INTRODUCTION
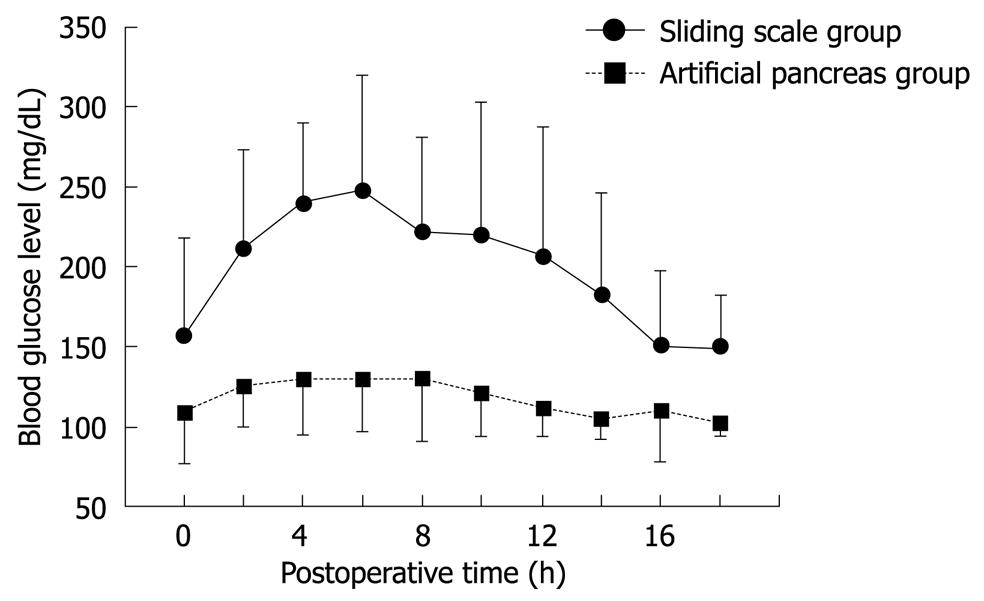
Figure 1 Postoperative blood glucose levels in hepatectomized patients in whom blood glucose was controlled using either the sliding-scale method or a closed-loop artificial endocrine pancreas system (Okabayashi et al[27] Diabetes Care 2009).
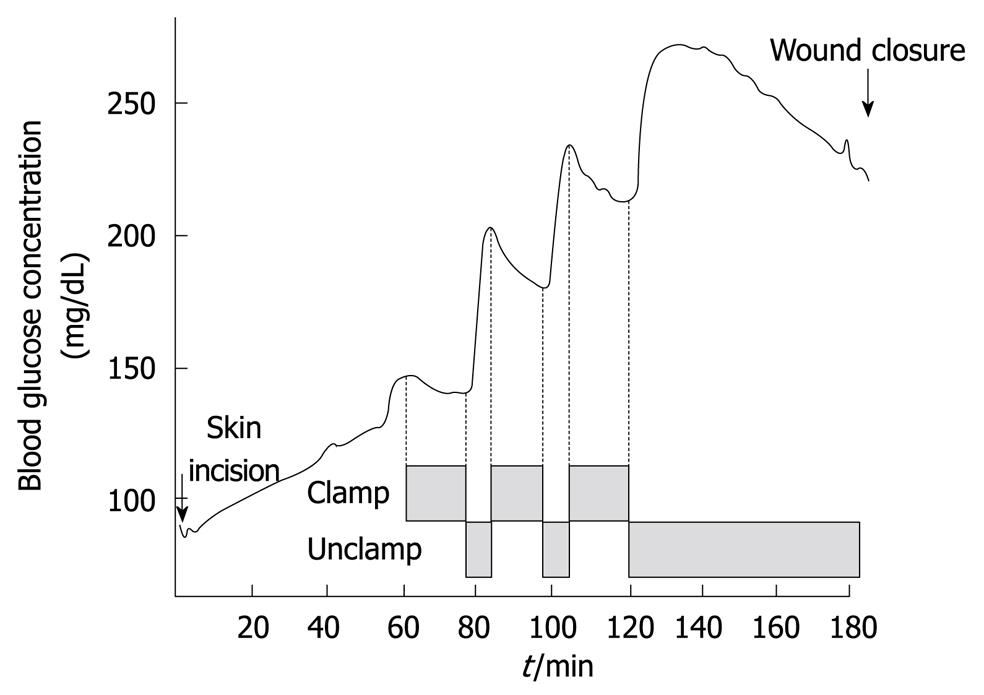
Figure 2 Typical trend of blood glucose concentrations during the Pringle maneuver for hepatic resection (Maeda et al[32] Am J Surg 2009).
