Published online Aug 28, 2009. doi: 10.3748/wjg.15.4023
Revised: July 27, 2009
Accepted: August 3, 2009
Published online: August 28, 2009
AIM: To identify possible predictive factors for rebleeding after angiographically negative findings in patients with acute non-variceal gastrointestinal bleeding.
METHODS: From January 2000 to July 2007, 128 patients with acute non-variceal gastrointestinal bleeding had negative findings after initial angiography. Clinical and laboratory parameters were analyzed retrospectively.
RESULTS: Among 128 patients, 62 had no recurrent gastrointestinal bleeding and 66 had recurrent gastrointestinal bleeding within 30 d. As determined by the use of multivariate analysis, an underlying malignancy, liver cirrhosis and hematemesis were significant factors related to recurrent gastrointestinal bleeding.
CONCLUSION: Clinical factors including underlying malignancy, liver cirrhosis, and hematemesis are important predictors for rebleeding after angiographically negative findings in patients with acute non-variceal gastrointestinal bleeding.
- Citation: Joo I, Kim HC, Chung JW, Jae HJ, Park JH. Risk factors for rebleeding after angiographically negative acute gastrointestinal bleeding. World J Gastroenterol 2009; 15(32): 4023-4027
- URL: https://www.wjgnet.com/1007-9327/full/v15/i32/4023.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4023
Acute non-variceal gastrointestinal bleeding accounts for approximately 20% of emergency room visits and 5% of admissions[1]. Although endoscopy (including the use of upper endoscopy and colonoscopy) has been used as a first-line treatment option in patients with gastrointestinal bleeding[23], angiographic intervention can be used as a safe diagnostic and treatment method in patients with gastrointestinal bleeding that is refractory to endoscopic treatment[4–6].
Angiography requires a bleeding rate of 0.5-1 mL/min for detection. When neither extravasation nor vascular abnormality such as pseudoaneurysm is found, the bleeding site cannot be embolized selectively. Thus, intermittent bleeding is likely to result in a negative angiographic study[78]. The incidence of rebleeding in patients with negative initial angiography has been reported in up to 60% of cases[9]. However, little is known about the predictive factors for rebleeding, to determine if further investigations should be performed. The aim of this retrospective study was to identify the factors related to rebleeding in patients with gastrointestinal bleeding and normal angiographic findings.
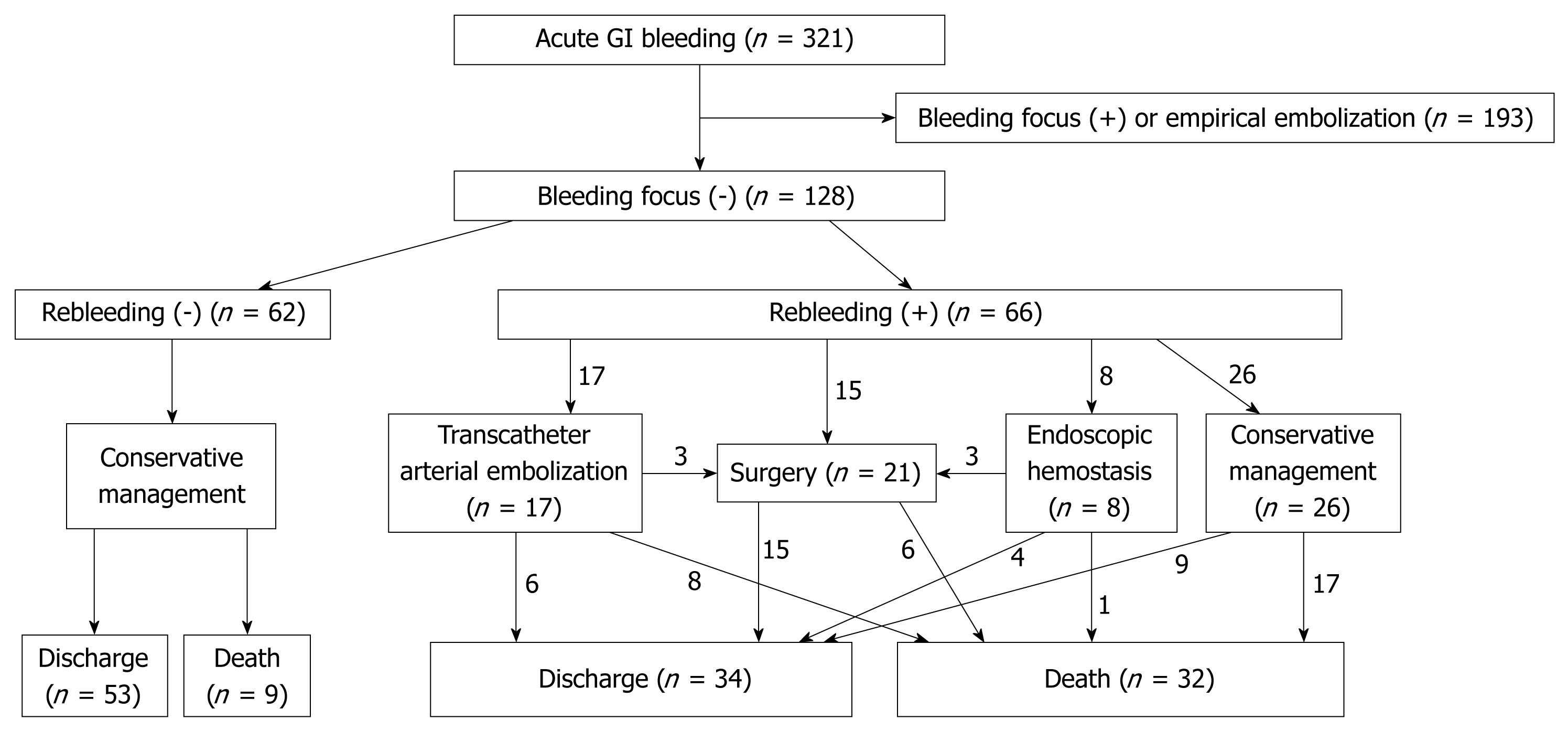
