Published online Jul 21, 2009. doi: 10.3748/wjg.15.3341
Revised: May 27, 2009
Accepted: June 3, 2009
Published online: July 21, 2009
Diarrhoea is an alteration of normal bowel movement characterized by an increase in the water content, volume, or frequency of stools. Diarrhoea needs to be classified according to the trends over time (acute or chronic) and to the characteristics of the stools (watery, fatty, inflammatory). Secretory diarrhoeas, mostly acute and of viral aetiology in more than 70% of cases, are by far the most important subtype of diarrhoeas in terms of frequency, incidence and mortality (over 2.5 million deaths/year in developing countries). Natural and synthetic opiates such as morphine, codeine, and loperamide which react with endogenous opiates (enkephalins, beta-endorphins, dynorphins) mainly act on intestinal motility and slow down transit. An antidiarrhoeal drug developed in recent years, racecadotril, acts as an enkephalinase inhibitor. Clinical studies have shown that it is just as effective as loperamide in resolving acute diarrhoea but with greater reduction in pain and abdominal distension. Some studies have explored the prevalence of diarrhoea in old age. An epidemiological study carried out in Italy by 133 General Practitioners on 5515 elderly outpatients reported a prevalence of diarrhoea, defined according to the Rome criteria, of 9.1%. Infectious diseases (19%) and drug use (16%) were the most common causes of diarrhoea in old age. Regardless of the cause, the treatment of elderly patients with diarrhoea must include rehydration and nutritional support. Every year, more than 50 million tourists travel from industrialized countries to places where hygiene levels are poor. At least 75% of those travelling for short periods mention health problems, and in particular traveller’s diarrhoea.
- Citation: Baldi F, Bianco MA, Nardone G, Pilotto A, Zamparo E. Focus on acute diarrhoeal disease. World J Gastroenterol 2009; 15(27): 3341-3348
- URL: https://www.wjgnet.com/1007-9327/full/v15/i27/3341.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.3341
A decrease in consistency (i.e. soft or liquid) and an increase in frequency of bowel movements to > 3 stools per day has often been used as a definition of diarrhoea for epidemiological investigations. Diarrhoea is an alteration of normal bowel movement characterized by an increase in the water content, volume, or frequency of stools. Intestinal water balance results from a complex regulation involving inflammatory mediators (prostaglandins, leukotrienes, bradykinin, nitric oxide), hormones, neuropeptides, integrity of the intestinal wall, efficiency of the circulatory system and of the enteric nervous system[1].
From a clinical point of view, diarrhoea needs to be classified taking into account certain characteristics such as trends over time (acute or chronic, using a limit of 4 wk to separate the two conditions) and the characteristics of the faeces (watery, fatty, inflammatory, etc)[2]. Using all these characteristics, a doctor can better understand the diarrhoea symptom and decide upon diagnosis and therapy more rationally. The duration of diarrhoea is important, because acute forms are usually due to some infectious agent, intoxication, or food allergy. However, acute diarrhoea may be a symptom of the onset of chronic organic or functional disease. Also important is a chemical/physical examination of the stools. Watery diarrhoea is a symptom of some defect in the re-absorption of water due to an imbalance between the secretion and absorption of electrolytes (secretory diarrhoea) or to the ingestion of substances which the intestine has failed to absorb (osmotic diarrhoea). Excessively fatty diarrhoea may be due to low intestinal absorption of lipids, which may be due in turn to poor digestion thereof, and inflammatory diarrhoea, if there is mucus and pus. The distinction between secretory and osmotic diarrhoea may be made clinically by trying to eliminate the various causes of osmotic diarrhoea, which are relatively few. The latter is due to the ingestion of salts (magnesium sulphate or phosphate) or polysaccharides (mannitol, sorbitol) which are not readily absorbed, or to some enzyme defect in the intestinal mucosa (e.g. a lack of lactase). Osmotic diarrhoea stops when the patient fasts, or when substances which cannot be readily absorbed are no longer ingested; secretory diarrhoea, however, continues even when the patient has stopped eating. Secretory diarrhoea may be caused by several factors, either endogenous or exogenous, which determine an imbalance between the absorption and secretion of electrolytes. Among the causes of secretory diarrhoea there are also intestinal motility abnormalities, both primitive and secondary to systemic neuro-endocrine or metabolic diseases. A significant proportion - usually about one third - of patients with irritable bowel syndrome (IBS) have diarrhoea as their main symptom. The clinical characteristics of this type of diarrhoea, called “functional,” are its periodicity, its occurrence solely during the day, after meals, and faecal urgency or incontinence. The pathogenic mechanisms most often seen in these patients are stress (via central or peripheral mediators, the most important of which is serotonin), food factors (allergies) and hormones (oestrogen, prostaglandin)[3].
Secretory diarrhoeas, mostly acute and due to infections (bacteria, viruses, parasites), are by far the most important subtype of diarrhoeas in terms of frequency, incidence and mortality (over 2.5 million deaths/year). In developing countries, they represent the primary cause of child mortality, whereas in developed and developing countries alike secretory diarrhoeas are an important cause of hospitalisation and health expenditure. It is estimated that about 200-300 million new cases occur yearly in the USA with 900 000 hospital admissions and an overall expenditure of about 23 million dollars[4]. Despite these numbers, the true prevalence of infectious diarrhoea is probably underestimated since the pathogen may not be searched for in stool samples or the patient may not seek medical or hospital attention[5]. A Canadian study showed that only 22% of patients with diarrhoea seek medical attention and only 5% of these has a stool examination[5]. Infectious diarrhoeas are of viral aetiology in more than 70% of cases. Rotavirus is the major cause of infantile gastroenteritis and each year causes 600 000-800 000 deaths worldwide[6]. The virus infects the mature enterocytes of the villus tip of the small intestine and induces watery diarrhoea. Rotavirus impairs activities of intestinal disaccharidases and Na+-solute transport and inhibits water reabsorption through the production of NSP4 enterotoxin. An additional secretion component is due to activation of the enteric nervous system, producing an increased chloride secretory response. The other viral etiologic agent is Norovirus which exerts a direct action on the activity of enzymes of the brush border[7].
