Published online Sep 14, 2009. doi: 10.3748/wjg.15.4278
Revised: July 16, 2009
Accepted: July 23, 2009
Published online: September 14, 2009
AIM: To evaluate the efficacy and the safety of combined 5-Fluorouracil, irinotecan, bevacizumab and sirolimus in refractory advanced colorectal carcinoma.
METHODS: We initiated a regimen with at day 1 an injection (iv) of bevacizumab at 5 mg/kg, followed by 180 mg/m2 irinotecan, followed by Leucovorin 400 mg/m2, followed by a 5-Fluorouracil bolus 400 mg/m² and a 46-h infusion 2400 mg/m2. Sirolimus was given orally as continuous administration of 2 mg twice a day every days. This treatment was repeated every 14 d.
RESULTS: A total of 12 patients were enrolled. All patients presented with metastatic disease that had failed at least three lines of chemotherapy that contained oxaliplatin, irinotecan and bevacizumab. Cetuximab failure was also observed in all K-Ras wild-type patients. The median number of cycles was 8.5 (range 2-20) and clinical benefit was observed in eight patients. The median time to progression was 5 mo and the median survival was 8 mo. Grade 3 neutropenia developed in four patients, and grade 3 diarrhea and stomatitis in two.
CONCLUSION: The combination regimen of 5-Fluorouracil, irinotecan, bevacizumab and sirolimus in advanced colorectal carcinoma after failure of classical treatment is feasible and promising. Further evaluation of this combination is required.
- Citation: Ghiringhelli F, Guiu B, Chauffert B, Ladoire S. Sirolimus, bevacizumab, 5-Fluorouracil and irinotecan for advanced colorectal cancer: A pilot study. World J Gastroenterol 2009; 15(34): 4278-4283
- URL: https://www.wjgnet.com/1007-9327/full/v15/i34/4278.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4278
| Inclusion criteria | Exclusion criteria |
| Histologically confirmed colorectal cancer (adenocarcinoma) | Thromboembolism that required therapeutic anticoagulation |
| Measurable disease | Central nervous system metastasis |
| No secondary malignancy | Major surgery within 6 wk |
| Age > 18 years | Nonhealing wounds |
| ECOG performance status 0-2 | Uncontrolled hypertension |
| Adequate hematological, hepatic and renal function (including urinary excretion of no more than 500 mg protein/d) | Pregnant or lactating women |
| Failure of bevacizumab, oxaliplatin and irinotecan-based treatment | Bleeding diathesis, active or recent |
| For wild-type K-Ras: failure with cetuximab and irinotecan combination was required | Cardiovascular disease |
| Failure caused by significant intolerance to either drug was allowed | Cerebrovascular accident |
| Characteristics | n |
| Median age (Range) (yr) | 62 (51-75) |
| Sex | |
| Male | 7 |
| Female | 5 |
| ECOG performance status | |
| 0–1 | 8 |
| 2 | 4 |
| CEA level (range) (ng/mL) | 882 (21-6327) |
| Primary site | |
| Colon | 10 |
| Rectum | 2 |
| Sites of metastasis | |
| Liver | 9 |
| Lung | 7 |
| Lymph nodes | 6 |
| Others | 5 |
| K-Ras status | |
| Wild-type | 9 |
| Mutated | 3 |
| NCI-CTC grade | ||||
| 1 | 2 | 3 | 4 | |
| Hematological | ||||
| Anemia | 1 | 3 | ||
| Leucopenia | 2 | 4 | ||
| Neutropenia | 2 | 4 | ||
| Thrombocytopenia | 1 | 2 | ||
| Non hematological | ||||
| Nausea/vomiting | 2 | 5 | ||
| Mucositis | 3 | 4 | 2 | |
| Diarrhea | 5 | 2 | ||
| Proteinuria | 1 | |||
| Asthenia | 3 | 5 | ||
| High blood pressure | 3 | |||
Colorectal cancer is the third most common cancer worldwide[1]. Approximately 25% of patients with colorectal cancer present with overt metastatic disease, and metastatic disease develops in 40%-50% of newly diagnosed patients. Standard first-line treatments include fluorouracil (5-FU) with leucovorin and irinotecan[2,3] or oxaliplatin[4], alone or combined with bevacizumab[5]. Cetuximab, a chimeric IgG1 monoclonal antibody against epidermal growth factor receptor (EGFR), has efficacy as monotherapy and in combination with irinotecan in irinotecan-resistant patients, if the tumor expresses wild-type K-RAS and B-RAF[6,7]. However if these standard treatments fail, there are no accepted treatment options, and for such patients historical estimation of progression-free survival (PFS) and overall survival (OS) are about 2 and 4 mo, respectively[8].
