Published online Jul 28, 2009. doi: 10.3748/wjg.15.3511
Revised: June 10, 2009
Accepted: June 17, 2009
Published online: July 28, 2009
AIM: To determine the prevalence and possible risk factors of Barrett’s esophagus (BE) in patients with chronic gastroesophageal reflux disease (GERD) in El Minya and Assuit, Upper Egypt.
METHODS: One thousand consecutive patients with chronic GERD symptoms were included in the study over 2 years. They were subjected to history taking including a questionnaire for GERD symptoms, clinical examination and upper digestive tract endoscopy. Endoscopic signs suggestive of columnar-lined esophagus (CLE) were defined as mucosal tongues or an upward shift of the squamocolumnar junction. BE was diagnosed by pathological examination when specialized intestinal metaplasia was detected histologically in suspected CLE. pH was monitored in 40 patients.
RESULTS: BE was present in 7.3% of patients with chronic GERD symptoms, with a mean age of 48.3 ± 8.2 years, which was significantly higher than patients with GERD without BE (37.4 ± 13.6 years). Adenocarcinoma was detected in eight cases (0.8%), six of them in BE patients. There was no significant difference between patients with BE and GERD regarding sex, smoking, alcohol consumption or symptoms of GERD. Patients with BE had significantly longer esophageal acid exposure time in the supine position, measured by pH monitoring.
CONCLUSION: The prevalence of BE in patients with GERD who were referred for endoscopy was 7.3%. BE seems to be associated with older age and more in patients with nocturnal gastroesophageal reflux.
- Citation: Fouad YM, Makhlouf MM, Tawfik HM, Amin HE, Ghany WA, El-khayat HR. Barrett’s esophagus: Prevalence and risk factors in patients with chronic GERD in Upper Egypt. World J Gastroenterol 2009; 15(28): 3511-3515
- URL: https://www.wjgnet.com/1007-9327/full/v15/i28/3511.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.3511
Barrett’s esophagus (BE) was first identified by N.R. Barrett in 1950, who described the replacement of the normal squamous mucosa of the distal esophagus by a columnar epithelium of both gastric and intestinal types[12]. The definition of BE has been modified over subsequent years to include only intestinal metaplasia within the tubular esophagus.
The exact cause of BE remains unclear. A popular explanation for the occurrence of BE is that it results from mucosal damage caused by gastroesophageal reflux[34]. When visible upon upper gastrointestinal endoscopy, this mucosal damage is termed erosive esophagitis. However, not all patients with gastroesophageal reflux and erosive esophagitis go on to develop BE, and not all patients with BE have a history of gastroesophageal reflux[5]. In most patients with reflux esophagitis, the epithelium heals through regeneration of the normal squamous lining[6]. Other patients, however, will develop BE with the risk of ultimately progressing to esophageal adenocarcinoma (EAC)[7].
The prevalence of BE varies in different geographic areas worldwide. Multiple risk factors for the development of BE besides reflux have been studied, including being Caucasian and/or male, a history of smoking, and hiatus hernia[8].
The aim of this study was to determine the prevalence and possible risk factors of BE in patients with chronic gastroesophageal reflux disease (GERD) symptoms in Upper Egypt.
This study was carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association. All patients provided informed written consent.
The study was performed at two clinical centers in Upper Egypt (Southern part of Egypt): the endoscopy units of the Department of Tropical Medicine at El Minya University, and the Department of Internal Medicine at Assuit University, from January 2006 to January 2008. Both centers are referral centers for a large number of patients in Upper Egypt.
In a prospective manner, 1000 consecutive patients with chronic GERD symptoms were recruited. All patients had a history of longstanding heartburn and or regurgitation for at least three times weekly for the last year. A questionnaire was completed by every patient, including age, sex, occupation, smoking and alcohol consumption. The symptom questionnaire also included the following criteria: primary referral symptom; frequency of GERD symptoms such as heartburn, regurgitation, and acid taste; extra esophageal symptoms; and history of systemic diseases such as scleroderma and diabetes.
