INTRODUCTION
Granular cell tumor (GCT) is a benign tumor with unknown histogenesis that is characterized by large, granular eosinophilic cells[1]. The tumor was first described by Abrikossoff[2] in 1926 as a muscle tumor; yet, a close association with nerves and immunohistochemical characteristics have identified GCT as a neural lesion[1]. A wide variety of cell types have been proposed as the cells of origin, including histiocytes, fibroblasts, myoblasts, neural sheath cells, neuroendocrine cells and undifferentiated mesenchymal cells[3–5]. Tumor cells have been shown recently to have positive expression of a number of new markers of neural differentiation, as well as several non-neural markers[6]. Vered et al[7] have proposed the possibility of GCT as a reactive lesion rather than as a true neoplasm. In the present case, extensive hyalinization and calcification within the GCT support this proposal. GCT is not common in the gastrointestinal tract, where the most common site for the tumor is the esophagus, followed by the duodenum, anus and stomach[89]. A few cases of GCT have been reported in the cecum[1011]. In this report, we present a case of GCT of the cecum with extensive hyalinization and focal dystrophic calcification, accompanied by multiple colonic polyps, including tubular adenoma. The immunoreactivity of granular cells based on the use of a broad panel of antibodies did not confirm any particular cell type for the histogenesis of GCT.
CASE REPORT
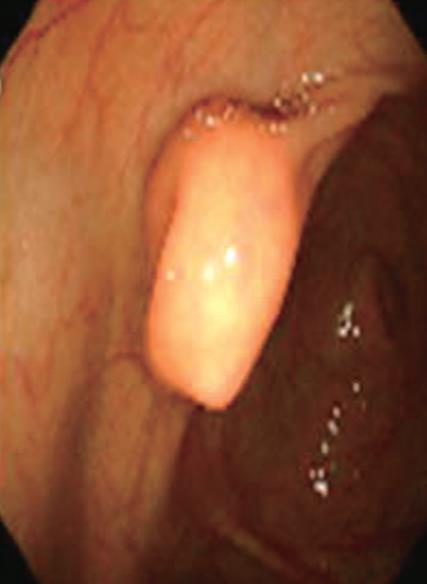