Recent results have suggested that inhibitors of mTor (mammalian target of rapamycin) signal are able to improve survival and induce tumor response in patients with poor-prognosis renal cell carcinoma[9], and combination of mTor inhibitors and classical chemotherapy is currently under clinical investigation. In colorectal carcinoma cell lines in vitro, rapamycin enhances the antitumor effect of irinotecan through Hypoxia-inducible factor-1α inhibition (a key transcription factor that regulates angiogenesis) and by disruption of tumor vasculature[10]. Therefore, we hypothesize that, in multi-treated colorectal patients, multimodal angiogenesis targeting using irinotecan, bevacizumab and the mTor inhibitor sirolimus can have a therapeutic effect.
The goal of this pilot study was to evaluate the safety and efficacy of sirolimus, bevacizumab, leucovorin, 5-FU, and irinotecan (FOLFIRI) in patients with advanced colorectal cancer that had progressed after treatment with 5-FU, irinotecan, oxaliplatin, bevacizumab and anti-EGFR therapy.
The eligibility and exclusion criteria, and pretreatment characteristics of the patients are presented in Table 1. Written informed consent was required before chemotherapy.
On day 1, irinotecan (180 mg/m2) was administered as a 2-h iv infusion. Then leucovorin 400 mg/m2 followed by a 5-Fluorouracil bolus 400 mg/m2 was administrated as a bolus iv injection. Then 5-Fluorouracil 2400 mg/m2 was given as a 46-h iv infusion, Bevacizumab was given at 5 mg/kg as an iv infusion every two weeks over 60 min before the beginning of the chemotherapy. Sirolimus was given orally as continuous administration of 2 mg twice a day every days. The doses used for fluorouracil, irinotecan and bevacizumab were the classical recommended doses[2-5]. For sirolimus, a dose of 4 mg/d was chosen because a phase I study has demonstrated that this is tolerable and sufficient to achieve mTor inhibition[11]. Dose modifications of irinotecan or 5-FU were made for hematological or non-hematological toxicity, on the basis of the most severe grade of adverse effect that occurred during the previous cycle. Treatment was delayed until the absolute number of neutrophils was > 1000/μL, platelets were > 100 000/μL, and mucositis, diarrhea, or skin toxicity had recovered to grade 1 or less. The 5-FU dose was reduced after the occurrence of National Cancer Institute Common Toxicity Criteria (NCI-CTC) grade 3 diarrhea, stomitis or dermatitis. For toxicity of grade 3 or higher, a 20% dose reduction for irinotecan was prescribed by the protocol. Bevacizumab was retained for uncontrolled hypertension or proteinuria of > 3 g in 24 h. Bevacizumab was discontinued for grade 3 or 4 hemorrhage, thromboembolic events that required full dose anticoagulation, or any grade 4 toxicity. Sirolimus was discontinued for grade 3 or 4 diarrhea or stomatitis. Treatment was administered until the disease progressed, unacceptable toxic effects developed, or the patient refused further treatment.
Pretreatment evaluation included physical examination, complete blood cell counts, blood chemistry, tumor marker level (carcinoembryonic antigen, CEA), and computed tomography (CT) within 15 d of starting chemotherapy. Tumor responses were determined by RECIST and Choi criteria[12,13]. Complete blood cell counts, serum chemistry, including liver and renal function, were performed at least every two weeks, and tumor assessment by CT was performed every three cycles.