Bacterial aetiology occurs in 1.5%-5.6% of cases. The most frequently identified bacteria are Campylobacter (2.3%), Salmonella (1.8%), Shigella (1.1%) or Escherichia Coli (0.4%). Symptoms such as fever and bloody diarrhoea are strongly suggestive of the presence of an invasive bacterium (Shigella spp, Salmonella spp, Camp. jejuni, Clostridium difficile). These orally ingested micro-organisms overtake immune defences and adhere to the intestinal wall. Subsequently they alter the metabolism of the cell by penetrating the cell, either directly or through the production of toxins. Both toxins and bacteria cause cell death and can invade bloody circulation with systemic symptoms such as fever, chills, hypotension, nausea and vomiting. Depending on the pathogenetic mechanism, infectious bacterial diarrhoeas can be divided in cytotonic (pathogens stimulate secretory function by activating intracellular enzymes without damaging the epithelial layer e.g. Vibrio cholerae, some strains of E. coli, Bacillus cereus) and cytotoxic (pathogens damage directly epithelial cell e.g. Shigella, C. perfringens, C. difficile, Staphylococcus aureus, Salmonella and Campylobacter)[8].
A striking example of infectious diarrhoea of bacterial origin is that caused by V. cholerae, a Gram-negative bacterium causing a severe acute watery diarrhoea burdened with a 25%-50% mortality rate[9]. Every year, more than 100 000 cholera cases and 2000-3000 deaths are officially reported to WHO. Cholera toxin activates adenylate cyclase and elevated intracellular cAMP levels provoke loss of water and electrolytes which is manifested as the typical diarrhoea. The massive outpouring of electrolyte-rich isotonic fluid into the bowel can lead to volume depletion and shock, followed by renal and cardiac failure. Rehydration therapy, either intravenous or oral, considerably decreases the number of deaths from 25%-50% to less than 1%[9].
E. coli, a normal saprophyte of the gastrointestinal tract, is another important cause of acute infectious diarrhoea[10]. According to pathogenetic mechanisms, as well as clinical syndromes, 5 strains can be distinguished (enterotoxigenic E. coli, enteropathogenic E. coli, enterohaemorrhagic E. coli, enteroinvasive E. coli, enteroaggregative E. coli). Transmission is via the orofaecal route or via direct person-to-person contact.
Shigellosis is a major cause of diarrhoea-related morbidity and mortality, especially in developing countries, with an estimated annual incidence of 165 million cases and 1 million deaths[11]. Transmission usually occurs via contaminated food and water or through person-to-person contact. Shigella bacteria multiply within colonic epithelial cells causing inflammation, mucosal ulceration, and bleeding. The symptoms of shigellosis include diarrhoea and/or dysentery with frequent mucoid bloody stools, abdominal cramps and tenesmus. The severity of the clinical picture is directly related to the infecting strain; Sh. sonnei causes mild diarrhoea, whereas Sh. dysenteriae and Sh. flexneri usually cause mucoid bloody diarrhoea[12].
C. difficile is an important nosocomial pathogen and the most frequently diagnosed cause of infectious hospital-acquired diarrhoea[13]. The causative organism is acquired by the oral route from an environmental source or by contact with an infected person or a health care worker who serves as a vector. Disruption of the bowel microflora, generally by antibiotics (clindamycin, cephalosporine and chinolonics) creates an environment that allows C. difficile to proliferate. Toxigenic strains usually produce toxin A and toxin B, which cause intense inflammation of the colonic mucosa with fluid and electrolyte secretion[14]. The syndrome that results includes severe watery diarrhoea, fever, abdominal pain, and leukocytosis, sometimes complicated by toxic megacolon.
Salmonella species are Gram-negative aerobic/anaerobic bacilli that cause substantial morbidity, mortality and burden of disease globally. Salmonella can colonize both the small bowel and colon causing different clinical pictures. Typhoid fever (S. typhi and S. parathyphi) and enteritis (S. enteritidis and S. typhimurium) are the most common disease syndromes. Typhoid fever is particularly frequent in under-developed countries. In year 2000, a total of 21.6 million cases occurred with more than 216 000 deaths[15]. Intestinal parasitic infections account for 20%-25% of infectious diarrhoeas, which are mostly chronic and endemic in developing countries[6]. Giardia lamblia is the most frequent cause of parasitic diarrhoea in immuno-competent patients. Giardiasis is common in developing countries but also in industrialized countries e.g. endemic areas in Russia[16]. G. lamblia, the cause of human giardiasis, is among the most common intestinal protozoa worldwide. Human infection may range from asymptomatic shedding of giardial cysts (60% of cases) to symptomatic giardiasis, causing abdominal cramps, nausea, acute or chronic diarrhoea, with malabsorption and failure of children to thrive. Factors affecting different clinical manifestations include virulence of the strain, amount of parasites ingested, and host immune response. Quite interestingly, giardiasis does not induce leukocytosis or eosinophilia.
Amoebiasis is the second leading cause of death from parasitic disease worldwide. The causative protozoan parasite, Entamoeba histolytica, is a potent pathogen infecting about 50 million people and resulting in 40 000 deaths per year[17]. The infection prevails in developing countries, particularly India, Africa, Mexico and South America. People at risk of infection include immigrants, travellers returning from countries of high endemicity, and men who have sex with men. Clinical manifestations range from asymptomatic carriage to invasive disease (bloody diarrhoea), to extraintestinal disease with liver abscess.
Acute infectious diarrhoea is among the most common illnesses worldwide, particularly in developing countries, and its associated morbidity and mortality are greatest among those at the extremes of age.