Endoscopic examination was performed using an Olympus Evis CLV-U 200 Videoscope (Olympus, Japan). All patients were examined in our units by a well-trained endoscopist. BE was diagnosed by the presence of columnar-lined esophagus at endoscopy and the confirmed presence of intestinal metaplasia upon biopsy. In addition, information on the presence of intestinal metaplasia, evidence of dysplasia (a premalignant condition characterized by increased cell growth, cellular atypia, and altered cell differentiation) and its severity, and the presence of coexistent EAC was obtained from histopathology records. We defined short-segment BE by the presence of less than 3 cm of columnar-lined esophagus at endoscopy. The distinction between long- and short-segment BE was made. We also recorded the presence of esophagitis or any esophageal lesions. Repeat endoscopy was done in patients with erosive esophagitis after complete healing, to confirm the presence of BE. Then the patients were classified into two groups according to presence or absence of BE.
Fresh endoscopic biopsy samples were obtained from the operating theatre and fixed in 10% formalin within 13 h at room temperature. Tissues were subjected to a series of processing steps, which included fixation, dehydration with ethanol, clearing with xylene, and wax impregnation with paraffin, and then stained with hematoxylin and eosin (HE).
Ambulatory pH monitoring was done for 40 patients (20 patients with BE and 20 without). All patients were studied with ambulatory pH monitoring using an antimony pH electrode placed 5 cm above the proximal border of the manometrically located lower esophageal sphincter, and another electrode placed 10 cm above this point in the proximal esophagus, connected to a portable Digitrapper (Synectics, San Antonio, TX, USA) data storage unit. Intraesophageal pH was recorded continuously, with sampling obtained every 4 s. All pH tracings were analyzed for the percentage time that the distal and proximal esophageal pH was < 4, determined for the upright and recumbent time periods in each study. Average esophageal acid clearance (EAC) time was calculated for each patient by dividing the total time (in minutes) that distal esophageal pH remained < 4 by the total number of GERD episodes. This was calculated for both the upright and supine periods.
Statistical analyses were performed using Stats Direct version 2.2.5 statistical software (Stats Direct Ltd., Sale, Cheshire, UK) and SPSS for Windows version 11.5 (SPSS, Inc., Chicago, IL, USA). All data are presented as means ± SD. χ2 and Fisher’s tests were used to compare BE and GERD groups according to sex, smoking and alcohol consumption. Student’s t test was used for some factors. ANOVA was used for pH measurement analysis. Significance was accepted at P ≤ 0.05.
This study included 1000 patients with chronic GERD symptoms (764 male and 236 female) with a mean age of 38.81 ± 14.52 years. Seventy-three of these patients (7.3%) had BE suspected endoscopically and detected histopathologically. The remaining 927 patients (92.7%) were negative for BE. Accordingly, we classified the patients into two groups: group A included patients with BE and group B included those with chronic GERD without BE. The mean length of BE was 5.3 ± 2.6 cm. Short-segment BE was present in 61 patients (84%), while long-segment BE was present in 12 (16%). Four cases with BE were detected in endoscopy-negative patients, while the remainder was detected in patients with esophagitis. EAC was detected in eight patients (six in group A and two in group B).
Regarding age and sex, the mean age of patients in group A (48.3 ± 8.2 years) was significantly older than that in group B (37.6 ± 13.4 years) (P < 0.05), and there was no significant difference regarding sex between the groups (Table 1). Although smoking and alcohol consumption were more frequent in the BE group, there was no significant difference from those without BE. Also, there was no significant difference detected between the groups regarding GERD symptoms (Table 1).
