INTRODUCTION
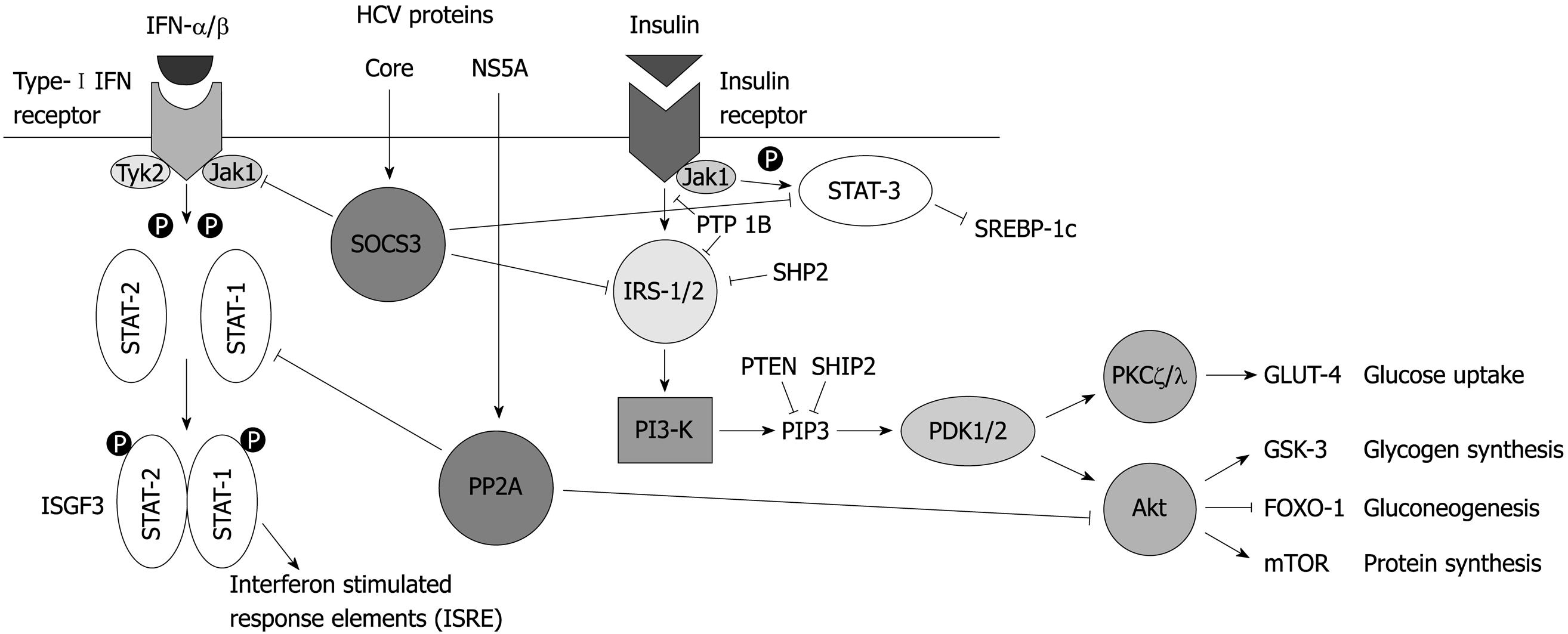
Figure 1 Hepatitis C virus core and NS5A proteins may inhibit insulin and interferon signaling.
Infection with the hepatitis C virus (HCV) is a leading cause of chronic liver disease, with over 3% of the world’s population (180 million people) infected and 130 million at risk of cirrhosis[1]. The majority of infected individuals (60%-80%) develop chronic hepatitis C (CHC), which is associated with progressive liver fibrosis and a 3%-9% risk of cirrhosis after 20 years as shown in community-based studies[2]. CHC is also associated with significant morbidity and mortality, accounting for 50%-76% of all liver cancer cases worldwide, and two thirds of liver transplants in the developed world[1].
It is now widely recognized that CHC is associated with insulin resistance (IR) and type 2 diabetes (T2DM), so can be considered a metabolic disease. Apart from the well-described complications of diabetes, IR in CHC predicts faster progression to fibrosis and cirrhosis that may culminate in liver failure and hepatocellular carcinoma (HCC). More recently, it has been recognized that IR in CHC predicts a poor response to antiviral therapy. The molecular mechanisms for the association between IR and HCV infection are not well defined. This review will elaborate on the clinical associations between CHC and IR and summarize current knowledge regarding the molecular mechanisms that potentially mediate HCV-associated IR.