The presence of leukocytes in the stools is extremely important for the differential diagnosis of infectious diarrhoea. Faecal leukocytes are present in patients with diarrhoea caused by Shigella, Campylobacter, enteroinvasive E. coli, and absent in cases of infection by V. cholerae, enterotoxigenic E. coli, Rotavirus, Norovirus, G. lamblia, Entamoeba histolytica, Staph. aureus, Clostridium.
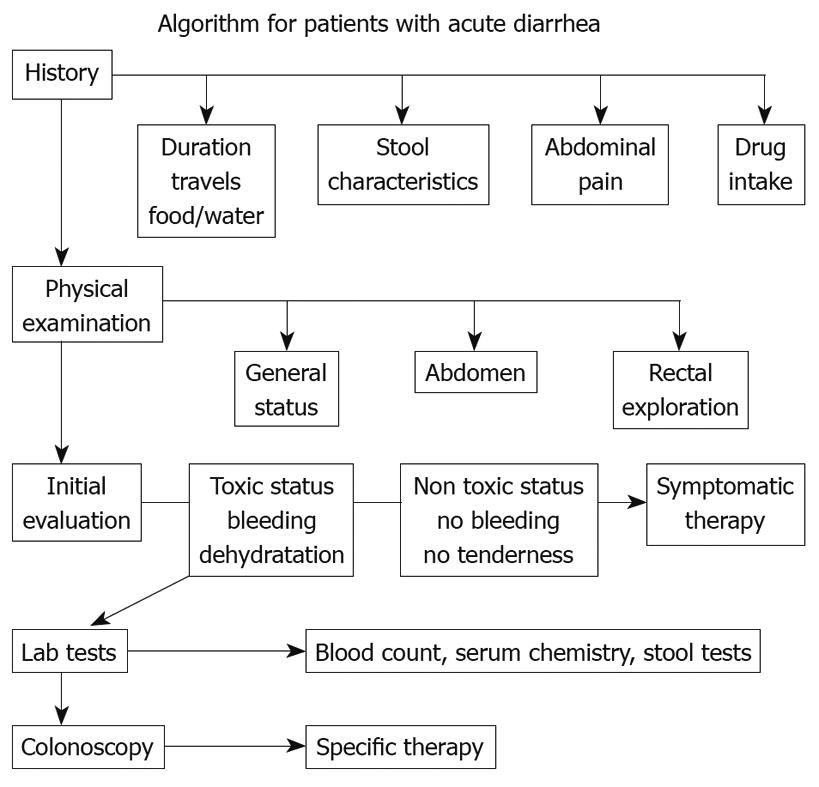
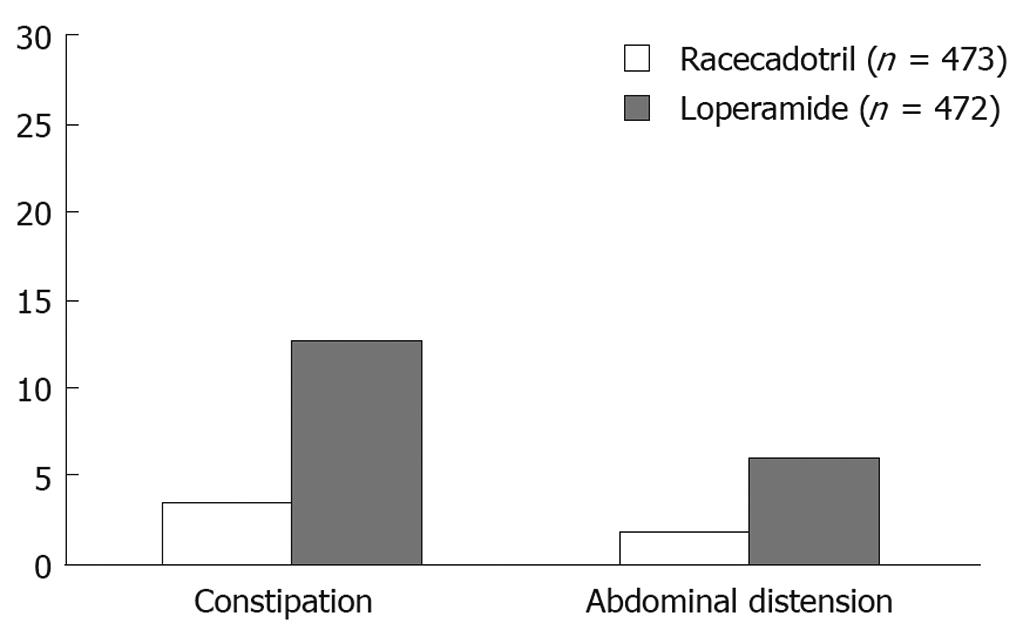
Clinical classification of diarrhoea and an understanding of the main pathogenic mechanisms that bring it about are vital for a diagnostic and therapeutic approach. The initial diagnostic strategy (Figure 1) in patients with acute diarrhoea is mainly based upon a proper collection of medical history and an evaluation of the patient’s clinical condition. This first diagnostic step often allows a good empirical therapy to be prescribed, whereas in patients with a poor general condition, or in those that do not respond to treatment, laboratory tests and, if required, an endoscopic examination of the intestine, must be undertaken. In both adults and children, re-hydration is essential in managing patients with acute diarrhoea. Millions of lives have been saved thanks to the introduction of oral re-hydration therapy but this therapeutic approach, although essential in correcting dehydration, does not resolve the process that is at the base of diarrhoea and does not change the volume of faeces and evacuation frequency. In patients where the diagnosis leads to ascertainment of the cause of diarrhoea, therapy is usually aimed at removing the identified cause or aetiological agent. In many patients, therapy must begin before all the diagnostic tests have been exhausted (empirical therapy), since acute diarrhoea can greatly affect the quality of a patient’s life and may compromise the health of children and elders. Anti-diarrhoeal therapy, known as symptomatic therapy, may be adopted alongside an aetiological therapy to improve the patient’s clinical condition. The rational basis for an empirical or symptomatic therapy is determined by the main pathogenetic mechanism, that is the inability of the intestine to re-absorb water. Table 1 lists drugs for treatment of diarrhoea. The most widely used drugs in treating the symptoms of diarrhoea are those containing substances which react with endogenous opiates. These are peptides present not only in the central nervous system but also in the EC cells and the nervous plexus of the intestinal wall. There are three main types of endogenous peptide opiates: (1) enkephalins, which are found in the epithelial cells of the intestine; when they bind with delta receptors, they reduce cAMP levels and thus reduce the secretion of water and electrolytes; (2) β-endorphins, which bind with the mu receptor and mostly act by blocking gastrointestinal motility; (3) dynorphins, which bind with kappa receptors and lower nociceptive sensitivity. Natural and synthetic opiates such as morphine, codeine, and loperamide, are ineffective as antidiarrhoeal drugs; however, they mainly act on intestinal motility and slow down transit. This is why they have some side-effects, such as secondary constipation and abdominal bloating, which, together with some effects on CNS, contraindicate their use in children and in the elderly. An understanding of the molecular and cellular mechanisms causing intestinal secretion has brought about developments in substances which can act selectively as anti-secretory drugs. Pharmacological research has recently been mainly oriented on enkephalins, which are endogenous opiates and are fairly widespread in the enteric nervous system. Enkephalinergic nerves reach the basolateral membrane of enterocytes where, via the delta receptors, they inhibit the action of adenylcyclase thus blocking the secretion