Published online Oct 14, 2009. doi: 10.3748/wjg.15.4829
Revised: August 31, 2009
Accepted: September 7, 2009
Published online: October 14, 2009
AIM: To compare the analgesic properties and efficacy of transnasal butorphanol with intramuscular meperidine after anal surgery.
METHODS: Sixty patients who underwent fistulectomy were enrolled in the study from January 2006 to December 2007. They were randomly divided into transnasal butorphanol (n = 30) or intramuscular meperidine (n = 30) treatment groups. Assessment of postoperative pain was made using a visual analogue scale (VAS). The VAS score was recorded 6 h after the completion of surgery, before receiving the first dose of analgesic, 60 min after analgesia and the next morning. Any adverse clinical effects such as somnolence, dizziness, nausea or vomiting were recorded. Satisfaction with narcotic efficacy, desire to use the particular analgesic in the future and any complaints were recorded by patients using questionnaires before being discharged.
RESULTS: Forty-two men and eighteen women were included in the study. There were no significant differences in VAS scores between the groups within 24 h. Length of hospital stay and the incidence of adverse effects between the groups were similar. In addition, most patients were satisfied with butorphanol nasal spray and wished to receive this analgesic in the future, if needed.
CONCLUSION: Butorphanol nasal spray is effective for the relief of pain after fistulectomy. However, it offered patients more convenient usage and would be suitable for outpatients.
- Citation: Mai CM, Wan LT, Chou YC, Yang HY, Wu CC, Jao SW, Hsiao CW. Efficacy and safety of transnasal butorphanol for pain relief after anal surgery. World J Gastroenterol 2009; 15(38): 4829-4832
- URL: https://www.wjgnet.com/1007-9327/full/v15/i38/4829.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4829
| Butorphanol group | Meperidine group | P value | |
| Age (yr) | 37.70 ± 10.76 | 38.90 ± 10.89 | 0.669 |
| Sex (%) | 0.778 | ||
| Male | 20 (66.7) | 22 (73.3) | |
| Female | 10 (33.3) | 8 (26.7) | |
| Weight (kg) | 62.70 ± 10.29 | 64.83 ± 12.01 | 0.463 |
| Height (cm) | 164.57 ± 7.03 | 165.73 ± 7.18 | 0.528 |
| Variable | Butorphanol group | Meperidine group | P value |
| Operative time (min) | 46.17 ± 15.59 | 48.43 ± 10.80 | 0.515 |
| Frequent of analgesia (times) | 3.03 ± 1.67 | 1.23 ± 0.504 | < 0.001 |
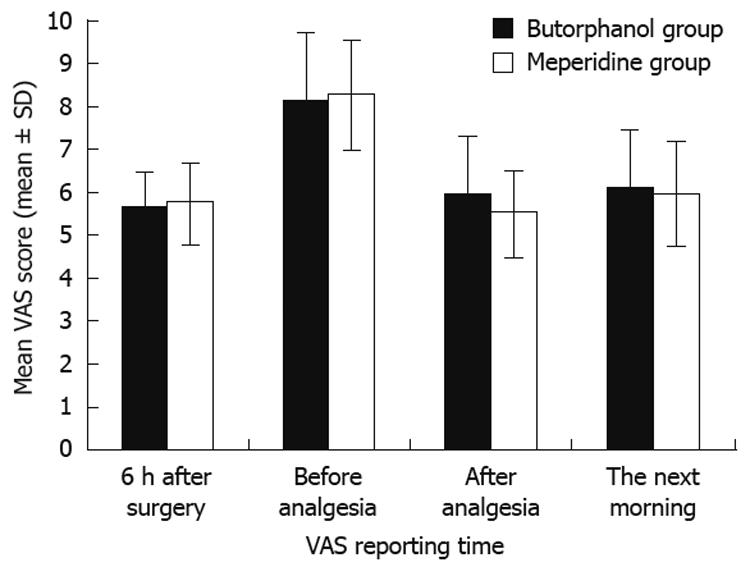
| VAS (6 h after surgery) | 5.67 ± 0.88 | 5.77 ± 1.00 | 0.684 |
| VAS (before analgesia) | 8.17 ± 1.66 | 8.31 ± 1.36 | 0.719 |
| VAS (after analgesia) | 5.93 ± 1.41 | 5.52 ± 1.12 | 0.216 |
| VAS (the next morning) | 6.13 ± 1.47 | 5.93 ± 1.14 | 0.560 |
| Hospital stay (d) | 3.63 ± 0.69 | 3.73 ± 0.74 | 0.585 |
| Adverse effect | Butorphanol group | Meperidine group | P value |
| Any side effect | 16 (53.3) | 10 (33.3) | 0.192 |
| Somnolence | 10 (33.3) | 5 (16.7) | 0.23 |
| Dizziness | 6 (20.0) | 4 (13.3) | 0.73 |
| Nausea | 5 (16.7) | 4 (13.3) | 1.00 |
| Vomiting | 2 (6.7) | 2 (6.7) | 1.00 |