INSULIN SIGNALING AND IR
Insulin is an anabolic hormone secreted by pancreatic β-cells that is required for the maintenance of glucose homeostasis. It inhibits hepatic glucose production and increases peripheral glucose uptake and glycogen synthesis. The principal signaling pathway (Figure 1) involves sequential activation of the insulin receptor, insulin receptor substrates (IRS), phosphatidylinositol-3-kinase (PI3K), Akt and protein kinase C isoforms ζ and λ[3,4]. Akt is phosphorylated initially at serine 473 by phosphoinositide-dependent kinase (PDK)2 and subsequently at threonine 308 by PDK1. Activated Akt promotes storage of excess glucose as glycogen by phosphorylating glycogen synthase kinase and suppresses gluconeogenesis by inhibiting phosphoenol-pyruvate carboxykinase and glucose-6 phosphatase. In striated muscle and adipose tissue, activated Akt promotes translocation of the glucose transporter GLUT4 to the plasma membrane, promoting glucose uptake[3].
Insulin promotes lipid synthesis via sterol regulatory element-binding protein 1c and fatty acid synthase, although importantly the pathways are probably different from those mediating insulin-stimulated glucose homeostasis[5]. With regard to protein, insulin promotes cell survival and protein synthesis, mediated by the mammalian target of rapamycin (mTOR) pathway[3].
Insulin resistance plays a fundamental role in the pathogenesis of T2DM. It results from defects at any level of the ligand-receptor-response pathway, including well-characterized defects at the level of the insulin receptor or IRS molecules, although these account for only a small minority of cases of IR in clinical practice[3,4]. These defects can result from either reduced levels of signaling proteins, or modulation of their activity by phosphorylation. For example, IRS-1 is activated by phosphorylation of tyrosine residues, but inhibited by phosphorylation of key serine residues including Ser307, Ser318, Ser636 and Ser639[6]. Negative feedback loops down-regulate the pathway in response to chronic glucose supply, including inhibition of PI3K by phosphatase and tensin homolog.
Two IRS proteins are important in human liver: IRS-1 and IRS-2[7]. They appear to have complementary but overlapping roles in that IRS-1 knockout mice exhibit growth retardation and IR, while IRS-2 knockouts develop T2DM due to β-cell failure and hepatic IR. While both knockouts are insulin-resistant, IRS-1 knockout mice have reduced peripheral glucose uptake, while IRS-2 knockouts have a more complex phenotype, with both peripheral and central IR[8]. This has led to the traditional view that IRS-2 is more important for insulin signaling and glucose homeostasis in the liver[9]. However, recent evidence suggests that IRS-1 may be more important for glucose homeostasis, while IRS-2 is more important for lipid metabolism[10]. Therefore, changes affecting either IRS could contribute to HCV-induced hepatic IR.
The roles of mTOR in insulin signaling are multiple and complex. Early studies showed that insulin signaling via Akt caused phosphorylation and activation of mTOR[11]. However it is now known that mTOR is present in at least two different mTOR complexes and plays multiple roles in insulin signaling, as reviewed recently[12,13]. The mTOR-raptor complex 1 (mTORC1) appears to mediate the downstream effects of insulin on cell growth and proliferation[12] and also provides negative feedback of insulin signaling by phosphorylating IRS-1 at inhibitory serine residues 636 and 639[14]. In contrast, mTOR associates with rictor to form a second complex mTORC2[12], the elusive “PDK2” that phosphorylates Akt at serine 473 in response to insulin[15]. mTORC2 is much less sensitive to rapamycin inhibition than mTORC1[12].
CLINICAL ASSOCIATIONS OF HCV AND IR
CHC and T2DM
It is now over 10 years since an association between HCV and diabetes was first described by Allison et al[16] who noted that people with cirrhosis and HCV had T2DM more commonly than those with cirrhosis from other causes. A subsequent study of cirrhotic patients confirmed that T2DM was present in 21% of patients with cirrhosis due to CHC but was present in only 12% of patients with cirrhosis and chronic hepatitis B (CHB)[17]. In the latter report, T2DM was particularly associated with HCV genotype 2a[17].
