Published online Sep 14, 2009. doi: 10.3748/wjg.15.4305
Revised: August 5, 2009
Accepted: August 12, 2009
Published online: September 14, 2009
AIM: To investigate the differences in clinicopathological features between patients with pancreatic cancer greater or less than 2 cm situated over the pancreatic head and the prognostic factors for survival of patients with pancreatic cancer < 2 cm over the pancreatic head.
METHODS: From 1983 to 2006, 159 patients with histologically proven pancreatic adenocarcinoma (PAC) at the pancreatic head undergoing curative resection at the Department of Surgery, Chang Gung Memorial Hospital, Taipei, Taiwan were reviewed, comprising 123 cases of large (L)-PAC (tumor > 2 cm) and 36 cases of small (S)-PAC (tumor ≤ 2 cm). We compared the clinicopathological characteristics and prognosis of L-PAC and S-PAC patients. The clinicopathological characteristics of S-PAC were investigated to clarify the prognosis predictive factors of S-PAC.
RESULTS: One hundred and fifty-nine PAC patients, aged 16-93 years (median, 59.0 years) with a tumor at the pancreatic head undergoing intentional curative resection were investigated. The S-PAC and L-PAC patients had similar demographic data, clinical features, and tumor markers (a similar positive rate of carcinoembryonic antigen and carbohydrate antigen 19-9). There were also similar rates of lymph node metastasis, portal vein invasion, stage distribution, tumor differentiation, positive resection margin, surgical morbidity and mortality observed between the two groups. During a follow-up period ranging from 1.0 to 122.7 mo (median, 10.9 mo), S-PAC and L-PAC patients had a similar prognosis after resection (P = 0.4805). Among the S-PAC patients group, patients with higher albumin level (> 3.5 g/dL) had more favorable survival than those with lower albumin levels, which was the only favorable predictive prognostic factor. Meanwhile, early-staged (stage I, II) S-PAC patients tended to have a more favorable outcome than late-stage (stage III, IV) S-PAC patients, but this was not statistically significant.
CONCLUSION: S-PAC patients should not be regarded as early PAC. Only higher albumin level (> 3.5 g/dL) and early stage disease (stage I, II) were the favorable prognosis factors for S-PAC patients.
- Citation: Chiang KC, Yeh CN, Lee WC, Jan YY, Hwang TL. Prognostic analysis of patients with pancreatic head adenocarcinoma less than 2 cm undergoing resection. World J Gastroenterol 2009; 15(34): 4305-4310
- URL: https://www.wjgnet.com/1007-9327/full/v15/i34/4305.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4305
| S-PAC (n = 36) | L-PAC (n = 123) | P | |
| Age (yr) | 61.9 ± 8.2 | 62.8 ± 10.5 | 0.601 |
| Sex (M/F) | 22/14 | 74/49 | 0.918 |
| Symptom (+/-) | 35/1 | 123/0 | 0.226 |
| Physical findings (+/-) | 32/4 | 99/24 | 0.244 |
| Albumin (g/dL) | 3.74 ± 0.58 | 3.71 ± 0.61 | 0.827 |
| AST (IU/L) | 178.8 ± 258.5 | 138.1 ± 127.7 | 0.204 |
| CEA (ng/mL) ≥ 5 | 8/27 (29.6) | 33/92 (35.9) | 0.549 |
