CASE REPORT
A 47-year-old woman, who was admitted to the radiology department of our hospital, presented with a 2 mo history of general illness, slight weight loss and a palpable mass in the right upper abdomen. Ultrasound and contrast-enhanced computed tomography (CT) of the abdomen were performed in another hospital. Ultrasound of the upper abdomen revealed a heterogeneous hypoechoic tumor in the liver. The CT scan showed a heterogeneous contrast-enhanced mass in segments 5 and 6 of the liver. Her past medical history included an appendectomy and hepatitis B positivity for more than 15 years. She was considered a so called healthy carrier of hepatitis B.
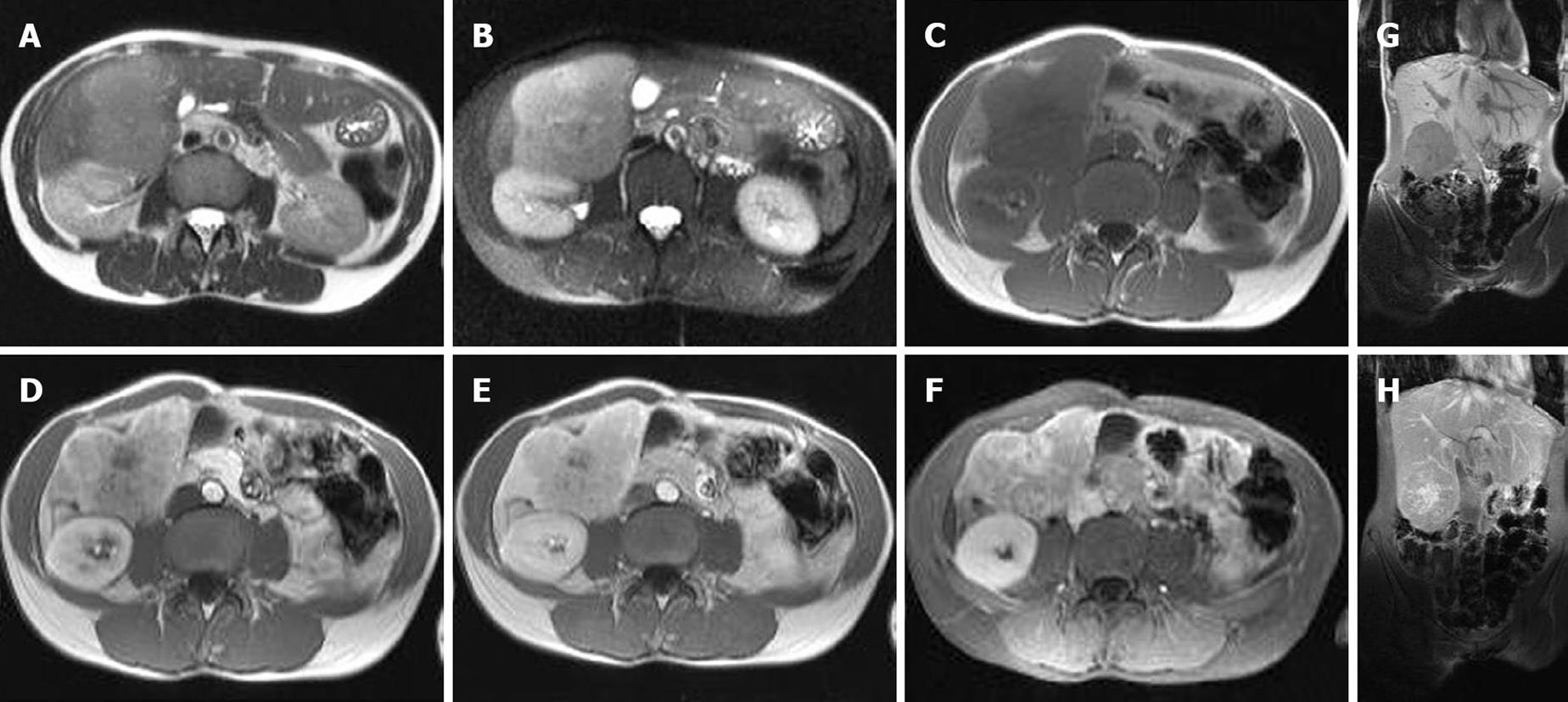
Magnetic Resonance Imaging (MRI) was performed in our hospital. On MRI a focal liver lesion inferior in the right lobe was visualized. The mass was homogeneous hypointense on T1 (Figure 1C and G) and slightly hyperintense on T2 (Figure 1A and B). The dynamic contrast-enhancement with gadolinium chelate (Magnevist®, Baeyer HealthCare Pharmaceuticals, Düsseldorf, Germany) established a relatively hypervascular heterogeneous nature with late enhancement of the central scar (Figure 1D-H). The contrast-enhancement and morphologies were consistent with focal nodular hyperplasia. Laboratory results, however, revealed an elevated α-fetoprotein level of 154 000 ng/mL.