Significantly, subsequent case control studies have confirmed that T2DM is associated with CHC even in the absence of cirrhosis[18-20]. Most recently, a large cross-sectional study of over 9000 individuals in the USA found that in persons over 40 years of age, those with HCV infection were over three times more likely to have T2DM than those without[21]. Of relevance, there was no association between CHC and type 1 diabetes, and no association of hepatitis B virus infection with T2DM, suggesting a virus-specific association of HCV with T2DM. Interestingly, the association of T2DM was with HCV genotype 1b. In a subsequent large cohort study, it was noted that HCV-associated T2DM mainly occurred in patients with other risk factors for diabetes, such as older age and a high body mass index[22]. Thus, among patients classified as “high risk” for T2DM, CHC increased diabetes risk more than 11-fold after 9 years[22], although further studies are required to firmly establish causality.
CHC and IR
Insulin resistance is present in > 90% of individuals before the onset of frank T2DM. While the euglycemic hyperinsulinemic clamp[23] is the “gold standard” for measuring glucose utilization and insulin sensitivity or resistance, a common clinical approximation is obtained using the homeostasis model of IR (HOMA-IR), calculated by the following equation: HOMA-IR = fasting glucose (mmol/L) × fasting insulin (μU/mL)/22.5[24,25]. Typically, a HOMA-IR value > 2 is used to signify significant IR[26].
In 2003, Hui et al[27] first reported that IR is increased in patients infected with HCV, particularly genotype 1 and that this may form the basis for the earlier observations regarding the association with T2DM. Subsequent studies have confirmed this association, including for genotype 4[28] and possibly also genotype 2a[29]. Since liver fibrosis irrespective of etiology can of itself cause hyperinsulinemia, it is important to exclude patients with advanced fibrosis in any assessment of the relationship between HCV and IR. This was undertaken in the study by Hui et al[27], who demonstrated that even patients with minimal fibrosis (F0 or F1) had a mean HOMA-IR of 2.4 compared to 1.9 for matched uninfected controls (P = 0.002).
Studies have shown that in people with HCV and IR who respond to treatment there is a reduction in HOMA-IR[26]. This improvement in insulin sensitivity is maintained for people with a sustained virological response (SVR)[26,30], and results in a reduced risk of subsequent diabetes[30,31]. However other groups have shown that the apparent reduction in diabetes risk may actually reflect the lower baseline risk of diabetes in patients who respond to interferon treatment, rather than an effect of treatment per se[32]. It is also worth noting that not all patients with CHC develop IR, suggesting a complex interaction between virus and host factors that is only partially understood.
In contrast to the specific association of IR with CHC, IR is not associated with CHB. A recent study found that in patients with CHB the HOMA-IR reflected their overall metabolic profile, but was not increased in people with CHB compared with matched healthy controls[33]. Another recent prospective study comparing patients with CHB and CHC confirmed that IR is a specific feature of hepatitis C genotype 1 and 4 infection, but not CHB; it was present in 35% of patients with CHC compared with only 5% with CHB[28].
Complications of HCV-induced IR
As well as predisposing to T2DM, the presence of IR in CHC predicts non-response to antiviral therapy[26], both for genotype 1[26,34-36] and genotypes 2 and 3 infection[37]. Central obesity, which can be associated with IR, has also been shown to predict non-response[38]. Furthermore, HCV-infected patients who respond to antiviral therapy show improved insulin sensitivity[39]. Similarly, improvements in CHC-induced steatosis have been observed following SVR[40]. Interestingly, IR has been associated in several studies with elevated HCV viral loads[28,41,42]. One possible mechanism involves p21-activated kinase 1, which suppresses HCV replication and is stimulated by PI3K/Akt signaling, via mTOR[43]. HCV-associated IR could decrease PI3K/Akt signaling and thus favor viral replication, although the mechanism by which HCV induces IR is unclear.
The interactions between HCV, IR, steatosis and hepatic fibrosis are complex and genotype specific. In CHC due to genotype 1, IR is associated with hepatic steatosis. In this setting, IR is either virus-mediated or due to host metabolic factors such as visceral obesity[44]. A similar association has been shown for genotype 4[45]. In contrast, steatosis in HCV genotype 3 infection is predominantly a direct effect of the virus, occurring in the absence of other metabolic risk factors[46,47]. It should be noted however, that in people with genotype 3 CHC and obesity, a proportion of the hepatic steatosis will be secondary to their metabolic dysregulation. In patients infected with HCV, steatosis and IR are predictive factors for the later progression to hepatic fibrosis and cirrhosis[28,48-51]; the latter predisposes to HCC. Likewise, diabetes mellitus itself has recently been shown to increase the risk of HCC[52]. While CHC-induced steatosis has been reported as a risk factor for hepatic fibrosis[53], subsequent studies suggest that IR is most important in this relationship, both for genotypes 1 and 3[46,48].
