Published online Jan 21, 2009. doi: 10.3748/wjg.15.369
Revised: November 8, 2008
Accepted: November 15, 2008
Published online: January 21, 2009
AIM: To investigate the inhibitory and anti-metastatic effect of mutant p27 gene (p27mt) on the growth of colorectal cancer xenografts in nude mice and its underlying mechanism.
METHODS: Inhibitory effect of p27mt gene on the growth of colorectal cancer xenografts was determined by measurement of tumor size before and after direct intra-tumoral injection of Ad-p27mt in a pre-established transplantation model of human colorectal cancer in nude mice. Cell cycle and apoptosis were detected by flow cytometry performed on single-cell suspension from an isolated tumor. Expression of MMP-9 in tumor tissue was detected by immunohistochemistry.
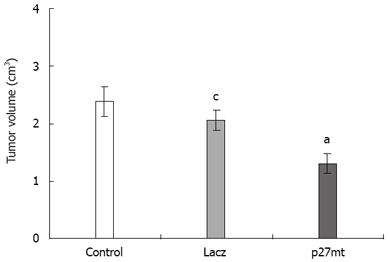
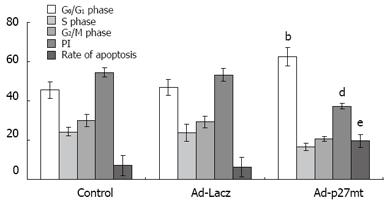
RESULTS: The average sizes of transplantation tumors were 1.94 ± 0.67 cm3, 2.75 ± 0.83 cm3 and 3.01 ± 0.76 cm3 in the Ad-p27mt, Ad-LacZ and control groups, respectively (P < 0.05). The average proliferation rates were 37.34% ± 1.45%, 53.16% ± 3.27% and 54.48% ± 2.43%, in the Ad-p27mt, Ad-LacZ and control groups, respectively (P < 0.05). The average apoptosis rates were 19.79% ± 3.32%, 6.38% ± 4.91% and 7.25% ± 5.20% in the Ad-p27mt, Ad-LacZ and control groups, respectively (P < 0.01). The average MMP-9 expression rates were 20%, 75% and 66.7% in the Ad-p27mt, Ad-LacZ and control groups, respectively (P < 0.01).
CONCLUSION: p27mt inhibits the growth of transplanted tumor by blocking the proliferation of cancer xenografts and by promoting apoptosis of transplantated tumor cells, as well as decrease transpl-anted tumor metastasis.
-
Citation: Chen J, Ding WH, Lu GX, Xu SY. Impact of
p27mt gene on transplantation model of human colorectal cancer in nude mice. World J Gastroenterol 2009; 15(3): 369-372 - URL: https://www.wjgnet.com/1007-9327/full/v15/i3/369.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.369
Along with the improvement of people’s living standard and change in diet, there has been a gradual increase in the incidence of colorectal cancer[1]. None of the current treatment modalities for colorectal cancer, including surgery, radiotherapy and chemotherapy, is effective. With the advent of post-genomic era, the function of genes has become a priority research area and brought the dawn in gene therapy for tumor. Since p27 is an anti-oncogene, this study was to evaluate the inhibitory and anti-metastatic effect of p27mt gene on the growth of colorectal cancer xenografts in nude mice and its underlying mechanism and to provide the theoretical basis for the use of p27 in clinical treatment of colorectal cancer.
Lovo cell line, purchased from the Center for Type Culture Collection of Wuhan University, was cultured in RPMI 1640 medium. Working density of Levo cells was 1 × 108/mL, with living cell count by trypan blue > 99%. Ad-LacZ was constructed and presented by Wang et al[2]. Ad-p27mt was self-constructed[3].
Thirty-six BALB/C nude mice, 4-6 wk old, and weighing 18-25 g, were purchased from the Laboratory Animal Management Center of Hubei Province. Lovo cell suspension (0.2 mL) was inoculated subcutaneously at the right back skin of each nude mouse. Upon tumor development, 27 nude mice whose tumor size was 0.5-1.5 cm in diameter were randomly assigned to control group, Ad-LacZ group or Ad-p27mt group. PBS (0.1 mL), Ad-LacZ (0.1 mL) with a virus density of 1010 pfu/mL, or Ad-p27mt (0.1 mL) with a virus density of 1010 pfu/mL was directly injected into the tumor of nude mice in the three groups, respectively, once every 3 d, for 28 d.
Transplanted tumor size was calculated according to the following formula: V = ab2/2, where a and b represent the length and width of the xenograft, respectively.