| CA 19-9 (IU/L) ≥ 37 | 20/29 (69.0) | 72/96 (75.0) | 0.518 |
| Size (median) | 1.5 | 3.5 | 0.0001 |
| Operation time (min) | 494.5 ± 141.5 | 469.2 ± 118.1 | 0.292 |
| LN metastasis | 23 (63.9) | 72 (58.5) | 0.565 |
| PV invasion | 4 (11.1) | 12 (9.8) | 0.760 |
| Positive margin | 10 (27.8) | 35 (28.5) | 0.244 |
| Staging | 0.459 | ||
| I | 12 (33.3) | 39 (31.7) | |
| II | 1 (2.8) | 12 (9.8) | |
| III | 22 (61.1) | 71 (57.7) | |
| IV | 1 (2.8) | 1 (0.8) | |
| Tumor differentiation | 0.723 | ||
| W-D | 11 (30.6) | 38 (30.9) | |
| M-D | 19 (52.8) | 59 (48.0) | |
| P-D | 6 (16.7) | 26 (21.2) | |
| Morbidity | 11 (30.6) | 33 (26.8) | 0.660 |
| Postoperative CT | 23 (63.9) | 58 (47.2) | 0.077 |
| Postoperative RT | 1 (2.8) | 11 (8.9) | 0.300 |
| Mortality | 1 (2.8) | 5 (4.1) | 0.760 |
| Factors | Survival time (mo) | P | |||
| Median (mo) | Mean (mo) | 3-yr (%) | 5-yr (%) | ||
| Gender | 0.8417 | ||||
| Male (n = 21) | 13.0 | 24.0 | 28.1 | 0 | |
| Female (n = 14) | 14.0 | 27.9 | 23.4 | 11.7 | |
| Age (yr) | 0.2931 | ||||
| ≤ 60 (n = 13) | 24.6 | 26.4 | 35.9 | 12.0 | |
| > 60 (n = 22) | 9.4 | 8.06-10.75 | 17.02 | 12.61 | |
| Physical examination | 0.9566 | ||||
| Positive (n = 31) | 13.0 | 28.1 | 26.7 | 8.0 | |
| Negative (n = 4) | 16.1 | 22.5 | 25.0 | 0 | |
| Bilirubin (mg/dL) | 0.5049 | ||||
| ≤ 2 (n = 8) | 14.0 | 15.6 | 14.3 | 0 | |
| > 2 (n = 27) | 16.1 | 29.4 | 39.6 | 7.7 | |
| Albumin (g/dL) | 0.0002 | ||||
| ≤ 3.5 (n = 13) | 5.9 | 7.6 | 0 | 0 | |
| > 3.5 (n = 22) | 16.6 | 32.7 | 33.6 | 11.2 | |
| Serum CEA (ng/mL) | 0.6741 | ||||
| ≤ 5 (n = 24) | 16.6 | 22.4 | 23.6 | 7.9 | |
| > 5 (n = 11) | 9.1 | 19.8 | 25.0 | 15.42 | |
| Serum CA 19-9 (IU/L) | 0.7592 | ||||
| ≤ 37 (n = 11) | 16.6 | 19.4 | 11.1 | 11.1 | |
| > 37 (n = 24) | 14.0 | 23.5 | 33.6 | 0 | |
| Operative procedure | 0.6500 | ||||
| Whipple (n = 30) | 16.1 | 27.9 | 28.8 | 7.2 | |
| PPPD (n = 5) | 14.0 | 15.5 | 0 | 0 | |
| Portal vein invasion | 0.1467 | ||||
| Positive (n = 2) | 4.0 | 9.0 | 0 | 0 | |
| Negative (n = 33) | 16.1 | 28.0 | 28.1 | 7.0 | |
| Resection margin | 0.6704 | ||||
| Positive (n = 10) | 10.9 | 19.5 | 30.0 | 0 | |
| Negative (n = 25) | 16.1 | 28.8 | 26.4 | 7.9 | |
| TNM staging | 0.0931 | ||||
| I + II (n = 13) | 16.6 | 40.2 | 50.0 | 15.0 | |
| III + IV (n = 22) | 13.0 | 17.6 | 14.4 | 0 | |
| Tumor differentiation | 0.3202 | ||||
| W-D (10) | 23.1 | 22.9 | 50.0 | 0 | |
| M-D (19) | 10.8 | 21.5 | 17.9 | 6.0 | |
| P-D (6) | 16.1 | 32.2 | 50.0 | 25.0 | |
| Post-operative CT | 0.7114 | ||||
| With (n = 23) | 16.1 | 23.9 | 25.3 | 0 | |
| Without (n = 12) | 10.8 | 28.8 | 27.8 | 13.9 | |
| Post-operative RT | 0.9385 | ||||
| With (n = 1) | 24.6 | 24.6 | 0 | 0 | |
| Without (n = 24) | 14.0 | 24.6 | 27.4 | 6.9 | |
Pancreatic adenocarcinoma (PAC), one of the most lethal malignant cancers, ranks fifth in mortality related to cancer worldwide with the 5-year survival after resection ranging from 10% to 29%[1-3]. Thirty-two thousand new PAC cases have been found in America each year[4].