Patients who undergo anal surgery always complain of intractable pain and appropriate analgesia is an important issue for these patients. Butorphanol is a synthetic opioid analgesic with agonist activity for the κ opioid receptor and antagonist activity for the μ opioid receptor[1,2]. Butorphanol nasal spray is an alternative method which avoids hepatic metabolism and provides easier use[1]. Several reports have demonstrated that butorphanol nasal spray is beneficial in treating pain after cesarean section, migraine headache, dental surgery, acute musculoskeletal pain and biliary colic[3-10]. However, no studies have been reported for pain control after fistulectomy. The prospective objective of this study was to evaluate the analgesic properties and efficacy of butorphanol nasal spray after fistulectomy.
After approval from the institutional review board of Tri-Service General Hospital and on receipt of each patient’s written informed consent, 60 patients (fistula in ano, intersphincteric type) scheduled for fistulectomy were enrolled from January 2006 to December 2007. Patients were randomly divided into the transnasal butorphanol treatment group (n = 30) and the intramuscular meperidine control group (n = 30) using a random number table. Exclusion criteria were an American Society of Anesthesiologists physical classification > II, any history of atrophy sinusitis or repeated epistaxis, previous anorectal surgery, inflammatory bowel diseases, hematologic disorders, significant cardiovascular disease, impaired renal function (serum creatinine > 1.5 mg/dL), hepatic disease (twice the upper normal limit of AST or ALT levels) or psychiatric disorders.
During surgery, a monitoring system was attached to each patient. This included pulse oximetry and noninvasive blood pressure measurement. A standardized heavy station intramuscular analgesia (meperidine 1 mg/kg, midazolam 0.08 mg/kg), local perianal anesthesia (2% lidocaine, 0.5% bupivacaine, 1:200 000 epinephrine) and surgical technique (fistulectomy) were prescribed for all patients. A gauze roll was placed in the anal canal for compression and this was removed 8 h later. At 6 h after completion of surgery, all patients were given oral analgesia (tolfenamic acid 10 mg). Subsequently, oral analgesia was prescribed regularly every 6 h. The patients in the butorphanol group received one spray (1 mg) at least every 4 h if they were in pain. In the control group, the patients received intramuscular meperidine (0.8 mg/kg) at least every 4 h if in pain.
Postoperative pain was assessed using a 10-point subjective visual analogue scale (VAS, 0 = “no pain” and 10 = “maximum pain”). The VAS score was recorded 6 h after the completion of surgery, before receiving the first dose of butorphanol or meperidine, 60 min after the analgesic prescribed and the next morning after removal of the gauze roll. Length of hospital stay and any adverse effects of the medicines such as somnolence, dizziness, nausea or vomiting were recorded. The VAS score and adverse effects were measured by an experienced nurse. Before discharge, the patients were asked three questions using questionnaires: (1) Are you satisfied with the narcotic efficacy? (2) How do you feel about the adverse effects? (3) Would you ask for the medicine again if necessary?
Statistical analysis was performed using SYSTAT (version 9.0, SPSS, Inc., Chicago, IL, USA). Each patient’s demographic information and the VAS scores were compared between groups using Student’s t or χ2 tests. Side effects between groups were analyzed using the Fisher’s exact test. The Wilcoxon rank-sum test was used to compare length of hospital stay between the groups and P < 0.05 was considered statistically significant.