The 28th day after virus injection into the transplanted tumor, all mice were sacrificed with their tumors removed, weighed and photographed. Tumor tissue (15 g) was used in preparation of single cell suspension. Two hundred &mgr;L DNA-PREPTM LPR was mixed with 100 &mgr;L single cell suspension, and 1000 &mgr;L DNA-PREP stain was added into the mixture 3 min after the mixture was set at room temperature and protected from light. Fifteen min later, cell cycle and apoptosis were determined with a Coulter Epics XL flow cytometer. Proliferation index (PI) was calculated according to the following formula[4]: PI = (S + G2/M)/(G0/G1 + S + G2/M) × 100%.
Anti-human mouse MMP-9 monoclonal antibody, S-P staining kit and DAB developer were obtained from Beijing Zhongshan Biotechnology, Co, Ltd. Since MMP-9 appears to be brown granules in cytoplasm, total cell number and the number of MMP-9 positive cells were counted in 5 visual fields of the matrix area around the tumor nest under microscope. Based on the scope and extent of staining, immunohistochemical results were logged according to the following criteria: “-” - no positively stained cells; “+” - cells lightly stained or < 10% cells stained; “++” - moderately stained or 10%-25% cells stained; “+++” - darkly stained or more than 50% cells stained, where - represents negative expression, +/++ stands for weakly positive expression, and + + + stands for strong expression.
One way-ANOVA was used in processing measurement data, which were expressed as mean ± SD. χ2 test was adopted in calculation of enumeration data.
The average size of transplanted tumor in the Ad-p27mt group (1.94 ± 0.67 cm3) was significantly smaller than that in the control group (3.01 ± 0.76 cm3) (P < 0.05), no statistical significance was found in the average size of transplanted tumor between the two groups (3.01 ± 0.76 cm3vs 2.75 ± 0.83 cm3) (Figure 1).
More cells at G0/G1 phase and less cells at S and G2/M phase were observed in the Ad-p27mt group than the other two groups (P < 0.05). However, the difference between the two groups was insignificant (P < 0.05, Figure 2).
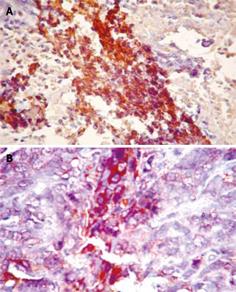
Since MMP-9 is mainly found in the matrix area around tumor nest, brown stained granules were observed in cytoplasm of cancer cells (Figure 3). MMP-9 expression rate for the Ad-p27mt group was significantly decreased compared with control group (Table 1).
While p27 is a negative regulator of cell cycle and a tumor suppressor[5], tumor may develop when abnormal (missing or decreased) expression of p27 and attenuated inhibition on cell cycle lead to uncontrolled cell growth and carcinogenesis[6]. The results of studies demonstrate that decreased p27 expression was associated with ubiquitin-mediated proteasome phosphorylation and abnormal activity of p27[78]. We investigated the in vivo inhibitory effect of p27 on transplanted tumor by intra-tumoral injection of mutated p27mt adenovirus.
Park et al[9] found that inhibition of mutant p27 (p27mt) on tumor cells seem stronger than that of wild type p27 (p27wt) as demonstrated in cells arrested in G0/G1 phase, and that the apoptosis promoting activity of p27mt is also stronger. Another study revealed that the half-life of p27mt is over 12 h, much longer than that of p27wt (2 h)[10]. Through determination of the size of transplanted tumor, this study displayed that p27mt gene significantly inhibited the growth of colorectal cancer by inhibiting cell proliferation and by promoting cell apoptosis, suggesting that p27mt can evidently suppress cell proliferation at G0/G1 phase. The apoptosis promoting activity of p27mt was more obvious in control group, while the apoptosis rate of p27wt was up to 37.9% ± 3.32%.
It was reported in our preliminary study that the expression level of p27 in colorectal cancer tissue is quite low[11]. In this study, the expression of MMP-9 was significantly decreased in p27mt group. MMP-9, in the form of proenzyme in cytoplasm, when released under physiological condition, may degrade extracellular matrix and is involved in development of human body and multiple physiological processes including tissue repair[12]. When disturbance of MMP-9 gene occurs, increased proenzyme leads to escalated degradation of extracellular components, including IV and V collagen and laminin, and undermined integrity of basement membrane. Therefore, MMP-9 plays a very important role in the process of tumor metastasis[13]. In this study, a reduced MMP-9 expression was observed after p27mt was injected. No tumor metastasis was found with in 28 d after transplantation of the tumor.
In conclusion, p27mt inhibits the growth of colorectal cancer by inhibiting cancer cell proliferation and promoting cell apoptosis as well as metastasis of colorectal cancer.