of water and chloride. Enkephalins are rapidly broken down by a specific enzyme, enkephalinase, which determines the biological half-life of these peptides. In this context, racecadotril is an antidiarrhoeal drug developed in recent years. Racecadotril acts as an enkephalinase inhibitor thus promoting the anti-secretory action of enkephalins at the gastrointestinal level. After ingestion racecadotril is rapidly absorbed and becomes an active metabolite (Tiorfan). The anti-diarrhoeal action of this substance has been studied many times in pre-clinical and clinical trials, and it has been shown that it is purely anti-secretory. In fact, this drug has no effect on intestinal motility. Clinical studies[18–21] have shown that it is just as effective as loperamide in resolving acute diarrhoea but with greater reduction in pain and abdominal bloating and less secondary constipation (Table 2, Figure 2). Racecadotril therefore represents a safe and rational therapy for acute diarrhoea, and is easy to use either alone or together with an aetiological therapy. The particular features of this drug make it very effective and suitable for use in children as well as in geriatric age groups.
| Opiates | Drug | Dose |
| &mgr;-receptor agonists | Diphenoxylate | 2.5-5 mg qid |
| Loperamide | 2-4 mg qid | |
| Codeine | 15-60 mg qid | |
| Morphine | 2-20 mg qid | |
| Enkephalinase inhibitors δ-receptor | Racecadotril | 1.5 mg/kg tid |
| Adrenergic agonists | Clonidine | 0.1-0.3 mg tid |
| Somatostatin analogue | Octreotide | 30-250 &mgr;g tid |
| Bile acids binding substances | Cholestyramine | 4-16 g/die |
| Fibers | Psyllium | 10-20 g/die |
| Author, yr | Patients (n) | Study design | Time to resolution (h) | P |
| Frexinos J, 1996 | 574 | d.b. randomized | 28.9 vs 26.8 | NS |
| Vetel JM, 1999 | 147 | d.b. randomized | 14.9 vs 13.7 | NS |
| Prado D, 2002 | 945 | s.b. randomized | 55 vs 55 | NS |
| Wang HH, 2005 | 62 | s.b. randomized | 19 vs 13 | NS |
Other drug categories include: (1) somatostatin analogues (octreotide), which are used in carcinoid syndrome or in other endocrinous diarrhoeas; (2) adrenergic agonists, such as clonidine, which affect intestinal motility and transport; (3) chelating agents, such as colestyramine, which are used with diarrhoea caused by bile acids, such as in post-cholecystechtomy or maldigestion syndromes; (4) dietary food supplements, such as psyllium, which increase stool consistency and are useful for patients with incontinence.
Some studies have explored the prevalence of diarrhoea in old age but the data does not give homogeneous results. A previous study performed in 328 non-institutionalized elderly subjects from U.S.[22] reported a prevalence of diarrhoea of 14.2%. More recently, a cross-sectional survey carried out in Australia, Canada, Ireland and the U.S. reported a prevalence of diarrhoea of 3.9% in elderly subjects aged 65 years or more[23]. Differences in the definitions of diarrhoea and the methodology of study recruitment may explain the discrepancies in the results. Indeed, in the first study, collection of data was made through a mailed self-administered questionnaire and patients with diarrhoea included in the survey were determined according to the Rome criteria for functional diarrhoea, i.e. both “the subjects with a stool frequency of more than three stools per day” and “the subjects who passed loose or watery stool and/or with urgent need for defecation”. In the second study, diarrhoea was defined according to the World Health Organization definition as three loose stools or bowel movements in any 24 h period during the 4 wk before the interview. In this study, moreover, data were collected by telephone call, thus excluding persons who did not have access to a fixed line telephone in their home and therefore probably the older and more disabled elderly people.