Surgery remains the only curative treatment of PAC and detection of PAC with small size (≤ 2 cm) (S-PAC) seems to be essential to improve the outcome, which is demonstrated in some reports[5-7]. The 5-year survival rate varied from 19% to 41% for PAC patients undergoing pancreatectomy with the highest survival in patients with small tumors confined to the pancreas. But the controversial question is “Dose PAC with tumor diameters of 2 cm or less necessarily indicate early-stage disease or good prognosis?” Recent data have shown the different opinions about this issue[8,9], which warrants further studies addressing this topic.
To collect an adequate number of resected cases of S-PAC was difficult due to limitations of diagnostic equipment, explaining why most of the previous studies were multi-institutional[7,10,11]. Recent advances in diagnosis have made it possible to detect PAC earlier and to increase the number of resected cases; consequently evaluation of S-PAC cases in one center becomes feasible, which could provide more accurate information.
We retrospectively reviewed 159 PAC cases with pancreatic head cancer undergoing pancreaticoduodenectomy from 1983 to 2006. Among them, 36 cases were diagnosed with S-PAC, and the remainder were PAC with tumor diameter > 2 cm, classified as large PAC (L-PAC). We compared the clinicopathological features and surgical outcomes between S-PAC and L-PAC to find the favorable prognostic factors.
From 1983 to 2006, 159 patients with histopathologically proven PAC located at the pancreatic head undergoing intentional curative resection at the Department of Surgery, Chang-Gung Memorial Hospital, Taipei, Taiwan were reviewed. Curative resection was defined as a negative resection margin observed during histopathological examination. The 159 PAC patients comprised 96 men and 63 women with a median age of 64.0 years (range, 16-93 years). Among them, 36 patients (22.6%) had a tumor size ≤ 2 cm classified as S-PAC and 123 PAC patients had tumor size > 2 cm (L-PAC). Tumor size was defined by histopathological examination. Surgical mortality was defined as death occurring within 1 mo after surgery. Laboratory tests were conducted on the day before surgery. Serum carbohydrate antigen 19-9 (CA 19-9) and carcinoembryonic antigen (CEA) were measured by radioimmunoassay. The tumors were preoperatively evaluated by abdominal ultrasonography, endoscopic retrograde cholangiopancreatography, percutaneous transhepatic cholangiography, computed tomography, magnetic resonance image with cholangiopancreatography, and angiography, as appropriate. Tumor stage was defined according to the pathological tumor node metastasis (pTNM system) classification proposed by the UICC. Stages I and II were classified as early-stage, and stages III and IV as advanced stage PAC. Adjuvant chemotherapy was systemic administration of either 5-flurouracil-based or gemcitabine-based regimen due to either the positive section margin or lymph node metastasis. Adjuvant radiotherapy was conducted by intra-operative radiotherapy, external beam radiotherapy and/or brachytherapy due to either a positive section margin or local recurrence.
All data are presented as percentage of patients or mean with standard deviation. Numerical data were compared by independent two-sample t-tests. Nominal data were compared by Pearson chi-square test, or multiple forward stepwise logistic regression when appropriate. Survival was calculated and plots constructed according to the Kaplan-Meier method. Sixteen clinicopathological variables were selected for survival analysis, including demographic data, clinical features, laboratory data, operative findings, and pathological features. The log-rank test was performed for a univariate analysis for prognosis by using log-rank test and multivariate analysis was conducted with Cox’s proportional hazard model. All statistical analyses were performed using the SPSS computer software package (Version 10.0, Chicago, IL, USA). A value of P < 0.05 was considered significant.
The S-PAC group contained 22 men and 14 women, with a mean age of 61.9 ± 8.2 years. In the L-PAC group, there were 74 men and 49 women, with a mean age of 62.8 ± 10.5 years. Both groups had a similar age distribution, gender ratio, and laboratory data. In terms of symptoms and physical examination, both groups possessed a higher positive rate (> 80%) for non-specific symptoms and signs irrespective of the tumor size. Regarding tumor markers (CEA and CA 19-9), L-PAC and S-PAC groups had similar positive rates (35.9% and 75%, and 29.6% and 69%, respectively). In the light of lymph node metastasis and portal vein invasion, the two groups had similar positive rates. Even in the S-PAC groups, lymph node metastasis and portal vein invasion rates reached 63.9% and 11.1%, respectively. Both groups had almost the same curative resection rate. Portal vein invasion and retroperitoneal extension of the tumor explained the reasons for the positive margin. Both groups also had similar distributions of tumor differentiation and stage.