MECHANISMS OF HCV-INDUCED IR
Inflammation and IR
Chronic inflammation plays a significant role in IR associated with metabolic liver disease, due to increased levels of interleukin (IL)-1, tumor necrosis factor (TNF)-α, IL-6 and leptin, and reduced levels of adiponectin[54]. Diet-induced IR in the context of obesity partly involves the inflammatory mediator IκB kinase β (IKKβ)[55]. Inflammatory cytokines such as IL-1, TNF-α and free fatty acids stimulate IKKβ. This induces proteasomal degradation of IκB, allowing nuclear translocation of the downstream effector molecule NFκB to stimulate secretion of IL-6[56]. IKKβ also induces IR by inhibitory phosphorylation of the insulin signaling molecule IRS-1 at serine 312[57]. High doses of aspirin and other salicylates inhibit IKKβ and can reduce IR in both rats and humans[58-60]. IR can similarly occur in other inflammatory conditions associated with elevated TNF-α, including inflammatory bowel disease[61], rheumatoid arthritis[62] and psoriasis[63].
Based on these well-described associations, it was initially proposed that IR in CHC may arise as a non-specific consequence of hepatic inflammation, possibly mediated by IKKβ. In support of this hypothesis, expression of HCV core protein in transgenic mice (genotype 1b) induces hepatic IR[64]. When fed a high-fat diet, these mice develop frank diabetes and hepatic steatosis that is associated with elevated circulating levels of TNF-α. The IR is reversed by administering antibodies against TNF-α, but the mechanism of this effect has not been well defined.
Early human studies were conflicting but more recent studies have shown that HCV-induced IR is NOT due to alterations in serum inflammatory cytokines or adipokines[65]. Rather, IR in CHC seems to be due to direct virus-specific effects on insulin signaling. Thus, initial studies noted increased serum levels of TNF-α in subjects with CHC[66-68] or showed a correlation with IR[69] but did not adequately correct for potential confounders. A later well-controlled study compared 154 HCV-infected non-diabetic males with 75 matched uninfected controls[65]. In that study, as expected, serum levels of TNF-α and IL-6 were higher in HCV-infected patients than controls but levels did not correlate with IR[65]. Serum levels of the adipocytokines leptin and adiponectin were likewise independently associated with IR (adiponectin inversely), but not with HCV infection itself. The authors therefore concluded that these adipocytokines could not account for the increased IR seen in HCV-infected subjects[65].
Direct effects of HCV in modulating insulin signaling HCV core protein
Although it was initially suggested that IR associated with CHC may be due to chronic inflammation, it is now known that HCV can induce IR directly, through specific viral effects[70]. Much of the published literature in this area has focused on the HCV core protein, which has been proposed to cause IR in hepatocytes by reducing the level or activity of molecules involved in insulin signaling, particularly IRS-1 and IRS-2. However, there is considerable disagreement concerning which of these molecules is more important, and whether altered signaling results from changes in IRS expression, degradation, or altered activity[64,71-75].
For example, one study found reduced activation (reduced tyrosine phosphorylation) of IRS-1 in liver biopsies from HCV-infected patients and reduced association of IRS-1 with its downstream effector PI3K but increased expression of IRS-1 protein[71]. In contrast, another report demonstrated reduced expression of both IRS-1 and IRS-2 in patient samples and in livers from transgenic mice expressing HCV core protein[73]. It was proposed that core protein stimulated increased levels of the molecule suppressor of cytokine signaling (SOCS) 3, leading to ubiquitination and proteasomal degradation of IRS-1 and IRS-2[73] (Figure 1). This is consistent with data showing IR in mice following over-expression of SOCS1 or SOCS3[76]. In support of their hypothesis, the same group found that in patients who responded to antiviral therapy hepatic levels of both IRS-1 and IRS-2 were increased, along with improved clinical insulin sensitivity[39]. One clinical study showed higher levels of SOCS3 in peripheral lymphocytes from people infected with HCV genotype 1 rather than genotype 2 and found that the level of SOCS3 was the best predictor of response to interferon therapy[77]. In a similar study from the same group, polymorphisms in the SOCS3 gene were shown to correlate with clinical response to interferon[78]. SOCS3 mRNA levels were higher in obese subjects with CHC than lean subjects and may contribute to their reduced response to IFN-α treatment[79].