Anatomically, the liver is situated downstream from the pancreas. It is a primary site for the metabolism of pancreatic hormones, such as insulin and glucagon, which have a central role in the regulation of peripheral blood glucose levels[1]. In addition, the liver is positioned downstream from the gut, from which it absorbs a large amount of ingested glucose, and is involved in glycogenolysis and gluconeogenesis. Accordingly, reduced liver function following hepatic resection using the Pringle procedure[2] may result in metabolic disturbances of the pancreatic hormones and glucose intolerance.
Hyperglycemia has a deleterious effect on cells that passively take up glucose independent of insulin, including hepatocytes, alveolar cells, endothelial cells, neurons, and immune cells. Postoperative hyperglycemia is common in critically ill patients, even in those without a prior history of diabetes mellitus[3-5]. It is well known that hyperglycemia induced by surgical stress often results in dysregulation of liver metabolism and immune function, leading to impaired postoperative recovery[6,7]. Thus, the prevention of glucose toxicity in the mitochondrial compartment is important[8].
In this Topic Highlight, we review the benefits and requirements of tight glycemic control in hepatic surgery, with a focus on postoperative infection control. We suggest that perioperative intensive insulin therapy using a closed-loop artificial endocrine pancreas system is safe and effectively improves surgical outcome after hepatic resection.
CURRENT STATE OF TIGHT GLYCEMIC CONTROL IN CRITICALLY ILL PATIENTS
In large randomized trials in which the use of tight blood glucose control (80-110 mg/dL) with intensive insulin therapy was compared with standard blood glucose control (< 200 mg/dL) in surgical intensive care unit (ICU) patients, strict control of postoperative blood glucose levels was shown to significantly reduce patient mortality and morbidity[9,10]. In addition, postoperative hyperglycemia has been shown to be associated with an increased risk of surgical site infection (SSI)[11,12]. Current evidence suggests that maintaining normoglycemia postoperatively improves surgical outcome and reduces mortality and morbidity in critically ill patients[8,11,12]. These observations led to several short-lived multicenter randomized controlled studies designed to evaluate the benefit of tight glycemic control with intensive insulin therapy[13,14]. The main reason for the early cessation of these clinical trials was the high incidence of hypoglycemia (10%-17%) induced by the intensive insulin therapy[15,16], which could not be prevented because of technical limitations at that time[17-19]. However, the subsequent development of accurate continuous blood glucose monitoring devices and closed-loop systems for computer-assisted blood glucose control in the ICU will likely reduce the incidence of hypoglycemia in these situations[8].
For the reasons described above, achieving tight glycemic control with intensive insulin therapy has come under increased scrutiny in the management of patients in the surgical ICU. Recently, we demonstrated in two retrospective studies and in one randomized clinical trial that perioperative tight glycemic control using a closed-loop glycemic control system for patients undergoing liver resection was safe and effective in decreasing the incidence of SSI without increasing the risk of hypoglycemia.
INSULIN THERAPY IN THE SURGICAL ICU
In all three studies, perioperative blood glucose concentrations were monitored continuously using the STG-22 system, developed by Nikkiso Co. (Tokyo, Japan). Patients were divided into two groups: one in which glucose levels were controlled by manual injection of insulin according to the commonly used sliding-scale method (SS group)[20,21], and a second group in which programmed infusions of insulin were administered as determined by the control algorithm of a closed-loop artificial endocrine system (AP group).