Surgical morbidity and mortality rates for the L-PAC group were 26.8% and 4.1%, respectively, similar to those of the S-PAC group which were 30.6% and 2.8%, respectively (Table 1). Almost the same percentage of patients received post-operative chemotherapy and radiation in the two groups, mainly for lymph node metastasis and positive margin.
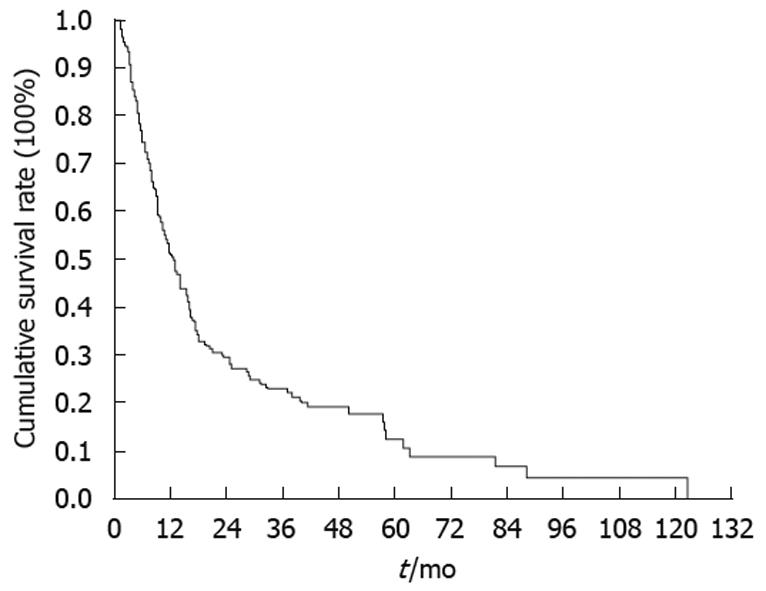
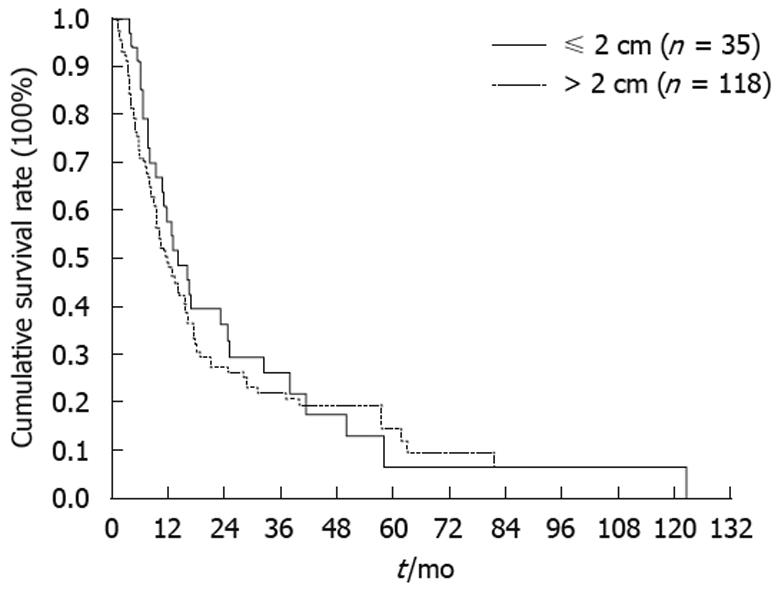
All of the 159 PAC patients undergoing resection were followed regularly until death with the duration of follow-up ranging from 1.1 to 213.5 mo (median, 16.4 mo). The 1-, 3- and 5-year survival rates of the 159 cases were 51.3%, 23.1% and 12.5%, respectively (Figure 1). The 3- and 5-year survival rates of the S-PAC and L-PAC patients were 26.4% and 6.6%, and 22.1% and 14.7%, respectively (Figure 2). The S-PAC patients had a similar overall survival rate to the L-PAC patients (P = 0.48) (Figure 2).