Efficacy analysis was performed according to the intention to-treat principle. Patients were considered assessable for response if they were eligible, had measurable disease, and had received at least one dose of study therapy. In the analysis of survival and subsequent treatment, all patients were followed until death, loss to follow-up, or termination of the study. PFS and OS were calculated using the Kaplan-Meier method. PFS was calculated from the date therapy started to the date of disease progression, and OS was calculated from the date therapy started to the date of death.
Between January 2008 and December 2008, a total of 12 patients were included in this pilot study at the Department of Medical Oncology, Georges-Francois Leclerc Cancer Center, Dijon, France. Demographic details of the patients included in the study are shown in Table 2. There were seven male and five female patients, median age 61 years (range 51-75). All patients had progressed after prior 5-FU, irinotecan and bevacizumab therapy, and 5-FU, oxaliplatin and bevacizumab therapy. Importantly, all patients had been treated previously with FOLFIRI bevacizumab chemotherapy and experienced progression according to the RECIST criteria following this treatment. Nine patients harbored wild-type K-Ras tumor and progressed on irinotecan plus cetuximab therapy. All 12 patients were assessable for response, for toxicity and survival.
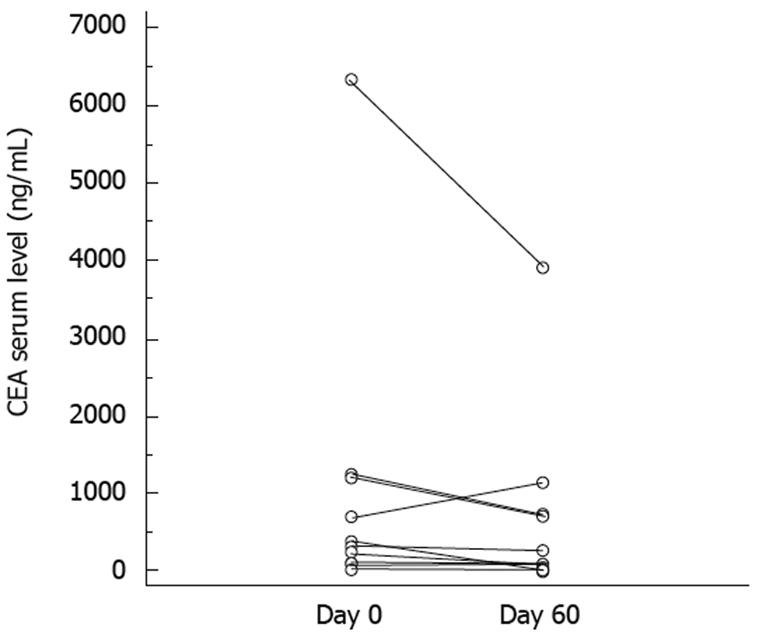
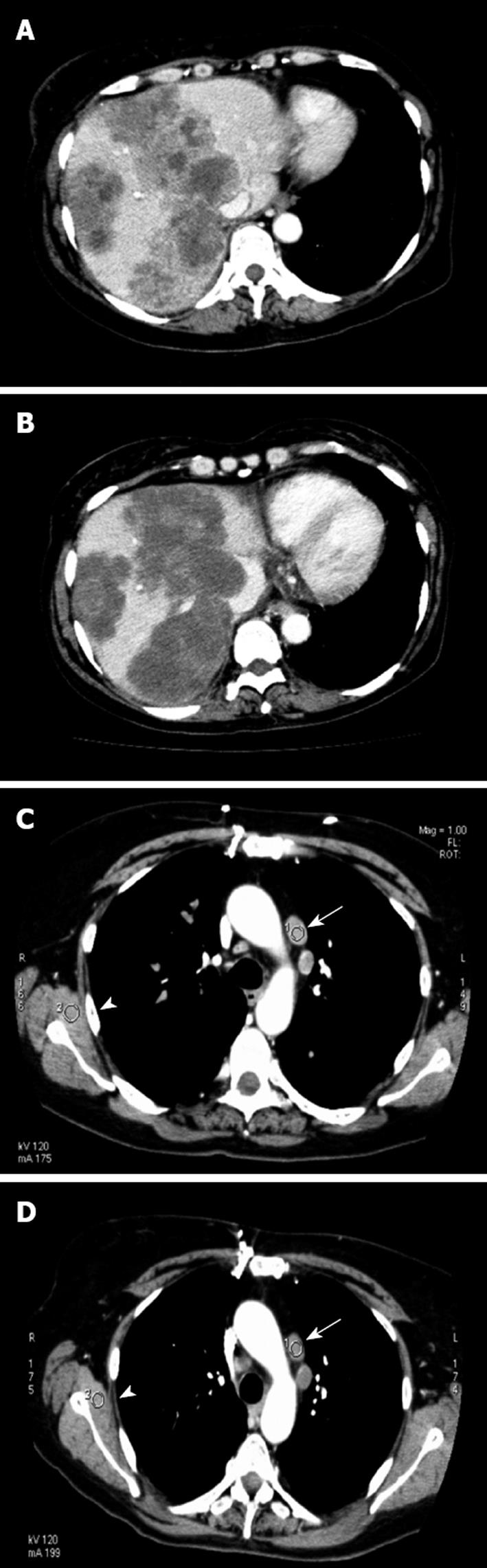
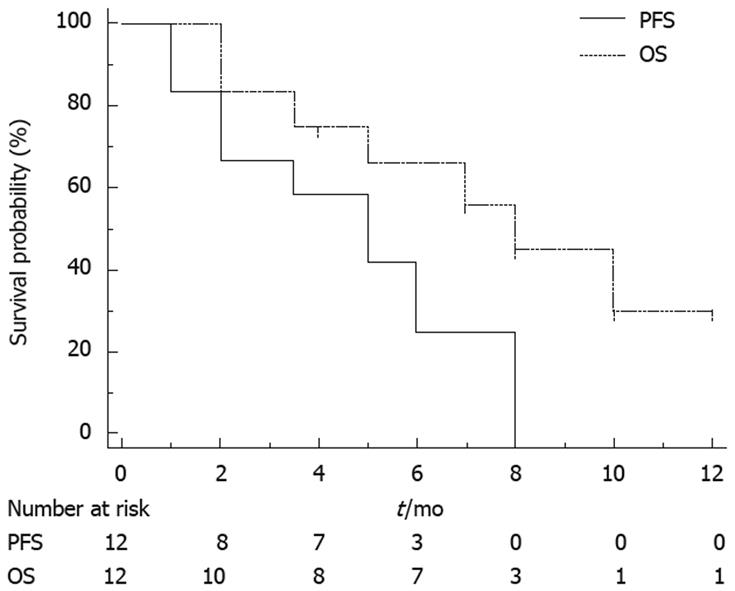
There were a median 8.5 cycles (range 2-20) of chemotherapy. Chemotherapy was stopped because of disease progression in 10 patients, and two discontinued because of toxicity (grade 3 diarrhea and stomatitis). Median follow-up duration was 9 mo. At 2 mo, CEA level decreased in all except one patient (mean level 882 ± 510 vs 579 ± 320; P = 0.025, Wilcoxon test; Figure 1). According to RECIST criteria, one patient had a partial response, seven had stable disease for > 3 mo, and four had progressive disease. According to the Choi criteria, three patients had a partial response, five had stable disease, and four had progressive disease (Figure 2). PFS was 5 mo (95% CI: 2.5-6), and median OS was 7 mo (95% CI: 4-NR). Figure 3 shows PFS and OS curves.