Figure 1 MRI images of combined hepatocellular and cholangiocellular carcinoma.
Axial T2 (A) and T2 fat saturated (B) MRI images showed a slightly well circumscribed lesion. The mass is hyperintense in comparison to the normal liver. Axial T1 (C) MRI images demonstrated a hypointense lesion. Early arterial (D), late arterial (E) and venous (F) MRI images displayed a hypervascular heterogeneous enhancement with central scar. The coronal T1 (G) image showed a hypointense lesion, while the coronal T1 MRI image after Gadolinium contrast (H) demonstrated a heterogeneous early enhancement of the lesion with delayed uptake in the scar.
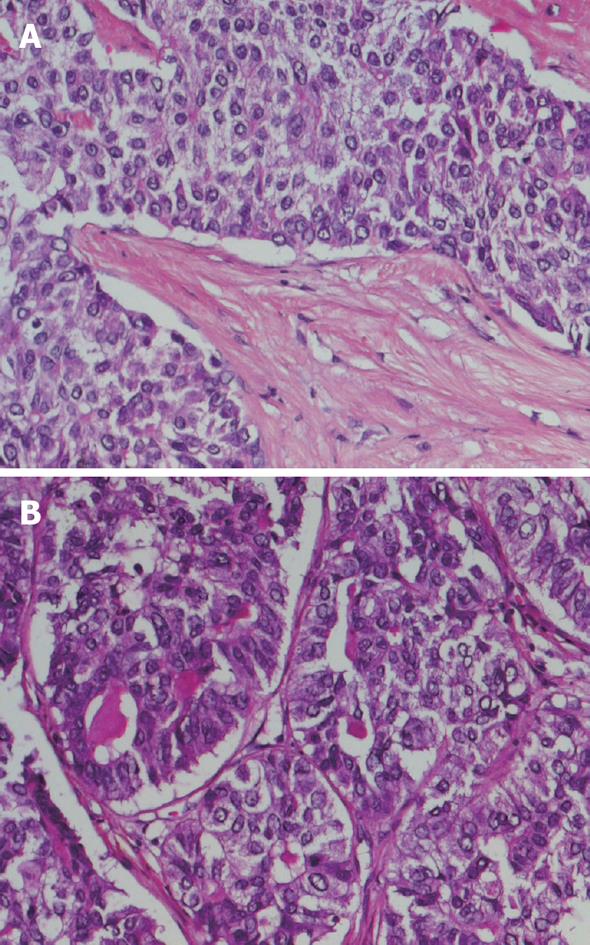
Right hepatectomy was performed. A white firm tumoral mass of 8.5 cm in diameter and one satellite nodule of 1 cm were identified during macroscopic examination of the resection specimen. The non-tumoral liver did not display signs of cirrhosis macroscopically. Microscopically, the tumor consisted of nests of tumor cells separated by broad bands of fibrous tissue, focally resembling a fibrous scar (Figure 2A). In most tumor cell nests, tumor cells displayed a trabecular growth pattern. Trabecular structures were several cell layers thick, in contrast to normal liver tissue in which trabeculae are only 1 to 2 layers in thickness. Focally, however, glandular structures containing secretory material were observed (Figure 2B). Tumor cells arranged in trabecular structures displayed positivity for HepPar1 and α-fetoprotein and immunohistochemistry with a polyclonal anti-CEA antibody revealed the presence of canalicular structures. These features are consistent with hepatocellular differentiation. Where tumor cells formed glandular structures, the presence of neutral mucin could be demonstrated. The secretory material displayed alcian blue positivity and was positive in a PAS-diastase reaction, compatible with cholangiocellular differentiation. Moreover, areas of glandular differentiation displayed positivity for CA19.9 and MUC1, while areas of hepatocellular differentiation were negative. In addition, positivity for CK7 and CK19 was observed in areas of glandular growth pattern, while areas with trabecular growth pattern displayed most often only positivity for CK8 and CK18. These histological features together with the immunohistochemical profile are consistent with a diagnosis of cHCC-CC.