S-PAC and L-PAC groups had the same prognosis in this study, which meant tumor size did not influence the outcome of this disease. So, we were interested in which factors could affect the prognosis of S-PAC patients. We chose 16 clinicopathological characteristics including demographic data, clinical biochemical laboratory values, tumor markers, operative procedure, tumor invasion, tumor differentiation, tumor stage, post-operative chemotherapy and radiation to determine which one could be the prognostic predictive factor (Table 2). Only the albumin level had a significant impact on survival of S-PAC patients (P = 0.002). The early stage S-PAC patients (Stage I, II) tended to have a favorable outcome compared with late stage S-PAC patients (Stage III, IV), but this difference did not reach statistical significance (P = 0.0931). Age, gender, operative procedures, biochemical data, tumor invasion, resection margin, and tumor differentiation did not associate with favorable prognosis.
PAC is one of most lethal human malignancies producing the fourth highest cancer-related mortality in the western world and the fifth highest cancer-related mortality rate worldwide[4]. The overall 5-year survival rate of PAC has been reported to be around 1%-4%, which was attributed to its aggressive growth behavior, early local spread, early metastasis, and resistance to radiation and most systemic chemotherapies[4]. Surgery remains the cornerstone of treatment. After resection, the 5-year survival rate can reach 10%-29%[1-3].
For most malignancies, a smaller tumor size is usually deemed as a good indicator of early stage, and should be diagnosed and treated as soon as possible to improve the outcome. Some reports have demonstrated that tumor size was one of most important determinants of resectability and prognosis for pancreas cancer[12-14]. Previously, Satake et al[15] stated that PAC with tumor size < 40 mm represented a better prognosis. However, according to our report, even PAC with tumor size < 20 mm did not necessarily mean early stage disease and should not be treated by surgery alone[7,9,16,17]. The reported 5-year survival for S-PAC patients ranges from 8% to 59% and is only 6.6% in our series[3,7,9,16,18,19].
Several reasons could be found to explain this dismal outcome. S-PAC patients (tumor size < 2 cm) had similar clinicopathological features including positive clinical symptoms, tumor differentiation pattern and positive lymph node invasion rate to L-PAC patients (Table 1). Contrary to previous reports[9,20], a well-differentiated type of adenocarcinoma is more frequently seen in PAC tumors with tumor size < 2 cm, but in our study, tumor size had no impact on tumor differentiation. S-PAC and L-PAC possessed the same distribution in terms of tumor differentiation. Jung et al[20] also indicated that pancreas tumor size < 2 cm would tend to be symptomless, however, in our study, we could not find this difference. Both groups had a very low negative rate of symptoms. Contrary to Shimada’s report[8], we showed the incidence of lymph node metastasis was similar between S-PAC and L-PAC (as high as 63.9%), demonstrating that S-PAC with tumor size < 20 mm did not necessarily mean early stage disease. Such findings showed that PAC was attributed to its aggressive growth behavior, early local spread and early metastasis no matter what its size.
CEA and CA 19-9 are widely used to screen malignancies in the general population. An 80% CA19-9 positive rate in pancreas cancer and high levels of CA19-9 associated with more advanced disease were reported[21-23]. Jung et al[20] reported that CA19-9 and CEA in patients with PAC tumor size > 6 cm would be higher than in smaller cancers. Steinberg revealed that CEA and CA 19-9 would not increase in patients with pancreas cancer < 2 cm[24]. But in our study, we found the sensitivity of CEA and CA 19-9 for S-PAC and L-PAC patients were 29.6% and 69%, and 35.9% and 75%, respectively. Tumor marker values did increase in the S-PAC group and both groups had similar positive rates of CEA and CA 19-9.