A 56-year-old man was admitted to the Gastroenterology Department of Chosun University Hospital with a 2-mo history of abdominal pain and diarrhea. Colonoscopy revealed a protruding mass approximately 2 cm in diameter, with central umbilication in the cecum (Figure 1), as well as a 5-mm polyp and tiny sized multiple polyps in the descending colon and rectum, respectively.
Figure 1 Endoscopic examination revealed an approximately 2-cm-sized protruding mass in the cecum.
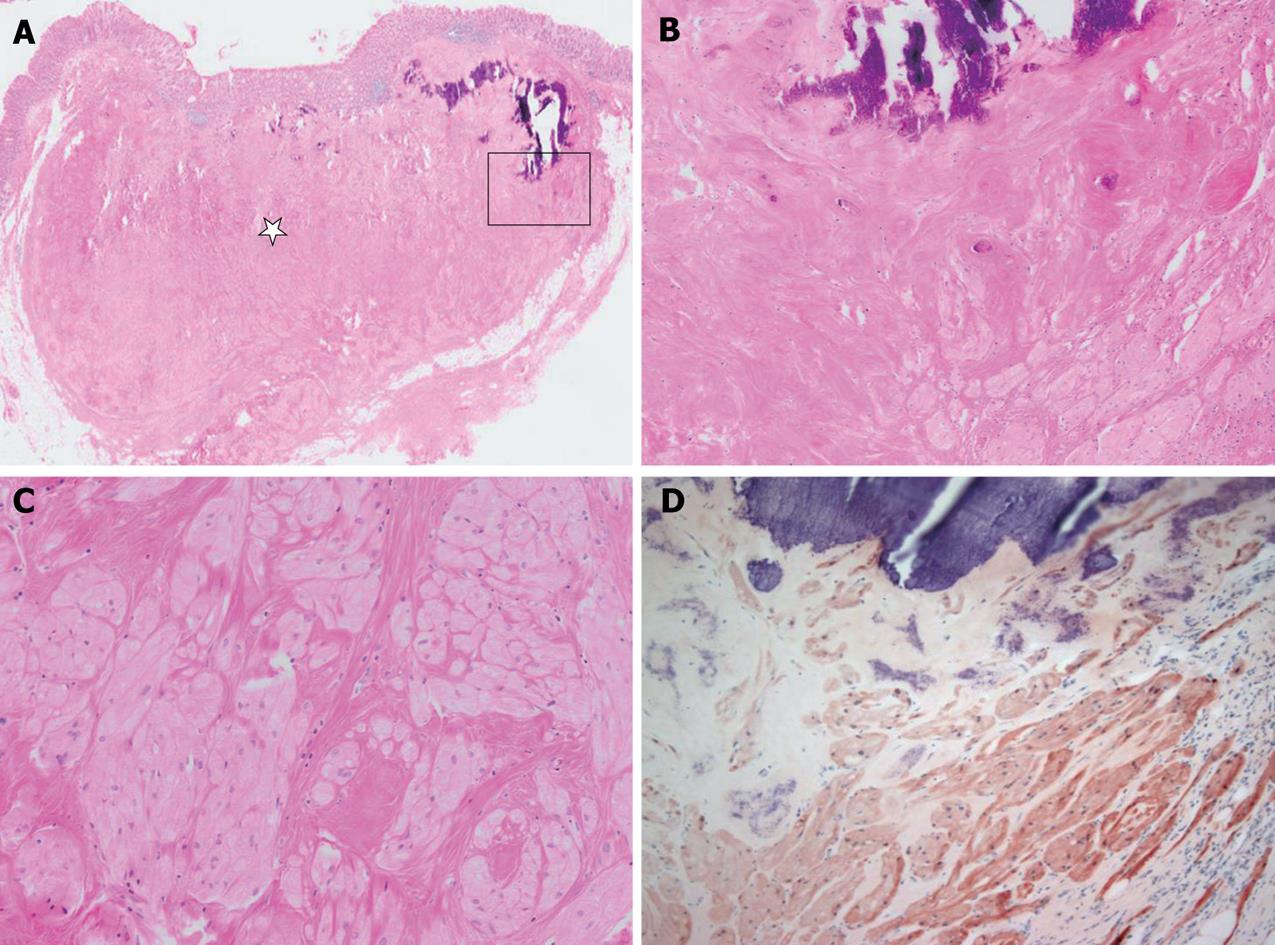
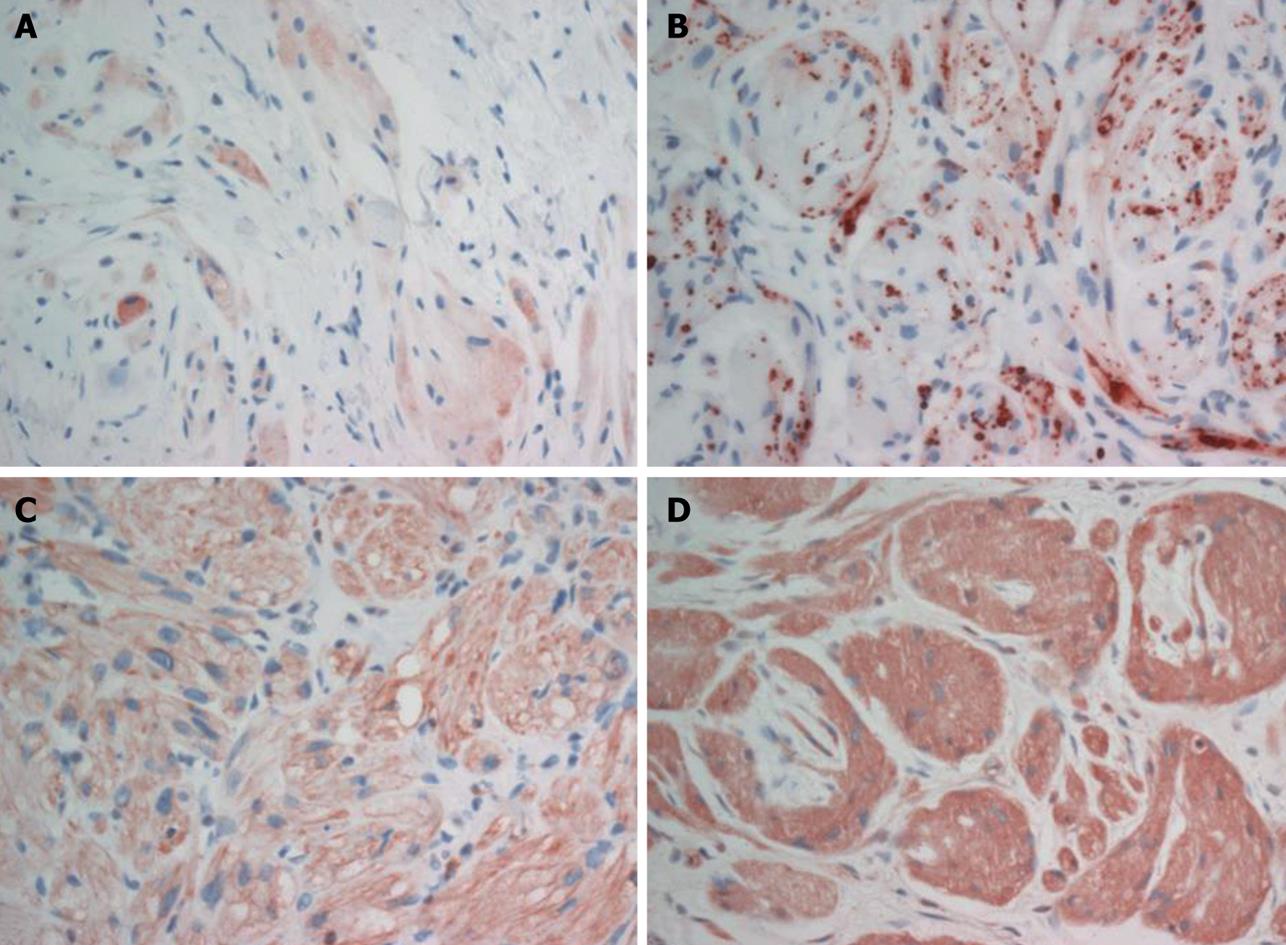
A polyp in the descending colon was removed by snare polypectomy, and the polyp was diagnosed histologically as tubular adenoma. The cecal mass was resected by laparoscopic myotomy. Grossly, the mass was 1.5 cm × 1.0 cm × 0.7 cm, yellowish-white in color, and hardly palpable. Microscopically, the well-circumscribed mass was located mainly between the submucosa and subserosa, with partial involvement of the mucosa (Figure 2A). The mass was composed of round to polygonal cells with abundant granular eosinophilic cytoplasm. Characteristically, much of the tumor showed extensive hyalinization and focal dystrophic calcification (Figure 2B and C). There was no tumor necrosis, spindling and vesicular nuclei with large nucleoli, increased mitotic activity, a high nuclear to cytoplasmic ratio, and pleomorphism. The tumor was seen as a small, well-circumscribed mass, but with no infiltrative growth pattern; findings that favor a benign rather than a malignant lesion. Immunohistochemical analysis demonstrated that the tumor cells were reactive for S-100 protein (Figure 