A total of 116 cycles of chemotherapy were administered. All patients received at least 1 mo of the chemotherapy and sirolimus regimen. Dose modifications or interruptions were required in three patients. The incidence of hematological and non-hematological toxicity is summarized in Table 3. The major grade 3/4 hematological toxicity included neutropenia in three patients (25%) and thrombocytopenia in two (16%). No neutropenic fever was observed. Grade 1/2 nausea, vomiting and diarrhea developed in five patients; however, this toxicity was mild and manageable. In two patients, sirolimus-related grade 3 stomatitis led to treatment discontinuation. Only grade 2 nose bleeding were observed and did not require therapeutic modification. Hypertension occurred in three patients and was managed without drug modification. Proteinuria > 2 g/24 h occurred in one patient and was treated by angiotensin-converting enzyme inhibitors. All patients with stable disease had minor grade 1/2 manageable adverse effects. There was no treatment-related death. Eight deaths were caused by disease progression.
Recent trials with advanced colorectal carcinoma have shown that patients may survive for 24-30 mo[14]. However, after failure of irinotecan, oxaliplatin, bevacizumab and anti-EGFR therapy, there are no accepted treatment options. Estimated PFS is about 2 mo and OS is 4 mo[8].
Few nonrandomized trial have demonstrated that association of fluorouracil or capecitabine with mitomycin may give some response in heavily pretreated patients[15-18]. Such treatments give an objective response rate of about 10% and PFS of 2-3 mo. Although, in these trials, patients did not benefit from new target therapies.
Recently, target therapy such as sorafenib and sunitinib have given some promising results in patients with end-stage colorectal cancer. Sunitinib was tested in a phase II trial in patients with previously treated colorectal metastases at a dose of 50 mg/d for 4 wk, followed by 2 wk off treatment[19]. Median time to progression was 2.5 mo and median OS was 7 mo in bevacizumab-pretreated patients and 10 mo in those not pretreated with bevacizumab. Sorafenib has been tested in association with oxaliplatin in a phase I trial in patients with oxaliplatin-refractory colorectal cancer. The recommended dosage was 130 mg/m2 oxaliplatin and continuous sorafenib 400 mg twice daily. This treatment showed promising efficacy[20].
The phosphoinositide 3-kinase/mTOR axis is a pivotal pathway in cell growth and cell-cycle progression, in response to different stimuli, such as nutrients and growth receptors[21]. The mTor pathway is activated aberrantly in around half of human tumors and plays a crucial role in angiogenesis. mTor inhibition is now considered as an important target for new anticancer drugs[22]. In a phaseItrial, mTor inhibitors showed minor efficacy in colorectal carcinoma[23]. In vitro and preclinical studies have demonstrated the synergistic efficacy of combined rapamycin and irinotecan[10]. In fact, irinotecan and rapamycin act on tumor growth by inhibiting angiogenesis by acting on Hypoxia inducible factor-1α[24,25]. Recently, combination of everolimus and bevacizumab has demonstrated some activity in patients with refractory metastatic colorectal cancer who had progressed on a bevacizumab-based regimen, which suggests that bevacizumab and mTor inhibitors overcome resistance to bevacizumab[26]. Therefore, we hypothesized that combination of sirolimus, bevacizumab and irinotecan may provide some synergistic antiangiogenic effects that can reverse resistance to bevacizumab- and irinotecan-based chemotherapy.
In our pilot study, combination of sirolimus, bevacizumab, 5-FU and irinotecan demonstrated that, for patients with advanced colorectal cancer, some clinical stabilization and prolonged stable disease can be obtained. CT monitoring suggested an antiangiogenic effect of the therapy, with tumor necrosis obtained in three patients. Median PFS of 5 mo and median OS of 8 mo suggest a potential clinical benefit of a such treatment. Despite its potent efficacy, this target therapy can have adverse effects, and we have to balance the efficacy, toxicity and cost of such therapy. Further studies will be needed to confirm our results.
Novel combinations of new chemotherapeutic agents and target therapies have demonstrated an improvement in tumor response and overall survival in patients with advanced colorectal cancer. Although, after failure of irinotecan, oxaliplatin, bevacizumab and cetuximab, no treatment demonstrates efficacy.