Conventional insulin therapy using the sliding-scale method
Blood glucose levels in patients in the SS group were monitored continuously by the artificial pancreas and patients were checked routinely by nursing staff every 2 h. Blood glucose levels in these patients were controlled by subcutaneous injections of regular human insulin, with the dose determined by the sliding-scale method and the target blood glucose level to avoid hypoglycemia set at 150-200 mg/dL[20,21].
Novel insulin therapy using a closed-loop artificial endocrine pancreas system
The STG-22 unit was developed in 1984 by Nikkiso Co. as a closed-loop artificial endocrine pancreas system. The STG-22 system is a reliable and accurate device that measures blood glucose concentrations continuously and is comparable to the ABL 800FLEX machine (Radiometer Medical ApS, Brønshøj, Denmark) recommended by the National Committee for Clinical Laboratory Standards[22,23]. The STG-22 closed-loop glycemic control system is composed of a glucose sensor for the detection and/or monitoring of glucose and pumps for the infusion of appropriate amounts of insulin or glucose[24,25]. The insulin and glucose pumps are regulated by a computer on the basis of target blood glucose values that are defined prior to initiation of the system. The STG-22 maintains stable blood glucose concentrations by automatic infusion of regular insulin or glucose into the circulation[24,25]. In the ICU, peripheral blood was sampled continuously at 2 mL/h over the first 18 h postoperatively to monitor glucose levels. In addition, the STG-22 was used to evaluate the patients’ insulin requirements.
Statistical analysis
Continuous variables are presented as the mean ± SD. Dichotomous variables are presented as both absolute numbers and percentages. Data were analyzed using Student’s t-test (two-tailed), with dichotomous variables analyzed by the χ2 test (two-tailed) or Fisher’s exact test (two-tailed). P < 0.05 was considered significant. All analyses were performed using SPSS software (SPSS, Chicago, IL, USA).
RETROSPECTIVE STUDIES
The benefits of using a closed-loop glycemic control system in patients after hepatectomy were investigated in two retrospective studies. The aim of the first study was to evaluate the usefulness of the closed-loop system in providing continuous monitoring and strict control of postoperative blood glucose levels in patients after hepatic resection[25]. The aim of the second study was to identify, using multivariate analysis, risk factors and predictors of SSI, as well as how to prevent the development of SSI, in a consecutive series of patients undergoing hepatic resection for liver disease in a single institution.
In the first study[25], postoperative blood glucose levels increased initially in the SS group, reaching a plateau of approximately 250 mg/dL between 4 and 7 h after hepatectomy. Thereafter, blood glucose levels decreased, returning to normal within 16 h after surgery. In the AP group, blood glucose decreased gradually, reaching target levels (90-110 mg/dL) within 12 h after surgery. Total insulin administered per patient during the first 16 h after surgery was significantly higher in the AP group compared with the SS group (183 ± 188 IU vs 8 ± 7 IU, respectively, P < 0.001). These data suggest that the sliding-scale method is not as effective as the closed-loop artificial endocrine system in preventing hyperglycemia resulting from disturbed glucose metabolism following liver resection.
In the second study[26], the association between SSI and various clinical parameters was investigated in 152 patients following hepatic resection. The incidence of SSI in these patients was 14.5%. Multivariate analysis identified four independent parameters that were correlated with the occurrence of SSI, namely (1) body mass index > 23.6 kg/m2, (2) estimated blood loss volume > 810 mL, (3) the presence of postoperative bile leak organ/space SSI; and (4) use of the sliding-scale method for postoperative glucose control. No SSI was observed after liver resection in patients in whom postoperative blood glucose levels were controlled by an artificial pancreas. The results of this second retrospective study demonstrated that a lack of postoperative glycemic control is associated with a significantly higher incidence of postoperative infectious complications and a longer period of hospitalization.