The effects of HCV genotype on insulin signaling are less well understood but are important given the clinical association of genotypes 1 and 4 with IR[27,28]. One study compared the effects of over-expressing genotype 1b and 3a core proteins in the Huh7 hepatoma cell line[75]. No difference in SOCS3 expression was detected, but cells expressing genotype 3a core contained higher levels of SOCS7 than cells expressing genotype 1b core, as well as reduced levels of IRS-1[75]. In contrast, cells expressing genotype 1b core had a smaller reduction in the amount of IRS-1, but increased phosphorylation of IRS-1 at inhibitory serine residues (636/639), as well as increased mTOR activity[75]. The authors therefore speculated that IR in the context of HCV genotype 1 infection is due to core-induced induction of the TORC1 mTOR/raptor complex, resulting in reduced IRS-1 signaling[75]. A different group has shown reduced insulin signaling in core-expressing cells, due to JNK-mediated inhibitory phosphorylation of IRS-1 at serine 312[72]. Although intriguing, the relevance of IRS-1 serine phosphorylation to clinical HCV-induced IR has yet to be confirmed.
PA28γ is an inducer of late proteasome activity that may play a role in HCV-induced IR as it is essential for the development of IR in HCV core transgenic mice[74]. Core transgenic mice display reduced insulin sensitivity, reduced activation (tyrosine phosphorylation) of IRS-1 and reduced expression (mRNA and protein) of IRS-2; factors which are restored to normal following knockout of the PA28γ gene[74]. TNF-α expression was increased in the livers of core transgenic mice and in human hepatoma cell lines expressing core protein, but knocking out or silencing PA28γ returned TNF-α expression to normal[74]. PA28γ has also been shown to play a critical role in the development of steatosis and HCC[80], and may provide a useful link between the different pathways affected by HCV.
HCV NS5A
Another molecule that may play a role in HCV-induced IR is protein phosphatase 2A (PP2A). PP2A can affect several cell pathways and is upregulated in HCV infection, possibly due to increased endoplasmic reticulum (ER) stress[81] or directly by the HCV non-structural protein NS5A[82] (Figure 1). PP2A has been shown to mediate HCV-associated IR by dephosphorylating and thus inactivating Akt[83]. In that study, PP2A levels were increased in HCV protein-expressing cell lines, the livers of transgenic mice expressing HCV proteins and in liver biopsies from HCV-infected patients. Impaired insulin signaling was demonstrated in each model, with reduced insulin-stimulated phosphorylation of Akt[83]. However, the authors were unable to show a correlation between reduced Akt signaling and IR in HCV-infected patients, as measured by HOMA-IR[83]. Interestingly, PP2A has been shown to inhibit interferon signaling and this has been proposed as another potential link between IR and reduced clinical response to interferon treatment in HCV-infected patients[84].
Peroxisome proliferator activated receptor (PPAR)-α and PPAR-γ
PPARs are nuclear receptors that modulate lipid and glucose metabolism, as reviewed recently[85]. PPARs form heterodimeric complexes with the retinoid X receptor (RXR) and bind to PPAR response elements (PPRE) on PPAR regulated genes, inhibiting their expression. Binding of PPAR ligands, including unsaturated fatty acids, eicosanoids, oxidised low density lipoprotein (LDL) and very LDL, causes dissociation of the PPAR-RXR complex, derepression and increased gene expression[85]. In this way PPARs react to lipid excess by stimulating differentiation of adipocytes, oxidation of fatty acids and glucose metabolism.
PPAR-α is the major PPAR isoform present in liver and is also found in brown fat and heart. It regulates cell energy by stimulating oxidation of fatty acids in mitochondria and peroxisomes[85]. Along with PPAR-δ, it stimulates expression of human adipose differentiation-related protein, thus promoting storage of cellular lipid in lipid droplets[86]. PPAR-γ is the dominant isoform in adipose tissue, colon, myeloid cells and placenta, where it stimulates adipocyte differentiation and lipid storage[85].
As well as their effects on lipid metabolism, PPARs may also play a role in HCV-induced IR[87]. Liver biopsies from patients with CHC show reduced levels of PPAR-γ and PPAR-α mRNA[88,89]. In an in vitro model of HCV-induced hepatic IR, cells expressing genotype 3 core protein, but not genotype 1b, had reduced levels of PPARγ mRNA[89]. In a follow-up study, treatment of genotype 3a core-expressing cells with the PPAR-γ agonist rosiglitazone improved insulin signaling[75]. Interestingly, PPAR-α is required for HCV core-induced steatosis in transgenic mice[90], suggesting overlapping mechanisms for IR and steatosis in people with CHC.