| Group A | Group B | P value | |
| Age (yr, mean ± SD) | 48.3 ± 8.2 | 37.6 ± 13.4 | < 0.05 |
| Sex | NS | ||
| Male | 68 (93) | 785 (85) | |
| Female | 5 (7) | 142 (15) | |
| Total | 73 | 927 | |
| Smoking (508 patients) | |||
| Smokers | 45 (61.6) | 463 (49.9) | NS |
| Non-smokers | 28 (38.4) | 464 (50.1) | |
| Alcohol consumption (60 patients) | |||
| Alcoholic | 6 (8) | 54 (6) | NS |
| Non-alcoholic | 67 (92) | 871 (94) | |
| Main symptoms | |||
| Heartburn | 71 (97) | 908 (98.1) | NS |
| Regurgitation | 61 (83.6) | 769 (82.9) | NS |
| Dyspepsia | 58 (80) | 742 (80.1) | NS |
| Epigastric pain | 58 (80) | 797 (86.2) | NS |
| Dysphagia | 23 (31.5) | 222 (24) | NS |
| Endoscopic findings | |||
| Hiatus hernia | 29 (39.7) | 389 (42.1) | 0.8 (NS) |
| Gastritis | 53 (72.7) | 667 (71.6) | 0.8 (NS) |
| Duodenitis | 21 (28.7) | 297 (31.8) | 0.7 (NS) |
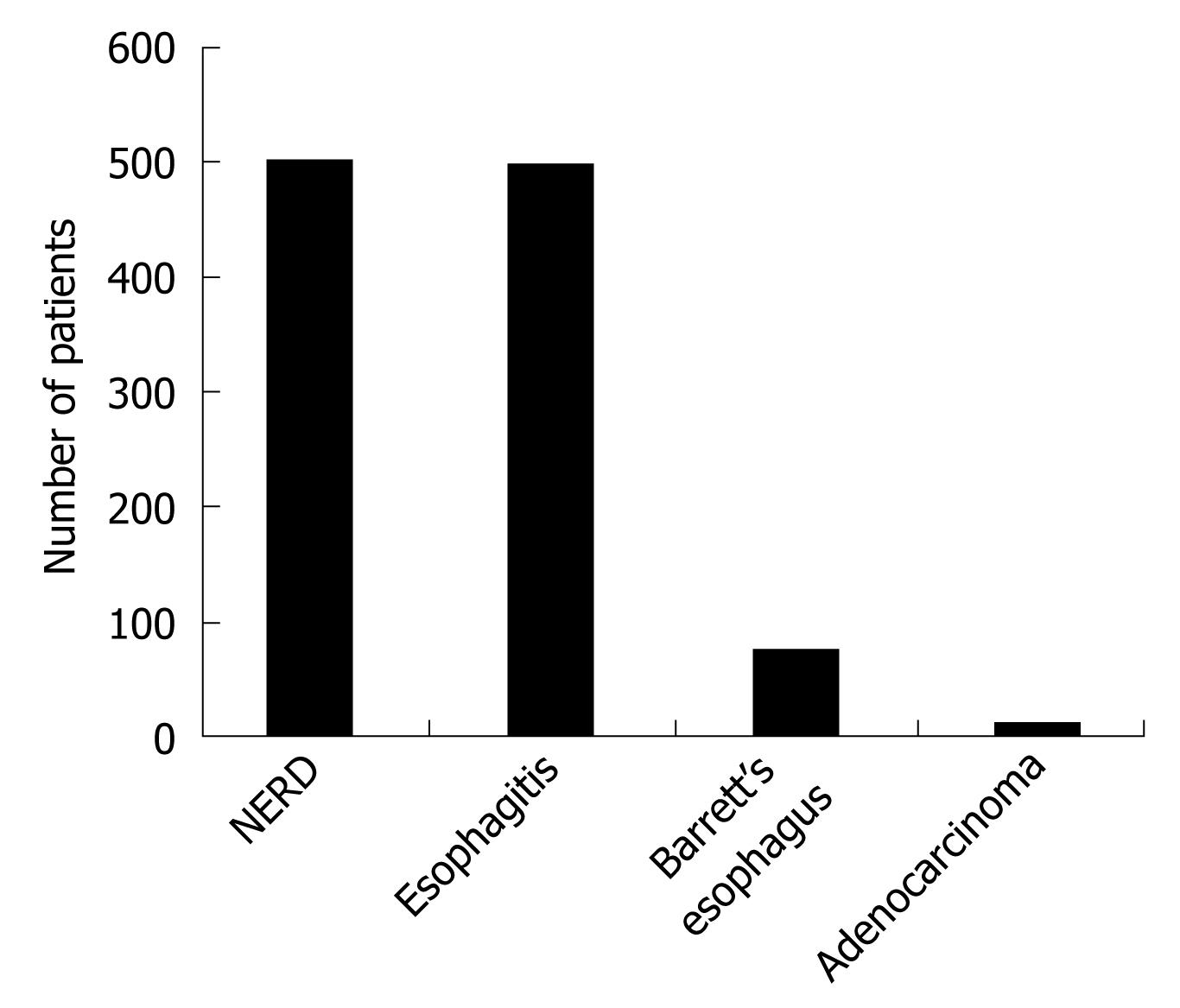
Endoscopic examination detected esophagitis in 498 patients (49.8%) and non-erosive reflux disease (NERD) was seen in 502 patients (Figure 1). Only four cases with BE were seen among patients with NERD.