2D), neuron-specific enolase (NSE), vimentin, calretinin and inhibin (Figure 3). The tumor cells were non-reactive for smooth muscle actin (SMA), c-kit (CD117) and CD34. Based on the morphological and immunohistochemical findings, the resected tumor was diagnosed as a benign GCT that occurred in the cecum.
Figure 2 Microscopically and immunohistochemically, the appearance of the tumor was compatible with that of granular cell tumor.
A: The tumor was located mainly between the submucosa and the subserosa. Much of the tumor showed extensive hyalinization (star) and dystrophic calcification. B and C: A high magnification view (square of A) showed dystrophic calcification, hyalinization and some viable tumor cell nests in the peripheral portion of the tumor. The tumor cells were composed of round to polygonal cells with abundant granular eosinophilic cytoplasm. D: Immunohistochemically, tumor cells were reactive for S-100 protein.
Figure 3 Tumor cells of the granular cell tumor were immunoreactive for inhibin-α (A), calretinin (B), vimentin (C) and NSE (D).
DISCUSSION
GCT is diagnosed only rarely, based on macroscopic and endoscopic examinations, as a result of its small size and shape that resemble a diminutive polyp[12]. Recently, endoscopic ultrasonography (EUS) has been used more frequently to determine the depth of tumor invasion in the gastrointestinal wall, and, it is useful for evaluating gastrointestinal tract submucosal tumors[13]. However, EUS cannot sufficiently distinguish a benign submucosal tumor from other tumors such as malignant neoplasia[14].