Of the 60 patients, 42 (70%) were men and 18 (30%) were women. The mean age of the patients in the butorphanol group was 37.7 years and was 38.9 years in the meperidine group. The mean surgical time was 46.17 min in the butorphanol group compared with 48.43 min in the meperidine group. The demographic details did not differ significantly between two groups (Table 1). The overall frequency of analgesic usage was 3.03 times in the butorphanol group compared with 1.23 times in the meperidine group (P < 0.001). The VAS scores were similar between the groups (Table 2). The mean VAS score 6 h after completion of surgery was 5.67 in the butorphanol group compared with 5.77 in the meperidine group. The mean VAS score before analgesic was 8.17 in the butorphanol group compared with 8.31 in the meperidine group. The mean VAS score after analgesia was 5.93 in the butorphanol group compared with 5.52 in the meperidine group. The mean VAS score the next morning was 6.13 in the butorphanol group compared with 5.93 in the meperidine group (Figure 1). The mean hospital stay was 3.63 d in the butorphanol group compared with 3.73 in the meperidine group.
Several adverse effects were recorded (Table 3). Sixteen patients complained of side effects in the butorphanol group and 10 patients complained of side effects in the meperidine group. The incidence of somnolence, dizziness and nausea was higher in the butorphanol group than in the meperidine group. However, no significant difference in adverse effects between the groups was observed. In the questionnaires, most patients reported good satisfaction with the analgesic they received; 22 patients in the butorphanol group (73%) would be happy to receive butorphanol nasal spray for analgesia in the future if necessary. However, in the butorphanol group, two patients felt they had a poor analgesic response and four patients complained of multiple side effects. Three patients in the meperidine group complained of multiple side effects such as somnolence, dizziness and nausea.
Butorphanol nasal spray proved effective in relieving pain after fistulectomy. These data also demonstrate that butorphanol was equivalent to meperidine for analgesia. This is consistent with the literature on the treatment of moderate to severe pain[3-10]. Butorphanol has been approved in an injectable formula in Taiwan since 1979. Initially, it was prescribed for intravenous or intramuscular administration to avoid the problem of hepatic first-pass metabolism[1]. In 1992, a transnasal formulation was developed to avoid the reduced bioavailability of oral administration. Compared with the oral formulations, transnasal administration produces higher maximum concentration, rapid absorption and better pain relief[1]. It is also 4-8 times more potent than morphine and 30-40 times more potent than meperidine[11]. Peak plasma concentration is reached 30-60 min after 1 mg transnasal administration[12,13]. Moreover, butorphanol nasal spray can be self-administered, is noninvasive and allows convenient usage for patients, especially outpatients.
In both treatment groups, the VAS scores were lower 6 h after completion of surgery. This could reflect the long anesthetic effect of bupivacaine. Subsequently, the VAS score increased and then decreased. The frequency of requests for butorphanol was higher than for meperidine (P < 0.001). However, the reduced VAS pain scores were similar in both groups. Moreover, transnasal butorphanol was noninvasive, convenient and more acceptable to patients. Thus, butorphanol nasal spray could be considered as an alternative device for patient-controlled analgesia.
From the questionnaires, we found that 22 patients (73%) would prefer to receive butorphanol nasal spray in the future, if necessary. However, two patients in the butorphanol group felt that they had a poor analgesic response and these patients developed severe adverse effects. Therefore, to achieve the maximum benefit from butorphanol nasal spray, clinicians should inform patients about the possible adverse effects. Patients must also be alerted to the sedative properties of butorphanol. Most importantly, patients should be cautioned to avoid activities such as driving or operating equipment until the analgesia has worn off.
This study had several limitations, the main limitation being the small sample size. A larger sample size would have offered better information on efficacy and adverse effects of the medications. In addition, with a larger sample size statistically significant differences between the groups may have been observed. Secondly, the VAS pain scores were subjective, possibly reflecting inadequate instruction. Moreover, we did not design a placebo group as it would be unethical to withhold analgesia. Thus, we cannot draw any conclusion as to how butorphanol or meperidine might compare with placebo treatment.
In conclusion, butorphanol nasal spray was effective for relief of pain after fistulectomy. In addition, it offers patient-controlled and more convenient usage than intramuscular meperidine.