From January 2000 to July 2007, 341 patients with acute non-variceal gastrointestinal bleeding were referred to the angiography unit of our institution for possible transcatheter arterial embolization. We excluded 193 patients as they had active bleeding detected by angiography, and these patients received selective or empirical embolization. Among 148 patients with negative findings upon initial angiography, 20 were excluded because of limited medical records or suspected variceal gastrointestinal bleeding. A total of 128 patients (94 male, 34 female; age range, 18-85 years, mean age, 57.8 years) who had no active bleeding detected by initial angiography were included in this study. Approval was obtained from the ethical board committee of Seoul National University Hospital and patient informed consent was waived because of the retrospective nature of the study.
Clinical and laboratory parameters were reviewed retrospectively. The variables assessed included the following: patient age, sex, history of hematemesis, hematochezia or melena, shock, hemoglobin level, platelet count, prothrombin time, partial thromboplastin time, recent surgery, potential bleeding diatheses (liver cirrhosis or chronic renal failure), underlying malignancy, use of nonsteroidal anti-inflammatory drugs, and use of other antiplatelet agents or anticoagulants at the time of evaluation. Rebleeding was confirmed by endoscopy or surgery, and was clinically defined as: (1) fresh hematemesis; (2) fresh melena with systolic blood pressure < 100 mmHg; (3) a decrease in hemoglobin level of > 4 g/dL within 24 h; and (4) a requirement of two more red blood cell transfusions within 24 h.
The univariate association between clinical and laboratory variables and rebleeding was examined using Fisher’s exact test for categorical variables and the t test for continuous variables. Variables with P < 0.25 as determined by univariate analysis were subjected to multiple logistic regression analysis with the use of a backward stepwise method. For univariate and multiple logistic regression analysis, P < 0.05 was regarded as statistically significant. Statistical analyses were performed using commercially available software (SPSS for Windows version 10.0 (Chicago, IL USA). All reported P values were two tailed.
Before the initial angiography examination, endoscopy was performed in 97 patients, endoscopic hemostasis was attempted in 18 patients, tagged red blood cell scintigraphy was performed in 21 patients, and a computed tomography (CT) scan was obtained for 40 patients. After initial angiography with negative findings, endoscopy was performed in 81 patients, endoscopic hemostasis was attempted in eight patients, tagged red blood cell scintigraphy was performed in 18 patients, a CT scan was obtained for 18 patients, and surgical treatment was performed in 21 patients.
The location of bleeding was the esophagus (n = 3), stomach (n = 31), duodenum (n = 25), small intestine (n = 12), colon (n = 13) and unknown (n = 44). The cause of bleeding was a benign ulcer (n = 50), gastrointestinal tumor (n = 7), ischemic enteritis (n = 3), radiation colitis (n = 2), iatrogenic injury (n = 2), angiodysplasia (n = 2) and undetermined (n = 62).