Regarding prognostic analysis for S-PAC patients, only albumin level could be a favorable prognostic factor for S-PAC statistically (P = 0.0002). Early stage S-PAC (Stage I, II) tended to have a better prognosis than late stage S-PAC (Stage III, IV), but this was not statistically significant (P = 0.0931). In our study, mos non-curative resection cases were due to portal vein invasion and retroperitoneum tumor extension. However, curative resection or not did not influence the final survival of S-PAC and tumor differentiation of S-PAC did not have any influence on survival either. Lymph node metastasis, poorly differentiated tumors, and positive margins were usually regarded as poor prognostic factors for pancreas cancer[13,25,26]. In this study, we did not find these as unfavorable factors for prognosis. Limited case numbers should be the main cause. In terms of these issues, we need more time to collect more cases to answer these questions. Detection of PAC with small size (≤ 2 cm) (S-PAC) still warrants more efforts to improve the outcome.
In conclusion, S-PAC and L-PAC had similar clinicopathological characteristics. They expressed similar tumor biology, such as lymph node metastasis, portal vein invasion and tumor differentiation. So both groups had the same survival rate, explaining why S-PAC should not be deemed as an early stage disease and treated by surgery alone. For S-PAC groups, only albumin level could be a prognostic predictor. Early stage S-PAC tended to have a more favorable prognosis than late stage S-PAC, although this did not reach statistical significance.
Pancreatic cancer is a devastating cancer. Previously, cancer size has been deemed as an important prognostic factor for pancreatic cancer. However, growing evidence recently demonstrated the controversial conclusion against the previous viewpoint. Herein, the authors collected data from pancreatic cancer patients with cancer on the pancreatic head and they compared the clinicopathologic characteristics and survival between small pancreatic adenocarcinoma (S-PAC) and large (L)-PAC groups. In addition, they also tried to find out the prognostic factor for S-PAC patients.
The tumor biology, including differentiation, lymph node metastasis, portal vein invasion and so on is similar between S-PAC and L-PAC patients, which would give rise to further studies of whether there are any different gene mutations in terms of the size of pancreatic cancer.
In this report, albumin level is the only prognostic factor for survival of S-PAC patients. Traditionally, lymph node metastasis and portal vein invasion are deemed as important prognostic factors for pancreatic cancer. Although such findings were not shown in their report, they considered these could be blamed partly on the limited number of cases.
Through the findings of their report, S-PAC should not be regarded as a early pancreatic cancer. Aggressive management such as post-operative chemotherapy and radiation are justified.
This is an interesting clinical report. However, due to the poor prognosis of S-PAC patients and thus the small number of cases who survived for 5 years, we have to be careful in making extended conclusions from the data.
Peer reviewer: Ming Li, Associate Professor, Tulane University Health Sciences Center, 1430 Tulane Ave Sl-83, New Orleans 70112, United States
S- Editor Tian L L- Editor O’Neill M E- Editor Zheng XM
| 1. | Trede M, Schwall G, Saeger HD. Survival after pancreatoduodenectomy. 118 consecutive resections without an operative mortality. Ann Surg. 1990;211:447-458. |
| 2. | Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, Hruban RH, Ord SE, Sauter PK, Coleman J. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248-257; discussion 257-260. |
| 3. | Nitecki SS, Sarr MG, Colby TV, van Heerden JA. Long-term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Ann Surg. 1995;221:59-66. |
| 4. | Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43-66. |
| 5. | Fernández-del Castillo C, Rattner DW, Warshaw AL. Standards for pancreatic resection in the 1990s. Arch Surg. 1995;130:295-299; discussion 299-300. |