Postoperative pain is the major patient complaint after anal surgery. Our purpose was to investigate the analgesic properties and efficacy of transnasal butorphanol after anal surgery.
Postoperative pain was assessed using a visual analogue scale (VAS). However, VAS scales are subjective and the results possibly reflect inadequate instruction.
Several reports have demonstrated that butorphanol nasal spray is beneficial in treating postoperative pain. This is the first study to report that it is also effective for pain relief after anal surgery.
As butorphanol nasal spray is safe and effective for relief of pain after anal surgery, it might offer convenient usage for outpatients.
It is a small and possibly worthwhile clinical trial with a novel analgesic regimen.
Peer reviewers: Dr. Marc D Basson, Department of Surgery, Wayne State University and John D Dingell VA Medical Center, Detroit, Michigan, 48201, United States; Walter Edwin Longo, Professor, Department of Surgery, Yale University, New Haven, Connecticut, 06510, United States
S- Editor Cheng JX L- Editor Webster JR E- Editor Zheng XM
| 1. | Gillis JC, Benfield P, Goa KL. Transnasal butorphanol. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in acute pain management. Drugs. 1995;50:157-175. |
| 2. | Vandam LD. Drug therapy: butorphanol. N Engl J Med. 1980;302:381-384. |
| 3. | Scott JL, Smith MS, Sanford SM, Shesser RF, Rosenthal RE, Smith JP, Feied CF, Ghezzi KT, Hunt DM. Effectiveness of transnasal butorphanol for the treatment of musculoskeletal pain. Am J Emerg Med. 1994;12:469-471. |
| 4. | Dale O, Hjortkjaer R, Kharasch ED. Nasal administration of opioids for pain management in adults. Acta Anaesthesiol Scand. 2002;46:759-770. |
| 5. | Hoffert MJ, Couch JR, Diamond S, Elkind AH, Goldstein J, Kohlerman NJ 3rd, Saper JR, Solomon S. Transnasal butorphanol in the treatment of acute migraine. Headache. 1995;35:65-69. |
| 6. | Rapoport AM, Bigal ME, Tepper SJ, Sheftell FD. Intranasal medications for the treatment of migraine and cluster headache. CNS Drugs. 2004;18:671-685. |
| 7. | Ladov MJ, Precheur HV, Rauch DM, Engel PS, Stern RK. An open-label evaluation of the efficacy and safety of Stadol NS with ibuprofen in the treatment of pain after removal of impacted wisdom teeth. J Oral Maxillofac Surg. 2000;58:15-18. |
| 8. | Wermeling DP, Grant GM, Lee A, Alexander N, Rudy AC. Analgesic effects of intranasal butorphanol tartrate administered via a unit-dose device in the dental impaction pain model: a randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2005;27:430-440. |
| 9. | Olsen JC, McGrath NA, Schwarz DG, Cutcliffe BJ, Stern JL. A double-blind randomized clinical trial evaluating the analgesic efficacy of ketorolac versus butorphanol for patients with suspected biliary colic in the emergency department. Acad Emerg Med. 2008;15:718-722. |
| 10. | Abboud TK, Zhu J, Gangolly J, Longhitano M, Swart F, Makar A, Chu G, Cool M, Mantilla M, Kurtz N. Transnasal butorphanol: a new method for pain relief in post-cesarean section pain. Acta Anaesthesiol Scand. 1991;35:14-18. |
| 11. | Oliveto A, Sevarino K, McCance-Katz E, Feingold A. Butorphanol and nalbuphine in opioid-dependent humans under a naloxone discrimination procedure. Pharmacol Biochem Behav. 2002;71:85-96. |
| 12. | Vachharajani NN, Shyu WC, Nichola PS, Boulton DW. A pharmacokinetic interaction study between butorphanol and sumatriptan nasal sprays in healthy subjects: importance of the timing of butorphanol administration. Cephalalgia. 2002;22:282-287. |
| 13. | Davis GA, Rudy AIa, Archer SM, Wermeling DP. Bioavailability of intranasal butorphanol administered from a single-dose sprayer. Am J Health Syst Pharm. 2005;62:48-53. |