Among 128 patients who had no active bleeding detected by angiography, 62 had no recurrent gastrointestinal bleeding and 66 had recurrent gastrointestinal bleeding. Figure 1 documents the clinical course of the 128 patients. For the 66 patients with rebleeding, 40 received interventions including surgery, transcatheter arterial embolization and endoscopic hemostasis. The 4-wk mortality rate was 48% (32/66) for patients with rebleeding and 15% (9/62) for those without rebleeding. Among 41 expired patients, the cause of death was uncontrolled bleeding in 11, pneumonia or sepsis in 13, aggravation of the underlying malignancy in 10, cardiac failure in one, hepatic failure in one, and unknown in five.
Based on univariate analysis, the hemoglobin level, partial thromboplastin time, use of antiplatelet medication, underlying malignancy, presence of liver cirrhosis and shock were significant factors related to recurrent gastrointestinal bleeding. Based on multivariate analysis, underlying malignancy (P = 0.002, OR = 3.81), liver cirrhosis (P = 0.017, OR = 4.81) and hematemesis (P = 0.042, OR = 2.59) were significant factors related to recurrent gastrointestinal bleeding (Table 1).
| Variable | Rebleeding | P value at univariate analysis | Multiple logistic regression | ||||
| Absent | Present | P value | OR | CI | |||
| Age (yr) | 59.2 ± 15.1 | 56.9 ± 15.9 | 0.414 | ||||
| Hemoglobin (g/dL) | 8.2 ± 1.9 | 7.3 ± 1.9 | 0.009 | ||||
| Platelet (× 1000/mm) | 173.5 ± 98 | 151 ± 140 | 0.301 | ||||
| PT (INR) | 1.25 ± 0.31 | 1.38 ± 0.66 | 0.063 | ||||
| aPTT (s) | 38.7 ± 9.9 | 48.6 ± 31.8 | 0.027 | ||||
| Sex | Female | 17 | 18 | 1.000 | |||
| Male | 45 | 48 | |||||
| NSAID | No use | 59 | 64 | 0.673 | |||
| Use | 3 | 2 | |||||
| Anticoagulation | No | 56 | 64 | 0.155 | |||
| Yes | 6 | 2 | |||||
| Antiplatelet therapy | No | 47 | 61 | 0.014 | |||
| Yes | 15 | 5 | |||||
| Malignancy | Absent | 42 | 26 | 0.002 | 0.002 | 3.81 | 1.63-8.91 |
| Present | 20 | 40 | |||||
| GI tumor bleeding | Absent | 57 | 56 | 0.275 | |||
| Present | 5 | 10 | |||||
| Recent surgery | Absent | 44 | 41 | 0.350 | |||
| Present | 18 | 25 | |||||
| Recent GI surgery | Absent | 54 | 52 | 0.247 | |||
| Present | 8 | 14 | |||||
| Past GI bleeding history | Absent | 47 | 51 | 1.000 | |||
| Present | 15 | 15 | |||||
| Chronic renal failure | Absent | 54 | 58 | 1.000 | |||
| Present | 8 | 8 | |||||
| Liver cirrhosis | Absent | 56 | 47 | 0.007 | 0.017 | 4.81 | 1.32-17.54 |
| Present | 6 | 19 | |||||
| Hematemesis | Absent | 46 | 38 | 0.063 | 0.042 | 2.59 | 1.04-6.07 |
| Present | 16 | 28 | |||||
| Hematochezia | Absent | 32 | 34 | 1.000 | |||
| Present | 30 | 32 | |||||
| Melena | Absent | 40 | 43 | 1.000 | |||
| Present | 22 | 23 | |||||
| Shock | Absent | 37 | 24 | 0.013 | |||
| Present | 25 | 42 | |||||
Acute non-variceal gastrointestinal bleeding is one of the common emergency conditions for inpatients as well as outpatients[1]. Superselective angiography and transcatheter embolization have been used widely for upper and lower gastrointestinal bleeding refractory to endoscopic therapy[45]. In the case of failure of endoscopic management caused by a large number of blood clots or poor bowel preparation, angiography may be the choice of diagnostic or therapeutic method[4]. The angiographic procedure can provide accurate localization of the bleeding focus and immediate hemostasis, and localization of the bleeding site prior to surgery can prevent “blind” bowel resection. In addition, the use of angiography is less invasive than surgery, and is a good option for poor surgical candidates[10]. Recently, the use of improved techniques and instruments has decreased the number of complications such as bowel ischemia within an acceptable range[56].