Figure 2 Histopathological images of combined hepatocellular and cholangiocellular carcinoma.
A: Nests of tumor cells with trabecular architecture, reminiscent of hepatocellular differentiation, separated by bands of fibrous tissue; B: Focally tumor cells formed glandular structures with mucinous secretory material, compatible with cholangiocellular differentiation.
DISCUSSION
cHCC-CC is a rare primary liver tumor type containing unequivocal elements of both hepatocellular carcinoma and cholangiocarcinoma[12]. It accounts for 1.0%-14.2% of all primary liver carcinomas[3]. In 1949, the first description of cHCC-CC was reported by Allen et al[4]. Some studies found that this tumor type is more frequently diagnosed in patients with chronic hepatitis and liver cirrhosis[5], although this could not be confirmed in other studies[1]. Most studies report that patients with cHCC-CC show similar clinical and pathological features as hepatocellular carcinoma patients, including a mean age onset in the sixth decade, male predominance, a high incidence of hepatitis B virus infection, underlying chronic liver disease and elevated serum α-fetoprotein levels[6]. Several reported patients were initially misdiagnosed[7]. Various factors, such as an atypical enhancing pattern on imaging, the small size of one of the components, and the presence of a tumor composed of intermediate tumor cells, were found to be the causes of misdiagnosis[8]. The tumor remains usually generally clinically silent and patients present with advanced illness[6]. A simultaneous elevation of serum levels of CA 19-9 and α-fetoprotein in patients with a liver mass on imaging may suggest the presence of cHCC-CC[7]. Ultrasound usually demonstrates a round or ovoid hypoechoic mass with a central hyperechoic area[9]. On contrast-enhanced CT, cHCC-CC lesions are divided into 3 classes of enhancement patterns. The first group of lesions, namely cHCC-CC resembling hepatocellular carcinoma, shows hyperenhancement in the early phase and hypoenhancement in the late phase due to washout of contrast (Type I lesions). The second group, Type II lesions, demonstrates peripheral enhancement in the early as well as in the late phase. Type III lesions present an area of hyperenhancement in the early phase and an area of slight delayed enhancement in the late phase[10]. The cHCC-CC lesions resembling cholangiocarcinoma, however, enable low attenuation in the arterial phase and low, iso- or high attenuation in the venous phase[11]. Preoperatively cHCC-CC is, however, usually only considered when a liver tumor has CT features of both hepatocellular carcinoma and cholangiocellular carcinoma[12]. In addition, Toh et al[13] reported a hypodense tumor with vague contrast enhancement associated with elevation of α-fetoprotein levels and multiple regional lymphadenopathies as a cHCC-CC. Finally, cHCC-CC has also been reported as presenting as a hypervascular tumor associated with increased serum CEA and CA 19-9[14].
On MRI, cHCC-CC has been reported to show low signal intensities on T1-weighted images and high signal intensities on T2-weighted images[15]. In the arterial phase, it has been described that the T1-weighted images describe an enhancing mass nearly isointense to the surrounding parenchyma with a central necrotic non-enhancing zone[16].
Final diagnosis of cHCC-CC can only be made by histological examination. Extensive sampling of tissue is required in order not to miss the diagnosis in case one of the tumor compounds represents only a minor component of the tumor. Surgery is the only effective treatment for this tumor. The median survival time following cHCC-CC surgery has been reported to be 48 mo[17]. Partial hepatectomy with lymph node dissection can result in 5-year survival rates exceeding 50% in patients with early stage disease[18]. Prognostic factors of poor outcome of cHCC-CC are distant metastasis, major vascular branch invasion, regional lymph node involvement, bilobar distribution, multiple tumors, tumor necrosis and ascites[19].
In conclusion, preoperative diagnosis of cHCC-CC remains difficult. It is of great importance to choose appropriate imaging, such as MRI, in combination with serum α-fetoprotein and CA 19.9 levels. In addition, extensive tissue sampling is required in order to not miss this diagnosis in cases where one of the two components is of small size. Most studies show that this tumor type has similar geographical distribution and age and sex distribution as hepatocellular carcinoma. Some authors, however, have reported a worse prognosis as compared to patients with pure hepatocellular carcinoma.