PROSPECTIVE RANDOMIZED CLINICAL TRIAL
A prospective randomized trial was conducted in patients undergoing hepatic resection to evaluate the postoperative condition of the patients and the effects of a closed-loop artificial pancreas on tight glycemic control during intensive insulin therapy after hepatectomy[27]. Patients were randomly assigned to receive intensive insulin therapy using a closed-loop glycemic control system (i.e. an artificial pancreas; target blood glucose 80-110 mg/dL; AP group) or conventional insulin therapy using the sliding-scale method (target blood glucose 150-200 mg/dL; SS group). Perioperative blood glucose levels were monitored continuously in both groups using a closed-loop system. Neither group experienced hypoglycemia (blood glucose < 40 mg/dL). Although perioperative blood glucose levels in the AP group were close to 100 mg/dL, those in the SS group were > 150 mg/dL, which is the same as in our first retrospective study[25] (Figure 1). The incidence of SSI was significantly lower in the AP than in the SS group (2.3% vs 18.2%, respectively, P = 0.030), the duration of hospitalization was significantly shorter for patients in the AP group compared with the SS group (14.3 ± 5.9 d vs 18.7 ± 11.7 d, respectively, P = 0.049). The impact on hospital costs is one of the most frequently discussed consequences of SSI within a clinical setting. A few studies have presented data on the incidence and costs of infection for the hospital and post-discharge periods. As there were no incidences of post-discharge SSI in the current study, the costs-of-illness during hospitalization were incorporated into the overall costs. Total hospital costs were significantly lower for patients in the AP group than in the SS group (16 407 ± 5284 $ vs 21 879 ± 15 784 $, respectively, P = 0.047). There is evidence suggesting that SSI prolongs the length of hospitalization for patients undergoing cardiac surgery, caesarean section, orthopedic surgery and general surgery, and increases the total costs of a patient’s treatment[28]. These results support the notion that intensive insulin therapy using a closed-loop glycemic control system after hepatic resection results in the maintenance of near normoglycemia, contributing to a reduction in both the incidence of SSI and total hospital costs per patient due to a decreased duration of hospitalization. Thus, the closed-loop system promises more effective and safer intensive insulin therapy in hepatectomized patients. An important question that has arisen from this study is how to choose candidates for intensive insulin therapy using a closed-loop system. This important issue must be addressed in future studies.
PROBLEMS ASSOCIATED WITH INTENSIVE INSULIN THERAPY IN LIVER SURGERY
The possible risks of targeting normoglycemia include intraoperative hyperglycemia, hypoglycemia, and the need for perioperative parenteral nutrition.
The Pringle maneuver was introduced in liver surgery to reduce hepatic hemorrhage[2] and is now widely used in hepatic resections to control intraoperative bleeding[29-31]. This inflow-occlusion technique involves total compression of the hepatoduodenal ligament, generally by clamping it for 15 min during hepatic parenchymal resections, followed by 5 min of unclamping[31,32]. The clamping-unclamping procedure is repeated until the hepatic resection is complete. Glucose concentrations just before the first Pringle maneuver were significantly higher than baseline, but decreased gradually with the first clamping of the hepatoduodenal ligament. After unclamping of the hepatoduodenal ligament, there was an immediate and marked increase in glucose levels. The decrease and subsequent increase in glucose levels are seen with following rounds of clamping and unclamping of the hepatoduodenal ligament. After the surgery is completed, glucose concentrations gradually decline (Figure 2)[32]. In future studies, we will determine whether the rapid fluctuations in blood glucose levels during the Pringle maneuver should be controlled.