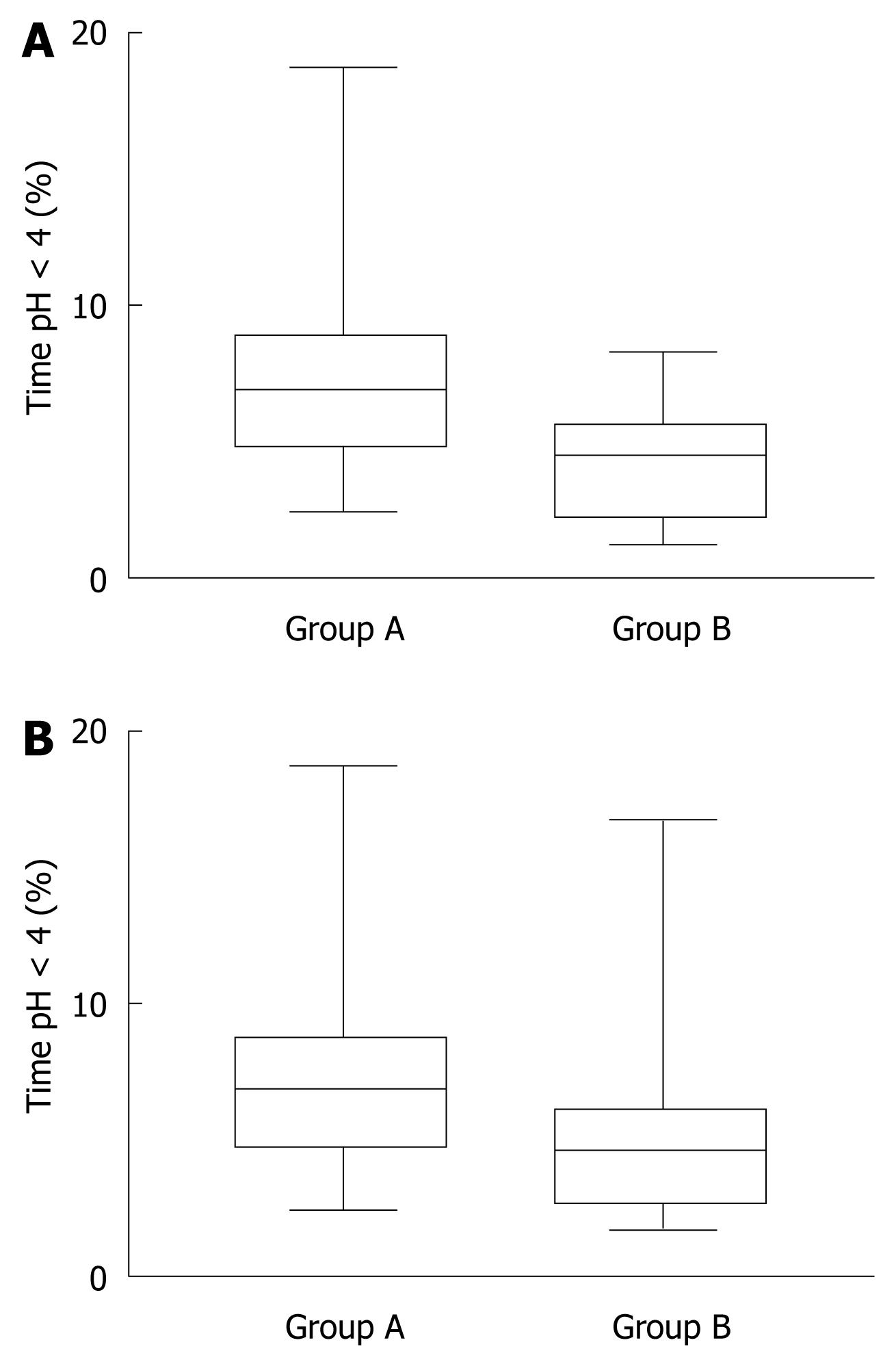
The percentage of patients with abnormal esophageal acid exposure was higher in the supine position in group A (16 patients, 80%) than in group B (nine patients, 45%). The median time at pH < 4 in the distal esophagus in the supine position was significantly longer in group A than group B, while no significant difference was detected in the upright position (daytime) between the groups (Figure 2A and B).