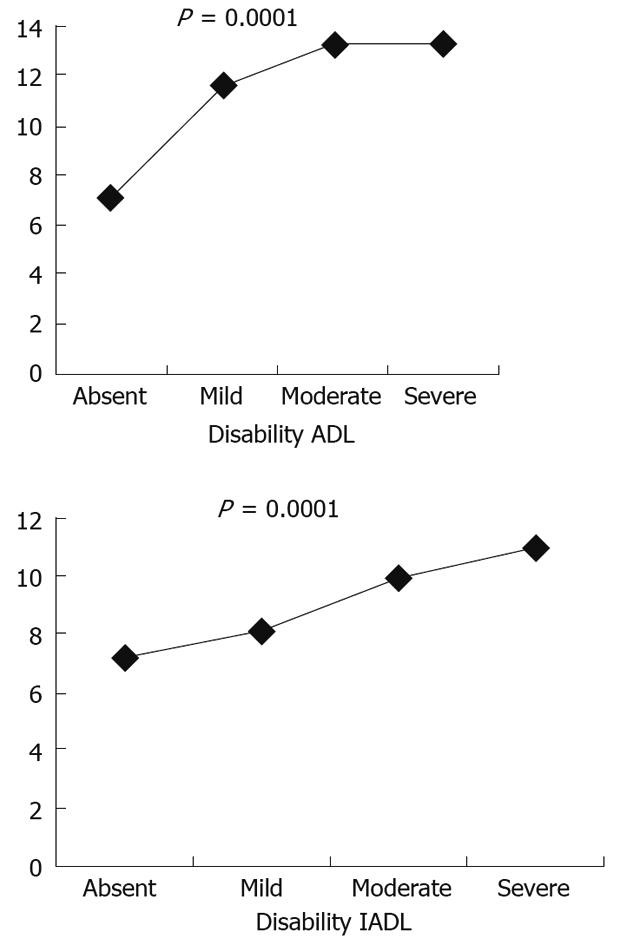
Very recently an epidemiological study carried out in Italy by 133 General Practitioners on 5515 elderly outpatients, reported a prevalence of diarrhoea, defined according to the Rome criteria, of 9.1%; the prevalence of diarrhoea significantly increased with an increase of both age and the grade of disability as evaluated by the ADL and the IADL (Figure 3). The study, moreover, demonstrated that, in the past 6 mo, elderly patients with diarrhoea underwent a significantly higher number of gastroenterological visits and instrumental examinations of the gastrointestinal tract such as barium X-ray, colonoscopies, abdominal ultrasound and CT than elderly subjects without diarrhoea. This finding is indirectly in agreement with previous studies supporting the concept that diarrhoea in old age may significantly impair the quality of life and functional status of affected individuals[24] and it may be a cause of morbidity and complications leading to a severe burden in hospitalized elderly patients[25].
A previous study carried out in the U.S. in hospitalized patients aged 70 years and over reported that infectious diseases (19%) and drug use (16%) were the most common causes of diarrhoea in old age. Gastrointestinal disorders, such as ischaemic colitis, malabsorption, diverticular disease, IBS and tumors of the colon and/or the small intestine accounted for about 15% of cases. Over 20% of diarrhoea observed in this population, however, was associated with the presence of constipation, and diarrhoea was a clinical expression of faecal incontinence in the presence of coprostasis[26].
Infectious diarrhoea: A study from the U.S. reported that Salmonella (16.1 cases/100 000 persons), Campylobacter (13.4 cases/100 000 persons), Shigella (10.3 cases/100 000 persons) and E. coli O157:H7 (1.7 cases/100 000 persons) were the pathogens most frequently associated with diarrhoea. Vibrio, Yersinia, Listeria and Cyclospora were found in less than one case per 100 000 subjects[8]. A positive stool culture was found in 1.5%-5.6% of cases when performed three days before the beginning of symptoms. After 72 h from the beginning of diarrhoea, the diagnostic accuracy of the stool culture was less than 0.8%. Thus the microbiologic evaluation of hospitalized patients or those with recent exposure to antibiotics in whom diarrhoea develops should focus on the diagnosis of toxigenic C. difficile, the most common cause of nosocomial diarrhoea. Testing for other pathogens in patients who have been hospitalized for more than 72 h is discouraged, except in elderly patients 65 years of age or older with comorbidity, patients with Human Immunodeficiency Virus infection, patients with neutropenia and patients with suspected systemic spread of enteric infection[27].
In elderly patients who have taken antibiotics the most frequent cause of infectious diarrhoea is C. difficile. Hospitalization and institutionalization are independent risk factors for C. difficile infection; indeed, in these settings the infection may be observed in 30% of subjects, even if asymptomatic in over 2/3 of subjects, with a high rate (10%-20%) of relapses. In elderly subjects C. difficile infection may have a nonspecific clinical presentation, including hyperpyrexia, abdominal pain or leukocytosis, and is sometimes complicated by toxic megacolon and sepsis.
Drug-related diarrhoea: Diarrhoea is a relatively frequent adverse event, accounting for about 7% of all drug adverse effects[28]. More than 700 drugs have been implicated in causing diarrhoea. Several mechanisms have been reported to be involved in inducing drug-related diarrhea, such as altered gastrointestinal defences, mucosal damage of the small and large intestine and/or disruption of normal pathophysiological processes of fluid and electrolyte absorption and secretion; sometimes more than one mechanism may be involved[29].
A recent epidemiological survey carried out in Italy demonstrated that elderly patients with diarrhoea were taking a significantly higher number of drugs than patients without diarrhoea. Moreover, a significant increase in the prevalence of diarrhoea occurred in patients who were treated with a progressively higher number of drugs, reaching a prevalence of 11.0% in patients who were taking 3-5 drugs and a prevalence of 11.7% in patients who were taking 6 drugs or more. In this elderly population of outpatients, the drugs significantly associated with the presence of diarrhoea were antibiotics, proton pump inhibitors, allopurinol, psycholeptics, selective serotonin reuptake inhibitors and the antihypertensive angiotensin II receptor blockers.
Rehydration and nutrition: Regardless of the cause, the treatment of elderly patients with diarrhoea must include rehydration and nutritional support. Patients should be encouraged to drink fluids and take salts both in liquids and in crackers. If necessary i.v. electrolyte solutions may be used.
