zazhi. 2006;44:333-335. |
| 7. | Brennan MF, Moccia RD, Klimstra D. Management of adenocarcinoma of the body and tail of the pancreas. Ann Surg. 1996;223:506-511; discussion 511-512. |
| 8. | Han SS, Jang JY, Kim SW, Kim WH, Lee KU, Park YH. Analysis of long-term survivors after surgical resection for pancreatic cancer. Pancreas. 2006;32:271-275. |
| 9. | Tian YT, Wang CF, Shan Y, Zhao DB, Wang GQ, Zhao XM, Ouyang H, Hao YZ, Sun YM, Qu H. [Prospective evaluation of ultrasonography, multi-slice spiral CT, endoscopic ultrasonography, and magnetic resonance imaging in assessment of TNM staging and assessment of resectability in pancreatic carcinoma]. Zhonghua Yixue Zazhi. 2008;88:2829-2832. |
| 10. | Wong JC, Lu DS. Staging of pancreatic adenocarcinoma by imaging studies. Clin Gastroenterol Hepatol. 2008;6:1301-1308. |
| 11. | Schwarz RE, Harrison LE, Conlon KC, Klimstra DS, Brennan MF. The impact of splenectomy on outcomes after resection of pancreatic adenocarcinoma. J Am Coll Surg. 1999;188:516-521. |
| 12. | Koukoutsis I, Tamijmarane A, Bellagamba R, Bramhall S, Buckels J, Mirza D. The impact of splenectomy on outcomes after distal and total pancreatectomy. World J Surg Oncol. 2007;5:61. |
| 13. | White SA, Sutton CD, Berry DP, Dennison AR. Value of splenic preservation during distal pancreatectomy for chronic pancreatitis. Br J Surg. 2000;87:124. |
| 14. | Govil S, Imrie CW. Value of splenic preservation during distal pancreatectomy for chronic pancreatitis. Br J Surg. 1999;86:895-898. |
| 15. | Kimura W, Moriya T, Ma J, Kamio Y, Watanabe T, Yano M, Fujimoto H, Tezuka K, Hirai I, Fuse A. Spleen-preserving distal pancreatectomy with conservation of the splenic artery and vein. World J Gastroenterol. 2007;13:1493-1499. |
| 16. | Shoup M, Brennan MF, McWhite K, Leung DH, Klimstra D, Conlon KC. The value of splenic preservation with distal pancreatectomy. Arch Surg. 2002;137:164-168. |
| 17. | Kloppel G, Adler G, Hruban RH, Kern SE, Longnecker DS, Partanen TJ. Ductal adenocarcinoma of the pancreas. World Health Organization classification of tumors, pathology of tumors of the digestive tract. Lyon: IARC Press 2000; 221-230. |
| 18. | Liu YB, Wang JW, Xu B, Li HJ, Tang Z, Fang HQ, Wu YL. Peng’s pancreaticojejunal anastomosis (binding pancreaticojejunostomy) for the pancreatoduodenectomy -A new procedure ensuring no leakage. Chin-German J Clin Oncol. 2002;1:65-67. |
| 19. | Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43-66. |
| 20. | Balcom JH 4th, Rattner DW, Warshaw AL, Chang Y, Fernandez-del Castillo C. Ten-year experience with 733 pancreatic resections: changing indications, older patients, and decreasing length of hospitalization. Arch Surg. 2001;136:391-398. |
| 21. | Fahy BN, Frey CF, Ho HS, Beckett L, Bold RJ. Morbidity, mortality, and technical factors of distal pancreatectomy. Am J Surg. 2002;183:237-241. |
| 22. | Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg. 1996;223:273-279. |
| 23. | Yeo CJ, Sohn TA, Cameron JL, Hruban RH, Lillemoe KD, Pitt HA. Periampullary adenocarcinoma: analysis of 5-year survivors. Ann Surg. 1998;227:821-831. |
| 24. | Benoist S, Dugué L, Sauvanet A, Valverde A, Mauvais F, Paye F, Farges O, Belghiti J. Is there a role of preservation of the spleen in distal pancreatectomy? J Am Coll Surg. 1999;188:255-260. |
| 25. | Shoup M, Winston C, Brennan MF, Bassman D, Conlon KC. Is there a role for staging laparoscopy in patients with locally advanced, unresectable pancreatic adenocarcinoma? J Gastrointest Surg. 2004;8:1068-1071. |
| 26. | Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg. 2003;237:74-85. |
| 27. | Lillemoe KD, Kaushal S, Cameron JL, Sohn TA, Pitt HA, Yeo CJ. Distal pancreatectomy: indications and outcomes in 235 patients. Ann Surg. 1999;229:693-698; discussion 698-700. |
| 28. | Yeo CJ, Abrams RA, Grochow LB, Sohn TA, Ord SE, Hruban RH, Zahurak ML, Dooley WC, Coleman J, Sauter PK. Pancreaticoduodenectomy for pancreatic adenocarcinoma: postoperative adjuvant chemoradiation improves survival. A prospective, single-institution experience. Ann Surg. 1997;225:621-633; discussion 633-636. |