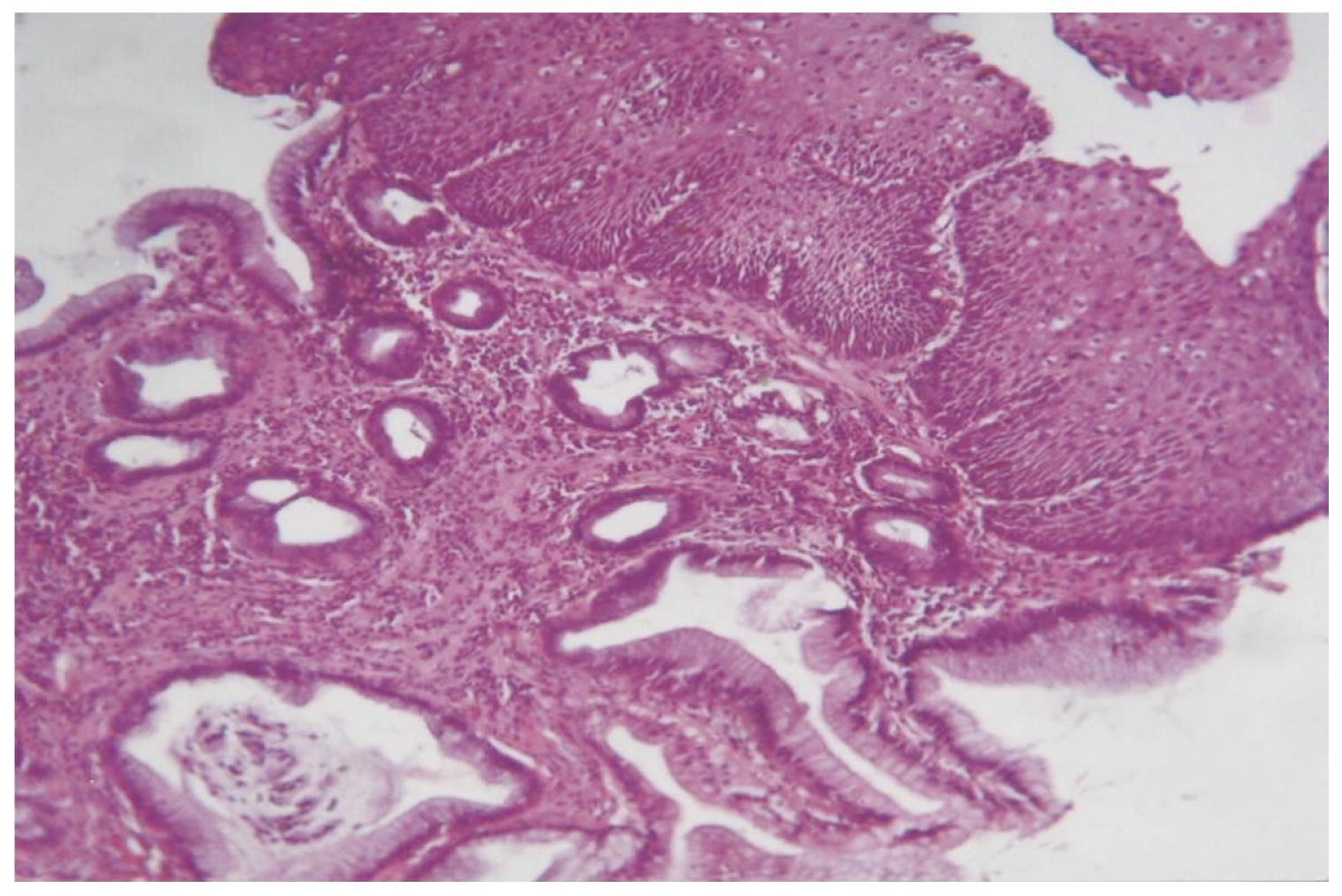
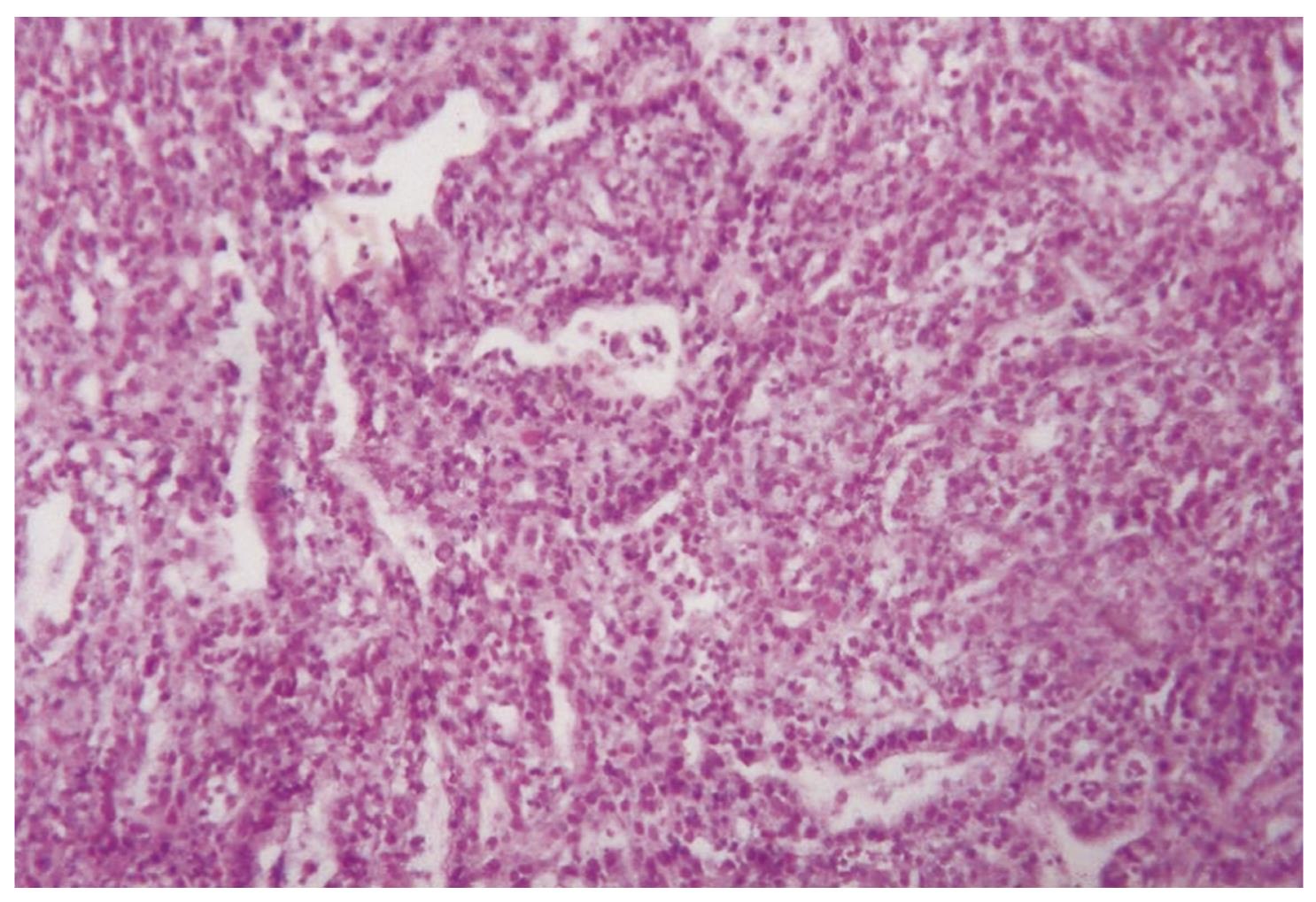
The histopathology of BE and EAC is shown in Figures 3 and 4.
To the best of our knowledge, this is the first study reporting the prevalence of BE in patients with chronic GERD symptoms in Upper Egypt. We recruited patients with GERD symptoms. We could not perform the study on the general population as it is difficult to convince asymptomatic people to undergo endoscopic procedures. One thousand consecutive patients were referred to endoscopy units and evaluated for chronic GERD symptoms by a well-trained endoscopist in a prospective manner.