Antimicrobials: Since in over 90% of cases of diarrhoea the pathogen may not be identified, the clinical benefit of an empiric antibiotic treatment should be evaluated taking into account the risk of adverse event reactions and the risk of harmful eradication of normal flora. In elderly patients with community-acquired diarrhoea with fever, dysentery and severe clinical conditions, and in whom diarrhoea is not thought to be attributable to fluoroquinolone-resistant bacteria, empirical treatment with an agent such as a fluoroquinolone is reasonable. Alternatively, treatment in severely ill elderly patients may include macrolides such as erythromycin or azithromycin[8]. The treatment of C. difficile-associated diarrhoea usually includes cessation of the initiating antibiotic. The choice to immediately re-treat the patient with another antibiotic is poor, supported by currently available evidence. Oral metronidazole is effective; vancomycin has been tested but it is more prone to serious adverse drug reactions. Recent evidence suggest that teicoplanin is better than vancomycin for bacteriological cure and that it has borderline greater efficacy in reducing symptomatology[30].
Symptomatic therapy: Over 300 over-the-counter products are currently used for their antidiarrhoeal properties; of these, only loperamide, bismuth subsalicylate and kaolin have been tested in controlled studies. Curiously, none of these studies have been carried out in elderly patients. Recently, a multicenter study carried out on 945 outpatients, reported that racecadotril, a potent inhibitor of enkephalinase which exerts an anti-hypersecretory effect without increasing intestinal transit time, was as effective as loperamide in reducing diarrhoea with a significantly lower prevalence of side effects such as constipation, anorexia and abdominal pain than loperamide[20].
Because of its characteristics, racecadotril can be an effective pharmacologic option in the treatment of diarrhoea in elderly patients; however, further studies are needed to extensively evaluate the role of racecadotril in the treatment of diarrhoea in elderly patients.
Every year, more than fifty million tourists travel from industrialized countries to places where hygiene levels are poor. At least 75% of those travelling for short periods mention health problems - mostly mild - but occasionally they require help from a doctor (7%) or even admission to a hospital (1%).
Traveller’s diarrhoea (TD) is the most frequent of these problems; it occurs during or immediately after a trip to a less hygienic country, lasts for 3-4 d, and is characterised by the elimination of watery or soft faeces.
TD is usually brought about by eating contaminated water or foodstuffs, and affects 20%-50% of those travelling to countries with poor hygiene standards. In certain geographical areas, up to 60% of tourists can be affected if they stay for longer than 2 wk.
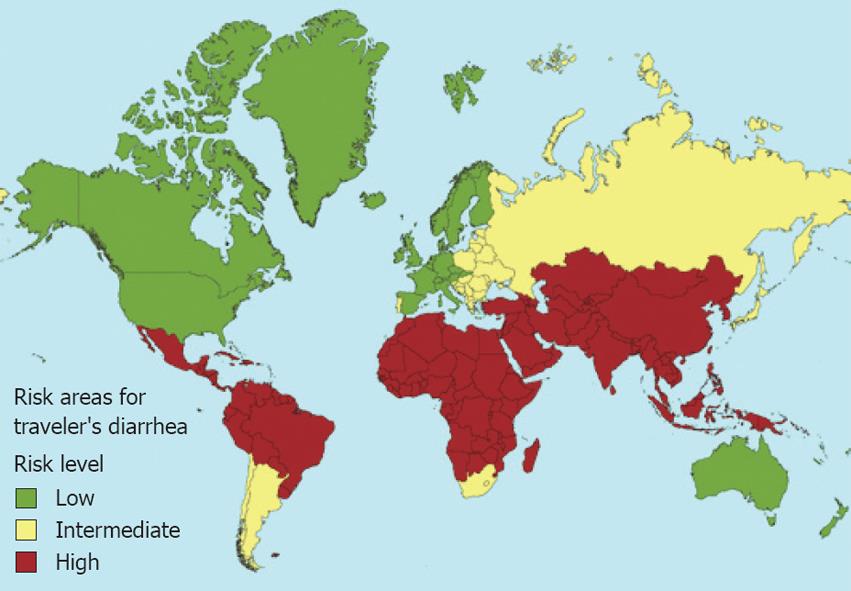
Based on hygiene conditions, there are 3 risk levels in the world: high risk [most of Asia, the Middle East, Turkey, Africa - except South Africa - Central America, and part of Latin America (20%-56%)], average risk (Eastern Europe, South Africa, some countries in Latin America, and some Caribbean islands) and low risk (Western Europe, the U.S., Canada, Australia, and Japan (4%-8%) (Figure 4).
The risk of contracting TD depends very closely upon the hygiene conditions in the country, and is very high for tourists from low-risk countries travelling to other countries (Table 3).
| Country of origin | Country of destination (%) | ||
| Low | Average | High | |
| Low | 2-4 | 10-20 | 20-40 |
| Average | 2-4 | Indeterminate | 901 |
| High | 2-4 | ND | 8-18 |
TD aetiology is varied, and includes viruses, bacteria, protozoans, and mycetes. The main aetiological agents of secretory diarrhoea are E. coli (enteropathogenic, enteroadhesive, enterotoxic), V. cholerae, C. difficile, Rotavirus or Norwalk astrovirus, and for dysentery-related diarrhoea they are E. coli (enteroinvasive, enterohaemorrhagic), Shigella spp, Camp. jejuni, S. typhi, S. paratyphi, other salmonellas, Aeromonas spp, Yersinia enterocolitica, non-cholera bacilli, Entamoeba histolytica, G. lamblia, Cryptosporidium, Cyclospora.