The final diagnosis of GCT is dependent on the pathological findings. The histological markers for GCT are plump histiocyte-like, bland-looking neoplastic cells with abundant granular eosinophilic cytoplasm, which contains acidophilic, periodic acid-Schiff-positive, diastase-resistant granules. The cells contain small, uniform nuclei where mitotic figures are absent and neural markers, including S-100 protein or NSE, are expressed uniformly[1516].
The histogenesis of GCT has remained enigmatic in spite of a vast number of immunohistochemical and ultrastructural studies[7]. Neural origin or differentiation, in particular of the Schwann cell type, is currently in favor. However, recent findings have cast doubt on the neural origin of these tumors[7]. Vered et al[7] have suggested that immunoreactivity of the granular cells to a broad panel of antibodies including S-100 protein, CD68, vimentin, calretinin, NKI/C3, protein gene product 9.5, nerve growth factor receptor and inhibin-α that characterize different tissue do not confirm any particular cell type for the histogenesis of GCT. In the present case, tumor cells were reactive for S-100 protein, NSE, calretinin, vimentin, and inhibin-α, which agrees with a previous study[7].
In most cases of colonic GCT, the tumor was less than 2 cm and well separated from the muscularis propria[17]. Since this tumor is considered as benign, and no patients with recurrence or metastasis have been documented, it is usually accepted that endoscopic tumor excision may be the best treatment for GCT in the gastrointestinal tract[14]. However, Nakachi et al[14] have suggested that GCT in the gastrointestinal tract is usually small and asymptomatic, and the tumor tends to be found incidentally during endoscopy performed for other reasons. Observation of these GCTs with the use of endoscopy and EUS is indicated unless the patient is symptomatic, or the tumor is larger than 2 cm or demonstrates atypical EUS or histological features. In the present case, there was extensive hyalinization and focal dystrophic calcification of tumor cells, which indicated a long-standing tumor with no atypical changes, which supports the above description. Vered et al[7] have advocated that GCT can be regarded as a lesion that reflects local metabolic or reactive changes rather than a true neoplasm. In the large series of GCTs evaluated by Vered et al[7], lesions displayed three main architectural patterns, including small and well-circumscribed nodules, larger and poorly circumscribed lesions, and an impressive infiltrative pattern with remote satellite nodules. The pattern of small and well-circumscribed nodules may represent benign mesenchymal tumors that have undergone extensive or complete granular cell change, and the remaining patterns may be compatible with a diffuse process of metabolically induced cytoplasmic granular changes in mesenchymal cells. Most lesions with an infiltrative pattern and positive margin almost never recur, whereas for a definite tumor, recurrent lesions are expected. It has been suggested that the lesions may be metabolic or reactive in nature and not neoplastic. The present case displayed extensive hyalinization and calcification in between granular cells, which suggests a long duration; findings that favor GCT with reactive changes, or a true neoplasm.
Several GCTs with adjacent benign or malignant tumors have been reported previously. In 2006, Eriksen et al[18] reported the first case of a synchronic adenoma and GCT, and Sarsik et al[19] also observed a tubular adenoma in the vicinity of a GCT. Caltabiano et al[20] reported a GCT covered by squamous cell carcinoma of the tongue. The present case also had adjacent multiple polyps, including tubular adenoma. Based on these findings, it is suggested that GCT shows reactive granular cell changes in the process of spontaneous regression of a preceding tumor. The coincidence of the adjacent tumor can be regarded as favoring a non-neoplastic or reactive process. However, to date, there is no evidence of any association or disposing factors between GCT in the colon and colonic adenoma or other malignancy[18].
In summary, we experienced a 1.5 cm × 1.0 cm × 0.7 cm cecal GCT that showed extensive hyalinization and focal dystrophic calcification with synchronous tubular adenoma in the descending colon. Immunohistochemical profiles did not confirm any particular cell type for the histogenesis of GCT. Endoscopists and pathologists should consider the possibility of this tumor in the gastrointestinal tract. When a patient is asymptomatic, and unless the tumor is larger than 2 cm or shows atypical features, observation of this tumor with the use of endoscopy and EUS is indicated.