Unfortunately, blood extravasation is not always visualized. In recent reviews of angiographic findings, blood extravasation or intraluminal blush was seen in 40%-60% of angiographic cases of non-variceal upper gastrointestinal bleeding[178]. There have been many studies of patients with normal angiograms, but a gold standard for management has not been determined. Some suggest that, in the case of negative angiographic findings in patients with intermittent or slow flow bleeding, use of nuclear scintigraphy seems reasonable to help confirm and localize the lesion[1112]. The use of CT angiography may add to the detection of intermittent bleeding with possible better localization of the source and etiology of the bleeding. Ettorre et al[13] have shown a detection rate of 72% in patients with obscure gastrointestinal bleeding, in whom endoscopic and nuclear imaging failed to localize the bleeding site. The use of angiography has been reported with intra-arterial or intravenous injection of vasodilators, heparin, and even thrombolytic drugs to improve the rate of positive angiographic findings in occult lower gastrointestinal bleeding, although these modifications have been considered provocative[14–16]. Some investigators have suggested that blind embolization of the left gastric artery after endoscopic localization can show a decrease in the rebleeding rate[17]. As a result of the safety of the procedure, empiric embolization of the upper gastrointestinal tract for acute bleeding has been recommended when guided by endoscopic findings.
We expect that determination of the predictive factors for rebleeding may help in the selection of patients for further work-up or treatment, and consequently, may increase the success rate and decrease the rate of complications. Several studies have demonstrated clinical and endoscopic factors including liver cirrhosis, recent surgery, hypovolemic shock, hematemesis, large ulcer size, non-bleeding visible vessel, and the presence of an adherent clot on an ulcer base as significant predictive factors for the recurrence of hemorrhage in patients with peptic ulcer[1819]. In our study, underlying malignancy, liver cirrhosis and hematemesis were significant factors related to recurrent gastrointestinal bleeding. As we did not perform endoscopy in all patients, and the study population was heterogeneous, including upper and lower gastrointestinal bleeding, endoscopic factors were not included in the analysis.
Rebleeding rates reported in the literature vary from 7% to 25% in patients with peptic ulcer or lower gastrointestinal bleeding[18–20]. In our study, the incidence of rebleeding within 1 mo was 52% (66/128). We think that the rebleeding rate was high because many severely ill patients were included in our study population.
The mortality rate in our study was 48% for patients with rebleeding and 15% for those without. Since there are many variables, we cannot state that the rebleeding itself affected mortality. However, prediction of rebleeding seems to have a relation to the prediction of prognosis.
This study had some limitations. First, the variable diagnostic and therapeutic modalities were performed without a settled sequence or principle. Most of the patients (120/128) received variable transfusions of red blood cells, fresh frozen plasma or platelet concentrate before angiography. Tagged red blood cell scintigraphy was performed in 21 patients and CT angiography in 40. Endoscopy was performed in 97 patients and 18 underwent endoscopic treatment. The selection of endoscopy or angiography in acute gastrointestinal bleeding is not well established. In our retrospective review, in cases in which postoperative CT showed an active bleeding focus, or the condition of the patient was inappropriate for endoscopy, angiography was performed as the first-choice method for diagnosis and treatment of acute gastrointestinal bleeding. Second, patients were enrolled in the study from only a single referral hospital. Many of the patients were elderly and had numerous medical problems. These conditions may have influenced the relatively high rebleeding rate and high mortality rate. Third, although the clinical features of upper gastrointestinal bleeding are quite different from lower gastrointestinal bleeding, both upper and lower gastrointestinal bleeding were included in this study population, as we could not determine the location of the bleeding site in 44 of 128 patients.
In conclusion, clinical factors including underlying malignancy, liver cirrhosis, and hematemesis are important predictors of recurrent bleeding after negative angiographic findings in patients with acute non-variceal gastrointestinal bleeding.
Acute non-variceal gastrointestinal bleeding is a common emergency condition. Superselective angiography and transcatheter embolization have been used widely for gastrointestinal bleeding. Unfortunately, blood extravasation is not always visualized.
To predict the risk of gastrointestinal rebleeding after negative angiography may be important clinically. However, little is known about the predictive factors for rebleeding, to determine if further investigations should be performed.
This study is believed to be the first to establish predictive factors for rebleeding after angiographically negative gastrointestinal bleeding.