| 6. | Cameron JL. Long-term survival following pancreaticoduodenectomy for adenocarcinoma of the head of the pancreas. Surg Clin North Am. 1995;75:939-951. |
| 7. | Tsuchiya R, Noda T, Harada N, Miyamoto T, Tomioka T, Yamamoto K, Yamaguchi T, Izawa K, Tsunoda T, Yoshino R. Collective review of small carcinomas of the pancreas. Ann Surg. 1986;203:77-81. |
| 8. | Shimada K, Sakamoto Y, Sano T, Kosuge T, Hiraoka N. Reappraisal of the clinical significance of tumor size in patients with pancreatic ductal carcinoma. Pancreas. 2006;33:233-239. |
| 9. | Egawa S, Takeda K, Fukuyama S, Motoi F, Sunamura M, Matsuno S. Clinicopathological aspects of small pancreatic cancer. Pancreas. 2004;28:235-240. |
| 10. | Satake K, Nishiwaki H, Yokomatsu H, Kawazoe Y, Kim K, Haku A, Umeyama K, Miyazaki I. Surgical curability and prognosis for standard versus extended resection for T1 carcinoma of the pancreas. Surg Gynecol Obstet. 1992;175:259-265. |
| 11. | Furukawa H, Okada S, Saisho H, Ariyama J, Karasawa E, Nakaizumi A, Nakazawa S, Murakami K, Kakizoe T. Clinicopathologic features of small pancreatic adenocarcinoma. A collective study. Cancer. 1996;78:986-990. |
| 12. | Yeo CJ, Cameron JL, Lillemoe KD, Sitzmann JV, Hruban RH, Goodman SN, Dooley WC, Coleman J, Pitt HA. Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg. 1995;221:721-731; discussion 731-733. |
| 13. | Nagakawa T, Nagamori M, Futakami F, Tsukioka Y, Kayahara M, Ohta T, Ueno K, Miyazaki I. Results of extensive surgery for pancreatic carcinoma. Cancer. 1996;77:640-645. |
| 14. | Tsiotos GG, Farnell MB, Sarr MG. Are the results of pancreatectomy for pancreatic cancer improving? World J Surg. 1999;23:913-919. |
| 15. | Satake K, Chung YS, Umeyama K, Takeuchi T, Kim YS. The possibility of diagnosing small pancreatic cancer (less than 4.0 cm) by measuring various serum tumor markers. A retrospective study. Cancer. 1991;68:149-152. |
| 16. | Manabe T, Miyashita T, Ohshio G, Nonaka A, Suzuki T, Endo K, Takahashi M, Tobe T. Small carcinoma of the pancreas. Clinical and pathologic evaluation of 17 patients. Cancer. 1988;62:135-141. |
| 17. | Moossa AR, Levin B. The diagnosis of "early" pancreatic cancer: the University of Chicago experience. Cancer. 1981;47:1688-1697. |
| 18. | Shimizu Y, Yasui K, Matsueda K, Yanagisawa A, Yamao K. Small carcinoma of the pancreas is curable: new computed tomography finding, pathological study and postoperative results from a single institute. J Gastroenterol Hepatol. 2005;20:1591-1594. |
| 19. | Ihse I, Andersson R, Axelson J, Kobari M, Andrén-Sandberg Å. Does tumor size influence early and late results after resection of pancreatic adenocarcinoma? J Hepatobiliary Pancreat Surg. 1995;2:371-375. |
| 20. | Jung KW, Kim MH, Lee TY, Kwon S, Oh HC, Lee SS, Seo DW, Lee SK. Clinicopathological aspects of 542 cases of pancreatic cancer: a special emphasis on small pancreatic cancer. J Korean Med Sci. 2007;22 Suppl:S79-S85. |
| 21. | Sawabu N, Watanabe H, Yamaguchi Y, Ohtsubo K, Motoo Y. Serum tumor markers and molecular biological diagnosis in pancreatic cancer. Pancreas. 2004;28:263-267. |
| 22. | Gattani AM, Mandeli J, Bruckner HW. Tumor markers in patients with pancreatic carcinoma. Cancer. 1996;78:57-62. |
| 23. | Kim HJ, Kim MH, Myung SJ, Lim BC, Park ET, Yoo KS, Seo DW, Lee SK, Min YI. A new strategy for the application of CA19-9 in the differentiation of pancreaticobiliary cancer: analysis using a receiver operating characteristic curve. Am J Gastroenterol. 1999;94:1941-1946. |
| 24. | Steinberg W. The clinical utility of the CA 19-9 tumor-associated antigen. Am J Gastroenterol. 1990;85:350-355. |
| 25. | Allison DC, Piantadosi S, Hruban RH, Dooley WC, Fishman EK, Yeo CJ, Lillemoe KD, Pitt HA, Lin P, Cameron JL. DNA content and other factors associated with ten-year survival after resection of pancreatic carcinoma. J Surg Oncol. 1998;67:151-159. |
| 26. | Cleary SP, Gryfe R, Guindi M, Greig P, Smith L, Mackenzie R, Strasberg S, Hanna S, Taylor B, Langer B. Prognostic factors in resected pancreatic adenocarcinoma: analysis of actual 5-year survivors. J Am Coll Surg. 2004;198:722-731. |