The phosphoinositide 3-kinase/mTOR (mammalian target of rapamycin) axis is activated aberrantly in around half of human tumors and plays a crucial role in angiogenesis. In a phase I trial, mTor inhibitors showed minor efficacy in colorectal carcinoma. In vitro and preclinical studies have demonstrated the synergistic efficacy of combined rapamycin and irinotecan. Recently, combination of everolimus (an mTor inhibitor) and bevacizumab have demonstrated some activity in patients with refractory metastatic colorectal cancer who had progressed on a bevacizumab-based regimen, which suggests that bevacizumab and mTor inhibitors overcome resistance to bevacizumab. Therefore, the authors hypothesized that combination of sirolimus, bevacizumab and irinotecan provides some synergistic antiangiogenic effects that can reverse resistance to bevacizumab- and irinotecan-based chemotherapy.
This is believed to be the first study to investigate the capacity of an mTor inhibitor to reverse resistance to conventional therapies. The data suggest that both treatment regimens demonstrate efficacy and tolerable toxicity in this setting.
These data are in accordance with the preliminary findings from ASCO 2009, which demonstrate that sirolimus may reverse resistance of colorectal cancer to bevacizumab therapy. The data suggest that the therapy may have some clinical efficacy but substantial adverse effects.
Sirolimus, also called rapamycin, is an mTor inhibitor. This drug is used as an immunosuppressant but demonstrates antitumor efficacy by inhibiting the mTor/AKT pathway. Irinotecan kills cells by inhibiting the enzyme topoisomerase I, which is also involved in DNA synthesis in proliferating cells. Bevacizumab is a monoclonal antibody that inhibits VEGF, which is expressed in some cases of colorectal cancer and aids the blood supply to tumors; bevacizumab inhibits this process and tumor cell growth.
This is an interesting small pilot study that investigated fourth-line chemotherapy in patients with advanced metastatic colorectal cancer that had failed at least three previous chemotherapeutic regimens. A major finding was that clinical benefit was observed in 8/12 patients with severe adverse effects and relatively short overall survival.
Peer reviewers: Peter L Lakatos, MD, PhD, Assistant Professor, 1st Department of Medicine, Semmelweis University, Koranyi S 2A, Budapest H1083, Hungary; Satoshi Osawa, MD, PhD, Assistant Professor, First Department of Medicine, Hamamatsu University School of Medicine, 1-20-1 Handayama, Higashi-ku, Hamamatsu, 431-3192, Japan
S- Editor Tian L L- Editor Kerr C E- Editor Ma WH
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. |
| 2. | Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041-1047. |
| 3. | Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905-914. |
| 4. | Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts SR. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23-30. |
| 5. | Van Cutsem E, Geboes K. The multidisciplinary management of gastrointestinal cancer. The integration of cytotoxics and biologicals in the treatment of metastatic colorectal cancer. Best Pract Res Clin Gastroenterol. 2007;21:1089-1108. |
| 6. | Lenz HJ, Van Cutsem E, Khambata-Ford S, Mayer RJ, Gold P, Stella P, Mirtsching B, Cohn AL, Pippas AW, Azarnia N. Multicenter phase II and translational study of cetuximab in metastatic colorectal carcinoma refractory to irinotecan, oxaliplatin, and fluoropyrimidines. J Clin Oncol. 2006;24:4914-4921. |
| 7. | Folprecht G, Lutz MP, Schoffski P, Seufferlein T, Nolting A, Pollert P, Kohne CH. Cetuximab and irinotecan/5-fluorouracil/folinic acid is a safe combination for the first-line treatment of patients with epidermal growth factor receptor expressing metastatic colorectal carcinoma. Ann Oncol. 2006;17:450-456. |
| 8. | Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626-1634. |