Risk depends greatly upon the foodstuffs consumed (Table 4). The place in which food is prepared is also essential: in fact, the risk increases from private homes to restaurants, to food purchased at the road side.
| Low risk (increasing) | High risk (decreasing) |
| Coffee, tea (served hot) | Puddings (> with unbaked creams) |
| Foods served at > 60°C | Tap water, ice |
| Fruit peeled by consumer | Cooked shellfish |
| Freshly-squeezed fruit | Cheese |
| Soft drinks in general | Cold collation |
| Bread | Spicy sauces |
| Bottled mineral water | Salads and raw vegetables |
| Butter | Milk |
When assessing risk, due account must be kept of certain variables which affect TD. Among the various predisposing factors are the type of trip, so an adventure holiday is more risky than one in a five-star hotel. Young people who do not eat at the table, especially children, seem to be at much greater risk than adults. There may also be certain genetic factors which might explain why some people are more easily affected by TD than others. Some conditions such as hypochlorhydria and achlorhydria should not be neglected: gastric acidity is an important barrier against pathogens. People with genetic or food-based hypochlorhydria, or who have had stomach surgery and need to take proton pump inhibitors, have a higher risk of TD[31].
Indeed, it is interesting to notice that a trip to a high-risk area less than 6 mo after a previous trip there has a much lower risk of TD. This may be due to some sort of immunity to gastroenteral pathogens developed since the previous visit.
TD prevention is based on: suitable treatment of food, vaccination, and good personal hygiene, especially washing one’s hands properly.
Treatment of food: To reduce the risk of TD as much as possible, one must take care when choosing food and drink, and avoid as far as possible any which do not appear to have been prepared properly.
These choices are extremely simple, but are very often ignored. Indeed, only a tiny fraction of all travellers take any notice of them. An inquiry carried out at big European airports[32] found that only 5.5% of travellers were willing to do anything about food safety.
Vaccinations: Anti-typhus, anti-hepatitis A, and anti-cholera vaccines can be effective against certain levels of TD. (1) Anti-typhus. There are two types of vaccines: tablets and injections. The first requires three tablets to be taken every other day. It is effective in 75% of cases, and provides protection for 2-3 years. The second type of vaccine requires a single injection, and is 77% effective for three years. The oral vaccine must not be taken if one is taking malaria tablets, because it reduces the effectiveness of both. The injection, however, can be given along with other vaccinations (including malaria). In the oral and injected forms, efficacy is 55%-80% for three years (Levine, 2001); (2) Anti-hepatitis A. A subcutaneous injection can be given, and it becomes very effective two weeks thereafter; it will continue to provide protection for up to 20 years. The vaccination requires two injections: the second is given 6-12 mo after the first; (3) Anti-cholera. Two types of oral vaccine are given against V. cholerae: live but weakened, genetically a sub-unit A (CVD 103 HgR), and inactive cells deactivated using a sub-unit B mix (WC/rBS). The latter, designed to protect travellers against cholera, has been proven effective against TD caused by enterotoxic E. coli, the most frequent cause of this type of disease. Protection against TD can reach 80%[3334].
Rest must be taken for 2-3 d, and only water drunk. If symptoms persist, intestinal disinfectants can be given. To reduce the risk of dehydration and the duration of diarrhoea, antidiarrhoeal drugs can be used. Loperamide is effective but has side effects such as constipation and meteorism and/or abdominal swelling, which may cause intestinal occlusion, especially in elderly patients[3536]. Racecadotril, recently made available in Italy, is equally effective as loperamide and does not cause any of these problems.
The General Medical Council in Italy has designed a solution to be taken by mouth, made up as follows: sodium chloride (3.5 g/L), potassium chloride (1.5 g/L), glucose (20.0 g/L), and sodium citrate (2.9 g/L). A solution using 2.5 g/L of sodium bicarbonate has a shorter half-life but is physiologically equivalent and can be found in most countries. Alternatively, the following solution can be used: 6 tsp. of sugar, 1 tsp. of kitchen salt, 2 pts. of drinking water[3738].
Antibiotics should only be given in serious cases, and where a specific bacterial aetiology is suspected.
In summary, diarrhoea is an alteration of normal bowel movement characterized by an increase in the water content, volume, or frequency of stools. Secretory diarrhoeas, mostly acute and due to infections (bacteria, viruses, parasites), are by far the most important subtype of diarrhoeas in terms of frequency, incidence and mortality. Clinical classification of diarrhoea and an understanding of its main pathogenic mechanisms are fundamental for a diagnostic and therapeutic approach. A symptomatic anti-diarrhoeal therapy may be adopted alongside an aetiological therapy to improve the patient’s clinical conditions and among the anti-diarrheal drugs racecadotril represents a safe and rational therapy. Acute diarrhoea is frequently reported in old age with a prevalence of 3.9%-14.2%. The treatment of elderly patients with diarrhoea, regardless of the cause, must include rehydration and nutritional support. TD may represent a relevant problem since every year more than fifty million tourists travel from industrialized countries to places where hygiene levels are poor and at least 75% of those travelling for short periods mention health problems.