The prevalence of BE was 7.3% in patients with GERD symptoms. Taking into considerations the type of subjects included, the prevalence could have been much lower in the general population because of the known association of BE and GERD. In Northern Egypt, Hak et al[9] have found a prevalence rate of 9.9% of BE in patients with GERD, which agrees with the results of our study in the Southern part of Egypt (Upper Egypt). Hak et al[9] recruited symptomatic patients with GERD, but with an emphasis on the effect of acid and bile reflux on the esophageal mucosa.
The prevalence of BE varies around the world and it seems to be higher in western than eastern countries. Focusing on patients who presented for their initial endoscopy in the setting of suspected GERD, Westhoff et al[8] studied 378 consecutive patients who had biopsies taken from areas suspicious for BE. The overall prevalence of BE was found to be 13.2%. The majority of patients diagnosed had short-segment BE, which agreed with previous data that showed the prevalence of endoscopically recognizable short-segment BE at 5%-7% vs 1%-3.4% for long-segment BE[910].
Ronkainen and colleagues have used a population-based study to estimate the prevalence of BE in Sweden. Of 19 000 subjects within a target age range of 20-80 years, a random sample of 3000 was surveyed by questionnaire. A random sub-sample of 1000 subjects then underwent upper digestive system endoscopy, in which an overall BE prevalence of 1.6% was observed. However, when reflux symptoms were present, the prevalence rose to 2.3%[11]. In another study in Korea, Kim et al[12] have found that, in the general population, the prevalence of BE was < 1%, and remained less common in Korea than in western countries.
In our study, the prevalence of BE did not differ significantly between men and women, although the number of men recruited was much higher than women. Probably, men have more reflux symptoms or seek medical advice and endoscopic evaluation more than women do. Lin et al[13] have studied 543 patients with GERD symptoms, and have shown that while male and female patients demonstrated an equal severity of erosive esophagitis, only 14% of female patients had BE, compared to 23% of male patients (P < 0.05). However, Banki et al[14] have shown that there was an equal prevalence of BE in men and women diagnosed with severe reflux by 24-h pH monitoring. A chart review of almost 22 000 first endoscopies identified 492 patients with BE, and suggested that there was a 20-year age shift between men and women in prevalence patterns, which resulted in a male to female OR of 4.15 (95% CI: 2.99-5.77)[15].
In our study, the mean age in patients with BE was significantly older than in those without BE. Other studies have demonstrated that increased age is a risk factor for developing BE, as well EAC[1617].
We found no significant difference between the groups regarding smoking and alcohol consumption, but it seemed that the number of smokers was high in both groups. While Ronkainen et al[11] and Kim et al[12] have found that alcohol consumption and smoking are significant risk factors, others have shown no significant importance of alcohol consumption and smoking in patients with BE[18–20].
Trying to explore the pattern of acid reflux in patients with BE, we found a significant difference between patients with BE and those with GERD for night-time acid reflux, which was more evident in patients with BE. The nocturnal gastroesophageal reflux that occurs in the recumbent position causes more injury to esophageal mucosa and may contribute to more severe chronic esophageal mucosal changes. Hak et al[9] have found more prolonged reflux periods in patients with BE than in those with GERD or NERD without BE. Gutschow et al[21] have reported that patients with BE have significantly more acid reflux events and a higher percentage of reflux time during the supine and upright phase than patients with NERD and GERD without BE. Also, Koek et al[22] in a multivariate analysis have found that BE is associated with male sex and exposure to both acid and duodenogastroesophageal reflux.
We conclude that BE is present in about 7.3% of patients with chronic GERD symptoms in our area. It may be associated with older age and nocturnal gastroesophageal reflux.
Barrett’s esophagus (BE) is gaining importance because of its association with esophageal adenocarcinoma (EAC). Its prevalence varies around the world. However, there are no data about its prevalence in upper Egypt.
This is the first study to report prevalence of BE in Upper Egypt.
This study may represent a future strategy for screening for BE and AEC in patients with chronic gastroesophageal reflux disease.