| 9. | Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi I. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271-2281. |
| 10. | Pencreach E, Guerin E, Nicolet C, Lelong-Rebel I, Voegeli AC, Oudet P, Larsen AK, Gaub MP, Guenot D. Marked activity of irinotecan and rapamycin combination toward colon cancer cells in vivo and in vitro is mediated through cooperative modulation of the mammalian target of rapamycin/hypoxia-inducible factor-1alpha axis. Clin Cancer Res. 2009;15:1297-1307. |
| 11. | Cloughesy TF, Yoshimoto K, Nghiemphu P, Brown K, Dang J, Zhu S, Hsueh T, Chen Y, Wang W, Youngkin D. Antitumor activity of rapamycin in a Phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med. 2008;5:e8. |
| 12. | Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205-216. |
| 13. | Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, Chen LL, Podoloff DA, Benjamin RS. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753-1759. |
| 14. | Grothey A, Sugrue MM, Purdie DM, Dong W, Sargent D, Hedrick E, Kozloff M. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE). J Clin Oncol. 2008;26:5326-5334. |
| 15. | Ross P, Norman A, Cunningham D, Webb A, Iveson T, Padhani A, Prendiville J, Watson M, Massey A, Popescu R. A prospective randomised trial of protracted venous infusion 5-fluorouracil with or without mitomycin C in advanced colorectal cancer. Ann Oncol. 1997;8:995-1001. |
| 16. | Chester JD, Dent JT, Wilson G, Ride E, Seymour MT. Protracted infusional 5-fluorouracil (5-FU) with bolus mitomycin in 5-FU-resistant colorectal cancer. Ann Oncol. 2000;11:235-237. |
| 17. | Seitz JF, Perrier H, Giovannini M, Capodano G, Bernardini D, Bardou VJ. 5-Fluorouracil, high-dose folinic acid and mitomycin C combination chemotherapy in previously treated patients with advanced colorectal carcinoma. J Chemother. 1998;10:258-265. |
| 18. | Vormittag L, Kornek GV, Gruhsmann B, Lenauer A, Foger A, Depisch D, Lang F, Scheithauer W. UFT/leucovorin and mitomycin C as salvage treatment in patients with advanced colorectal cancer - a retrospective analysis. Anticancer Drugs. 2007;18:709-712. |
| 19. | Saltz LB, Rosen LS, Marshall JL, Belt RJ, Hurwitz HI, Eckhardt SG, Bergsland EK, Haller DG, Lockhart AC, Rocha Lima CM. Phase II trial of sunitinib in patients with metastatic colorectal cancer after failure of standard therapy. J Clin Oncol. 2007;25:4793-4799. |
| 20. | Kupsch P, Henning BF, Passarge K, Richly H, Wiesemann K, Hilger RA, Scheulen ME, Christensen O, Brendel E, Schwartz B. Results of a phase I trial of sorafenib (BAY 43-9006) in combination with oxaliplatin in patients with refractory solid tumors, including colorectal cancer. Clin Colorectal Cancer. 2005;5:188-196. |
| 21. | Rohde J, Heitman J, Cardenas ME. The TOR kinases link nutrient sensing to cell growth. J Biol Chem. 2001;276:9583-9586. |
| 22. | Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489-501. |
| 23. | Tabernero J, Rojo F, Calvo E, Burris H, Judson I, Hazell K, Martinelli E, Ramon y Cajal S, Jones S, Vidal L. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol. 2008;26:1603-1610. |
| 24. | Wouters BG, Koritzinsky M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat Rev Cancer. 2008;8:851-864. |
| 25. | Yin MB, Li ZR, Toth K, Cao S, Durrani FA, Hapke G, Bhattacharya A, Azrak RG, Frank C, Rustum YM. Potentiation of irinotecan sensitivity by Se-methylselenocysteine in an in vivo tumor model is associated with downregulation of cyclooxygenase-2, inducible nitric oxide synthase, and hypoxia-inducible factor 1alpha expression, resulting in reduced angiogenesis. Oncogene. 2006;25:2509-2519. |
| 26. | Bullock KE, Hurwitz HI, Uronis HE, Morse MA, Blobe GC, Hsu SD, Zafar SY, Nixon AB, Howard LA, Bendell JC. Bevacizumab plus everolimus in refractory metastatic colorectal cancer. J Clin Oncol. 2009;27:15. |