Oxidative stress and IR
Oxidative stress may contribute to IR in HCV-infected people, as well as to steatosis. In vitro studies demonstrate increased mitochondrial reactive oxygen stress in hepatoma cells over-expressing core protein[91] and in HCV core transgenic mice[91,92]. Further, in HCV-infected patient serum, thioredoxin, a marker for oxidative stress, has been shown to correlate with clinical IR, independent of obesity[93]. However the interactions between oxidative stress, IR, metabolic syndrome and steatosis are complex, with each potentially influencing the other. A recent study showed that in patients infected with HCV genotype non-3, HOMA-IR (P < 0.01), fibrosis (P < 0.01) and oxidative stress (P < 0.05) were independently associated with steatosis, whereas steatosis was independently associated with oxidative stress (P < 0.03) and HOMA-IR (P < 0.02)[94]. The authors concluded that in genotype non-3 infection “oxidative stress and IR contribute to steatosis, which in turn exacerbates both IR and oxidative stress and accelerates the progression of fibrosis”[94].
Treatment of IR in people infected with HCV
It has been shown that response to interferon-based treatment can be improved by diet-induced weight loss, which improves insulin sensitivity[95]. Given the association between IR and poor treatment response in CHC, clinical trials of insulin-sensitizing drugs have been proposed to improve treatment response[29]. Since the mechanisms of HCV-induced IR are not well understood it is not clear whether it is better to use thiazolidenediones that target PPAR-γ, or metformin, which activates AMP-activated protein kinase[96] and may also stimulate the insulin receptor to signal via IRS-2[97].
A recent pilot study adding the PPAR-γ agonist pioglitazone (15 mg) to pegylated interferon alpha and ribavirin was undertaken in patients with HCV-induced IR who had previously failed standard treatment[98]. Although most of the treated patients showed improvement in their HOMA-IR on pioglitazone, none had a satisfactory virological response after 12 wk and the trial was terminated. A recent case report suggests that reducing IR prior to antiviral therapy, by pre-treating with insulin sensitizing drugs, may improve outcomes[99]. The authors administered a high dose of pioglitazone (45 mg) to a patient with genotype 3a CHC who had previously failed antiviral treatment. After 5 mo of pioglitazone, the patient’s HOMA-IR reduced from 4.8 to 1.3 and subsequent treatment with pegylated interferon and ribavirin for 48 wk produced a SVR[99]. It is not clear whether this outcome was due to the higher dose of pioglitazone, the sequential treatment approach or the patient’s genotype 3 infection[100]. Several similar studies are underway to address these issues and their results are eagerly awaited.
CONCLUSION
There is increasing evidence that CHC is a metabolic disease, strongly associated with IR and T2DM. Insulin resistance is most strongly associated with HCV genotype 1, in contrast to hepatic steatosis which is associated with genotype 3 infection. Although the precise mechanisms of HCV-associated IR are unclear, several possibilities have been suggested and the mechanisms may be multi-factorial, including both virus and host factors. Early studies in transgenic mice suggested IR may result from chronic inflammation and elevated TNF-α, but subsequent human studies have suggested a virus-specific effect. HCV core protein has the most evidence to support its role in IR, with likely effects on insulin signaling at the level of IRS. Core-induced increases in SOCS3 or SOCS7 expression may cause IRS destruction, probably requiring PA28γ, but other proposed alternatives include feedback inhibition of IRS-1 by activated mTOR or JNK. Insulin resistance may also result from core-induced alterations in PPAR-α and PPAR-γ, especially in genotype 3 infection. PP2A can cause IR and in CHC its activity may be increased, either in response to ER stress or to the HCV non-structural protein NS5A, which interacts with many cellular pathways.
Since IR in CHC is associated with a reduced response to antiviral treatment, clinical trials are underway to determine whether reducing IR improves treatment outcomes. Preliminary data from pilot studies of PPAR-γ agonists have been disappointing, but case reports suggest that more aggressive treatment may be successful, particularly if IR is reduced prior to commencing antiviral therapy. Trials of other insulin-sensitizing drugs including metformin are also in progress and their results are eagerly awaited. Since the underlying mechanisms of HCV-induced IR are still not clear, ongoing research is essential to guide a more rational, targeted approach to therapy.