Along with the improvement in people’s living standard and changes in diet, there has been a gradual increase in the incidence of colorectal cancer in China. However, no effective therapeutic modalities are available for it. Gene therapy for restoration of p27 expression is a promising therapy for it. A mutant type of p27 gene, with mutant of Thr-187/Pro-188 to Met-187/Ile-188, can inhibit degradation of p27 protein through the ubiquitin-mediated pathway. The inhibitory effect of mutant p27 (p27mt) seems stronger than that of wild type p27 (p27wt) on tumor cells, as demonstrated by cells arrested in the G0/G1 phase. The apoptosis promoting activity of p27mt is also stronger. However, no study about its effect on colorectal cancer is available.
p27, a cyclin-dependent kinases inhibitor, a tumor suppressor gene, and a promoter of apoptosis, has been widely investigated. Anti-tumor activity of p27 has been demonstrated in breast, lung, and oral cancer. However, the anti-tumor bioactivity of p27mt has not been studied on colorectal cancer.
The results of this study indicate that p27mt gene has a strong anti-tumor bioactivity on colorectal cancer in vivo and in vitro.
This gene may be developed into a new therapeutic agent for colorectal cancer.
This study showed the effect of over-expression of a mutant form of p27 on colorectal cancer growth in a xenotransplantation model. The results are largely descriptive and the effect of p27mt seems modest on tumor growth. Further study is needed to show the expression of transfected p27mt gene.
| 1. | Zheng S, Cai SR. Colorectal Cancer Epidemiology and Prevention Study in China. Chin Ger J Clin Oncol. 2003;2:72-75. |
| 2. | Wang JN, Huang YZ, Kong X, Guo LY. The construction of recombinant adenoviral plasmid by homologous recombination in bacteria and the preparation of recombinant adenovirus expressing β-galactosidase. Yunyang Yixueyuan Xuebao. 2004;23:1-5. |
| 3. | Chen J, Xu SY, Deng CS, Wang JN, Huang YZ. Efficient generation of human mutant p27 recombinant adenovirus by homologous recombination in bacteria. J Fourth Mil Med Univ. 2004;5:406-409. |
| 4. | Cai FG, Xiao JS, Ye QF. Effects of ischemic preconditioning on cyclinD1 expression during early ischemic reperfusion in rats. World J Gastroenterol. 2006;12:2936-2940. |
| 5. | Nan KJ, Jing Z, Gong L. Expression and altered subcellular localization of the cyclin-dependent kinase inhibitor p27Kip1 in hepatocellular carcinoma. World J Gastroenterol. 2004;10:1425-1430. |
| 6. | Fukunaga M. Immunohistochemical characterization of cyclin E and p27KIP1 expression in early hydatidiform moles. Int J Gynecol Pathol. 2004;23:259-264. |
| 7. | Ganoth D, Bornstein G, Ko TK, Larsen B, Tyers M, Pagano M, Hershko A. The cell-cycle regulatory protein Cks1 is required for SCF(Skp2)-mediated ubiquitinylation of p27. Nat Cell Biol. 2001;3:321-324. |
| 8. | Troncone G, Martinez JC, Iaccarino A, Zeppa P, Caleo A, Russo M, Migliaccio I, Motti ML, Califano D, Palmieri EA. p27Kip1 is expressed in proliferating cells in its form phosphorylated on threonine 187. BMC Clin Pathol. 2005;5:3. |
| 9. | Park KH, Seol JY, Kim TY, Yoo CG, Kim YW, Han SK, Shim YS, Lee CT. An adenovirus expressing mutant p27 showed more potent antitumor effects than adenovirus-p27 wild type. Cancer Res. 2001;61:6163-6169. |
| 10. | Hurteau JA, Brutkiewicz SA, Wang Q, Allison BM, Goebl MG, Harrington MA. Overexpression of a stabilized mutant form of the cyclin-dependent kinase inhibitor p27(Kip1) inhibits cell growth. Gynecol Oncol. 2002;86:19-23. |
| 11. | Sun ZQ, Xu SY, Deng CS, Zhang L, Tian L. Expression of p38 Protein and Its Clinical Significance in Human Colorectal Carcinomas. Yunyang Yixueyuan Xuebao. 2005;24:151-153. |
| 12. | Zhang YF, Wang ZR, Niu XJ, Wang L, Li HL. Expression of MMP-9 and CD44v6 in gallbladder carcinoma and implication. Zhonghua Shiyan Waike Zazhi. 2002;19:405-406. |
| 13. | Wan YL, Rong L, Liu YS, Wang X, Wu T, Pan YS, Yao HW, Ye JM, Tang JQ, Zhou J. The role of tissue factor in the invasion of colorectal carcinoma and its relationship with matrix metalloproteinase-9. Zhonghua Shiyan Waike Zazhi. 2004;21:1087-1088. |