| 1. | Guerrant RL, Van Gilder T, Steiner TS, Thielman NM, Slutsker L, Tauxe RV, Hennessy T, Griffin PM, DuPont H, Sack RB. Practice guidelines for the management of infectious diarrhea. Clin Infect Dis. 2001;32:331-351. |
| 2. | Gadewar S, Fasano A. Current concepts in the evaluation, diagnosis and management of acute infectious diarrhea. Curr Opin Pharmacol. 2005;5:559-565. |
| 3. | Farthing MJ. Functional diarrhea. Curr Gastroenterol Rep. 2005;7:350-357. |
| 4. | Guerrant RL. Why America must care about tropical medicine: threats to global health and security from tropical infectious diseases. Am J Trop Med Hyg. 1998;59:3-16. |
| 5. | Feldman RA, Banatvala N. The frequency of culturing stools from adults with diarrhoea in Great Britain. Epidemiol Infect. 1994;113:41-44. |
| 6. | Ball JM, Tian P, Zeng CQ, Morris AP, Estes MK. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science. 1996;272:101-104. |
| 7. | Musher DM, Musher BL. Contagious acute gastrointestinal infections. N Engl J Med. 2004;351:2417-2427. |
| 8. | Thielman NM, Guerrant RL. Clinical practice. Acute infectious diarrhea. N Engl J Med. 2004;350:38-47. |
| 9. | Marcos LA, DuPont HL. Advances in defining etiology and new therapeutic approaches in acute diarrhea. J Infect. 2007;55:385-393. |
| 10. | Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142-201. |
| 11. | Kotloff KL, Winickoff JP, Ivanoff B, Clemens JD, Swerdlow DL, Sansonetti PJ, Adak GK, Levine MM. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull World Health Organ. 1999;77:651-666. |
| 12. | Echeverria P, Sethabutr O, Pitarangsi C. Microbiology and diagnosis of infections with Shigella and enteroinvasive Escherichia coli. Rev Infect Dis. 1991;13 Suppl 4:S220-S225. |
| 13. | Hookman P, Barkin JS. Review: Clostridium difficile-associated disorders/diarrhea and Clostridium difficile colitis: the emergence of a more virulent era. Dig Dis Sci. 2007;52:1071-1075. |
| 14. | Warny M, Pepin J, Fang A, Killgore G, Thompson A, Brazier J, Frost E, McDonald LC. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366:1079-1084. |
| 16. | Ortega YR, Adam RD. Giardia: overview and update. Clin Infect Dis. 1997;25:545-549; quiz 550. |
| 18. | Frexinos J, Sallenave J-R. Comparison of loperamide-oxide and acetorphan in acute diarrhoea [abstract]. Gut. 1996;39 Suppl 3:A173. |
| 19. | Vetel JM, Berard H, Fretault N, Lecomte JM. Comparison of racecadotril and loperamide in adults with acute diarrhoea. Aliment Pharmacol Ther. 1999;13 Suppl 6:21-26. |
| 20. | Prado D. A multinational comparison of racecadotril and loperamide in the treatment of acute watery diarrhoea in adults. Scand J Gastroenterol. 2002;37:656-661. |
| 21. | Wang HH, Shieh MJ, Liao KF. A blind, randomized comparison of racecadotril and loperamide for stopping acute diarrhea in adults. World J Gastroenterol. 2005;11:1540-1543. |
| 22. | Talley NJ, O'Keefe EA, Zinsmeister AR, Melton LJ 3rd. Prevalence of gastrointestinal symptoms in the elderly: a population-based study. Gastroenterology. 1992;102:895-901. |
| 23. | Scallan E, Majowicz SE, Hall G, Banerjee A, Bowman CL, Daly L, Jones T, Kirk MD, Fitzgerald M, Angulo FJ. Prevalence of diarrhoea in the community in Australia, Canada, Ireland, and the United States. Int J Epidemiol. 2005;34:454-460. |
| 24. | O'Keefe EA, Talley NJ, Zinsmeister AR, Jacobsen SJ. Bowel disorders impair functional status and quality of life in the elderly: a population-based study. J Gerontol A Biol Sci Med Sci. 1995;50:M184-M189. |
| 25. | Faruque AS, Malek MA, Khan AI, Huq S, Salam MA, Sack DA. Diarrhoea in elderly people: aetiology, and clinical characteristics. Scand J Infect Dis. 2004;36:204-208. |
| 26. | Ratnaike RN. Diarrhea in old age. Interdisciplinary Topics in Gerontology. Aging and the Gastrointestinal Tract. Basel: Karger 2003; 187-199. |
| 27. | Bauer TM, Lalvani A, Fehrenbach J, Steffen I, Aponte JJ, Segovia R, Vila J, Philippczik G, Steinbrückner B, Frei R. Derivation and validation of guidelines for stool cultures for enteropathogenic bacteria other than Clostridium difficile in hospitalized adults. JAMA. 2001;285:313-319. |
| 29. | Ratnaike RN, Jones TE. Mechanisms of drug-induced diarrhoea in the elderly. Drugs Aging. 1998;13:245-253. |
| 30. | Nelson R. Antibiotic treatment for Clostridium difficile-associated diarrhea in adults. Cochrane Database Syst Rev. 2007;13:CD004610. |
| 31. | Oberhelman RA, McLellan SLF, Behrens RH. Special hosts: children, pregnant women, immunocompromised patients, the elderly traveler. Travelers' Diarrhea. Hamilton: BC Decker 2003; 240-257. |
| 32. | Castelli F. Human mobility and disease: a global challenge. J Travel Med. 2004;11:1-2. |
| 33. | Steffen R, Acar J, Walker E, Zuckerman J. Cholera: assessing the risk to travellers and identifying methods of protection. Travel Med Infect Dis. 2003;1:80-88. |
| 34. | Peltola H, Siitonen A, Kyrönseppä H, Simula I, Mattila L, Oksanen P, Kataja MJ, Cadoz M. Prevention of travellers' diarrhoea by oral B-subunit/whole-cell cholera vaccine. Lancet. 1991;338:1285-1289. |
| 35. | Shlim DR. Self diagnosis and treatment of traveler's diarrhea. Travel medicine. St. Louis: Mosby 2003; 201-204. |
| 36. | Luby S, Mintz E. Cholera. Health Information for International Travel 2003-2004. Department of Health and Human Services, Centers for Disease Control and Prevention. 2003;51-52. |
| 37. | Löscher T, Connor BA. Clinical presentation and treatment of travelers' diarrhea. Travel Medicine. Mosby: Philadelphia 2004; 191-199. |
| 38. | WHO/CAH Diarrhoea treatment guidelines including new recommendations for the use of ORS and zinc supplementation for clinic-based healthcare workers. UNICEF, MOST, USAID, Geneva. 2005; Available from: URL: http://www.mostproject.org. |