The most feared risk associated with intensive insulin therapy is postoperative hypoglycemia, which may cause convulsions, coma, and brain damage, as well as cardiac arrhythmias[15]. In ICU studies, the risk of severe hypoglycemia (glucose < 40 mg/dL) has been shown to increase from 5.0% to 18.7% with intensive insulin therapy[10,15,16]. Van den Berghe[8] suggested that clinical outcome by intensive insulin therapy (targeting blood glucose level of 80-110 mg/dL) was more effective for reducing hospital mortality and morbidity in critically ill adult patients compared with moderate intensive insulin therapy (targeting blood glucose level of 110-150 mg/dL). However, a large international randomized trial in 2009 showed that a blood glucose target of less than 180 mg/dL resulted in lower mortality than a target of 81-108 mg/dL[16]. Furthermore, contrary to this report[8], a recent meta-analysis[15] did not support the benefits of intensive insulin therapy that it was not associated with significantly reduced hospital mortality but was associated with an increased risk of hypoglycemia. These randomized trials had several issues as follows: (1) trials were not blinded, (2) unusually high mortality in the usual care group, (3) parenteral nutrition (different administration of energy at the ICU), and (4) a markedly increased risk of hypoglycemia. To address this issue on the focus of a more effective blood glucose control method under impartial conditions without hypoglycemia, we are constructing a prospective randomized comparison study between intensive insulin therapy and moderate intensive insulin therapy using a closed-loop artificial endocrine pancreas. There were no occurrences of hypoglycemia during intensive insulin therapy using a closed-loop glycemic control system in our series of studies. In our experience of more than 200 patients in the surgical and medical ICU, we have not seen hypoglycemia develop in any patient when intensive insulin therapy is administered using a closed-loop glycemic control system (data not shown).
Enteral feeding was started as soon as possible after patients were hemodynamically stable. However, if the energy intake target could not be achieved, parenteral feeding was initiated to compensate for the deficit. In our series, all patients were given parenteral nutrition following surgery[33], with the total caloric requirement calculated according to the Harris-Benedict equation[34]. Based on the results of our studies, maintaining an adequate calorie level, and controlling postoperative glucose levels with insulin therapy contributed to a reduction in the incidence of SSI. Moreover, the duration of hospitalization after liver surgery was reduced in patients who received perioperative tight glycemic control with intensive insulin therapy, and it is likely that this is related to a reduction in postoperative complications due to infection (SSI). In our studies, tight glycemic control was maintained for 18 h in patients in the surgical ICU after liver resection, and excellent glucose control was achieved without hypoglycemia using the closed-loop system.
Another outcome of our studies was that a brief period of glycemic control impacted on the incidence of SSI. Bacterial growth curves for Escherichia coli, Streptococcus, Proteus, Staphylococcus and Pseudomonas, among others, indicate that under optimum conditions the greatest growth occurs between 2 and 18 h[35]. We strongly believe that by instigating perioperative tight glycemic control for a brief period (at least 18 h) after surgery, postoperative infectious morbidity is decreased.
FUTURE DIRECTIONS
Clearly, we support a recent report that suggests that the development of accurate, continuous blood glucose monitoring devices (preferably closed-loop systems) for computer-assisted blood glucose control in the ICU will help prevent hypoglycemia[17]. However, using currently available technology, tight glucose control (i.e. targeting blood glucose levels of 80-110 mg/dL) using a closed-loop system in the surgical ICU was not achieved in 100% of cases, with the reported range being 60%-100%. Thus, further studies are needed to determine a better algorithm with which more accurate tight glucose control can be achieved. Regardless, we believe that by using the closed-loop glycemic control system during intensive insulin therapy in the ICU, the incidence of hypoglycemia and/or problems of nutritional support are reduced, as is the burden on nursing staff that is normally associated with the requisite frequent monitoring of blood glucose levels.
CONCLUSION
Intensive insulin therapy using a closed-loop artificial endocrine pancreas system during hepatic resection not only maintained near normoglycemia, but also contributed to a reduction in the rate of SSI and a decrease in total hospital costs due to shortened hospitalization. The closed-loop glycemic control system promises to revolutionize intensive insulin therapy for patients with disturbed glucose metabolism after liver resection.
Peer reviewers: Giulio Marchesini, Professor, Department of Internal Medicine and Gastroenterology, “Alma Mater Studiorum” University of Bologna, Policlinico S. Orsola, Via Massarenti 9, Bologna 40138, Italy; Giovanni Tarantino, MD, Professor, Department of Clinical and Experimental Medicine, Federico II University Medical School, VIA S. PANSINI, 5, Naples 80131, Italy
S- Editor Tian L L- Editor Webster JR E- Editor Zheng XM