This was a well-conducted and interesting study that investigated the prevalence of BE in Upper Egypt.
| 1. | Barrett NR. Chronic peptic ulcer of the oesophagus and 'oesophagitis'. Br J Surg. 1950;38:175-182. |
| 3. | Winters C Jr, Spurling TJ, Chobanian SJ, Curtis DJ, Esposito RL, Hacker JF 3rd, Johnson DA, Cruess DF, Cotelingam JD, Gurney MS. Barrett's esophagus. A prevalent, occult complication of gastroesophageal reflux disease. Gastroenterology. 1987;92:118-124. |
| 4. | Fass R, Sampliner RE. Barrett's esophagus and other mucosal evidence of reflux in asymptomatic subjects with abnormal 24-hour esophageal pH monitoring. Dig Dis Sci. 1994;39:423-425. |
| 5. | Locke GR, Zinsmeister AR, Talley NJ. Can symptoms predict endoscopic findings in GERD? Gastrointest Endosc. 2003;58:661-670. |
| 6. | Spechler SJ. The natural history of dysplasia and cancer in esophagitis and Barrett esophagus. J Clin Gastroenterol. 2003;36:S2-S5; discussion S26-S28. |
| 7. | Spechler SJ, Goyal RK; Barrett's esophagus: pathophysiology, diagnosis, and management. Elsevier Science: New York 1985; 1-221. |
| 8. | Westhoff B, Brotze S, Weston A, McElhinney C, Cherian R, Mayo MS, Smith HJ, Sharma P. The frequency of Barrett's esophagus in high-risk patients with chronic GERD. Gastrointest Endosc. 2005;61:226-231. |
| 9. | Hak NG, Mostafa M, Salah T, El-Hemaly M, Haleem M, Abd El-Raouf A, Hamdy E. Acid and bile reflux in erosive reflux disease, non-erosive reflux disease and Barrett's esophagus. Hepatogastroenterology. 2008;55:442-447. |
| 10. | Mashimo H, Wagh MS, Goyal RK. Surveillance and screening for Barrett esophagus and adenocarcinoma. J Clin Gastroenterol. 2005;39:S33-S41. |
| 11. | Ronkainen J, Aro P, Storskrubb T, Johansson SE, Lind T, Bolling-Sternevald E, Vieth M, Stolte M, Talley NJ, Agréus L. Prevalence of Barrett's esophagus in the general population: an endoscopic study. Gastroenterology. 2005;129:1825-1831. |
| 12. | Kim JH, Rhee PL, Lee JH, Lee H, Choi YS, Son HJ, Kim JJ, Rhee JC. Prevalence and risk factors of Barrett's esophagus in Korea. J Gastroenterol Hepatol. 2007;22:908-912. |
| 13. | Lin M, Gerson LB, Lascar R, Davila M, Triadafilopoulos G. Features of gastroesophageal reflux disease in women. Am J Gastroenterol. 2004;99:1442-1447. |
| 14. | Banki F, Demeester SR, Mason RJ, Campos G, Hagen JA, Peters JH, Bremner CG, Demeester TR. Barrett's esophagus in females: a comparative analysis of risk factors in females and males. Am J Gastroenterol. 2005;100:560-567. |
| 15. | van Blankenstein M, Looman CW, Johnston BJ, Caygill CP. Age and sex distribution of the prevalence of Barrett's esophagus found in a primary referral endoscopy center. Am J Gastroenterol. 2005;100:568-576. |
| 16. | Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825-831. |
| 17. | Cameron AJ. Epidemiology of columnar-lined esophagus and adenocarcinoma. Gastroenterol Clin North Am. 1997;26:487-494. |
| 18. | Robertson CS, Mayberry JF, Nicholson DA, James PD, Atkinson M. Value of endoscopic surveillance in the detection of neoplastic change in Barrett's oesophagus. Br J Surg. 1988;75:760-763. |
| 19. | Gray MR, Donnelly RJ, Kingsnorth AN. The role of smoking and alcohol in metaplasia and cancer risk in Barrett's columnar lined oesophagus. Gut. 1993;34:727-731. |
| 20. | Ritenbaugh C, Sampliner R, Aickin M, Garewal H, Meyskens F. Risk factors for Barrett's oesophagus: a life history approach to behavioural assessment in the distant past. Eur J Cancer Prev. 1995;4:459-468. |
| 21. | Gutschow CA, Bludau M, Vallböhmer D, Schröder W, Bollschweiler E, Hölscher AH. NERD, GERD, and Barrett's esophagus: role of acid and non-acid reflux revisited with combined pH-impedance monitoring. Dig Dis Sci. 2008;53:3076-3081. |