Determination of the predictive factors for rebleeding may help in the selection of patients for further work-up or treatment, and consequently, might increase the success rate and decrease the rate of complications.
This study evaluated retrospectively the risk factors for gastrointestinal rebleeding after negative angiography.
| 1. | Burke SJ, Golzarian J, Weldon D, Sun S. Nonvariceal upper gastrointestinal bleeding. Eur Radiol. 2007;17:1714-1726. |
| 2. | Martins NB, Wassef W. Upper gastrointestinal bleeding. Curr Opin Gastroenterol. 2006;22:612-619. |
| 3. | Beejay U, Marcon NE. Endoscopic treatment of lower gastrointestinal bleeding. Curr Opin Gastroenterol. 2002;18:87-93. |
| 4. | Defreyne L, Vanlangenhove P, De Vos M, Pattyn P, Van Maele G, Decruyenaere J, Troisi R, Kunnen M. Embolization as a first approach with endoscopically unmanageable acute nonvariceal gastrointestinal hemorrhage. Radiology. 2001;218:739-748. |
| 5. | Lee CW, Liu KL, Wang HP, Chen SJ, Tsang YM, Liu HM. Transcatheter arterial embolization of acute upper gastrointestinal tract bleeding with N-butyl-2-cyanoacrylate. J Vasc Interv Radiol. 2007;18:209-216. |
| 6. | Burgess AN, Evans PM. Lower gastrointestinal haemorrhage and superselective angiographic embolization. ANZ J Surg. 2004;74:635-638. |
| 7. | Dempsey DT, Burke DR, Reilly RS, McLean GK, Rosato EF. Angiography in poor-risk patients with massive nonvariceal upper gastrointestinal bleeding. Am J Surg. 1990;159:282-286. |
| 8. | Aina R, Oliva VL, Therasse E, Perreault P, Bui BT, Dufresne MP, Soulez G. Arterial embolotherapy for upper gastrointestinal hemorrhage: outcome assessment. J Vasc Interv Radiol. 2001;12:195-200. |
| 9. | Defreyne L, Vanlangenhove P, Decruyenaere J, Van Maele G, De Vos M, Troisi R, Pattyn P. Outcome of acute nonvariceal gastrointestinal haemorrhage after nontherapeutic arteriography compared with embolization. Eur Radiol. 2003;13:2604-2614. |
| 10. | Patel TH, Cordts PR, Abcarian P, Sawyer MA. Will transcatheter embolotherapy replace surgery in the treatment of gastrointestinal bleeding?(2)(2). Curr Surg. 2001;58:323-327. |
| 11. | Howarth DM, Tang K, Lees W. The clinical utility of nuclear medicine imaging for the detection of occult gastrointestinal haemorrhage. Nucl Med Commun. 2002;23:591-594. |
| 12. | Zettinig G, Staudenherz A, Leitha T. The importance of delayed images in gastrointestinal bleeding scintigraphy. Nucl Med Commun. 2002;23:803-808. |
| 13. | Ettorre GC, Francioso G, Garribba AP, Fracella MR, Greco A, Farchi G. Helical CT angiography in gastrointestinal bleeding of obscure origin. AJR Am J Roentgenol. 1997;168:727-731. |
| 14. | Koval G, Benner KG, Rösch J, Kozak BE. Aggressive angiographic diagnosis in acute lower gastrointestinal hemorrhage. Dig Dis Sci. 1987;32:248-253. |
| 15. | Bloomfeld RS, Smith TP, Schneider AM, Rockey DC. Provocative angiography in patients with gastrointestinal hemorrhage of obscure origin. Am J Gastroenterol. 2000;95:2807-2812. |
| 16. | Ryan JM, Key SM, Dumbleton SA, Smith TP. Nonlocalized lower gastrointestinal bleeding: provocative bleeding studies with intraarterial tPA, heparin, and tolazoline. J Vasc Interv Radiol. 2001;12:1273-1277. |
| 17. | Lang EV, Picus D, Marx MV, Hicks ME, Friedland GW. Massive upper gastrointestinal hemorrhage with normal findings on arteriography: value of prophylactic embolization of the left gastric artery. AJR Am J Roentgenol. 1992;158:547-549. |
| 18. | Hsu PI, Lin XZ, Chan SH, Lin CY, Chang TT, Shin JS, Hsu LY, Yang CC, Chen KW. Bleeding peptic ulcer--risk factors for rebleeding and sequential changes in endoscopic findings. Gut. 1994;35:746-749. |