Published online Jul 14, 2009. doi: 10.3748/wjg.15.3261
Revised: June 5, 2009
Accepted: June 12, 2009
Published online: July 14, 2009
AIM: To investigate whether birch pollen allergy symptoms are linked with gut microbiota changes and whether probiotics have an effect on these.
METHODS: Forty seven children with confirmed birch pollen allergy were randomized to receive either a probiotic combination of Lactobacillus acidophilus (L. acidophilus) NCFMTM (ATCC 700396) and Bifidobacterium lactis (B. lactis) Bl-04 (ATCC SD5219) or placebo in a double-blind manner for 4 mo, starting prior to onset of the birch pollen season. Symptoms were recorded in a diary. Blood samples were taken for analysis of cytokines and eosinophils. Fecal samples were analysed for microbiota components, calprotectin and IgA. Nasal swabs were taken for analysis of eosinophils.
RESULTS: The pollen season induced a reduction in Bifidobacterium, Clostridium and Bacteroides which could not be prevented by the probiotic intervention. During the intervention, significantly higher numbers of B. lactis 11.2 × 107± 4.2 × 107vs 0.1 × 107± 0.1 × 107 bacteria/g feces (P < 0.0001) and L. acidophilus NCFMTM 3.5 × 106± 1.3 × 106vs 0.2 × 106± 0.1 × 106 bacteria/g feces (P < 0.0001) were observed in the probiotic group compared to the placebo group. During May, there was a tendency for fewer subjects, (76.2% vs 95.2%, P = 0.078) to report runny nose, while during June, fewer subjects, 11.1% vs 33.3%, reported nasal blocking in the probiotics group (P = 0.101). Concomitantly, fewer subjects in the probiotic group had infiltration of eosinophils in the nasal mucosa compared to the placebo group, 57.1% vs 95% (P = 0.013). Eye symptoms tended to be slightly more frequent in the probiotic group, 12.5 d [interquartile range (IQR) 6-18] vs 7.5 d (IQR 0-11.5) (P = 0.066) during May. Fecal IgA was increased in the placebo group during the pollen season; this increase was prevented by the probiotics (P = 0.028).
CONCLUSION: Birch pollen allergy was shown to be associated with changes in fecal microbiota composition. The specific combination of probiotics used was shown to prevent the pollen-induced infiltration of eosinophils into the nasal mucosa, and indicated a trend for reduced nasal symptoms.
- Citation: Ouwehand AC, Nermes M, Collado MC, Rautonen N, Salminen S, Isolauri E. Specific probiotics alleviate allergic rhinitis during the birch pollen season. World J Gastroenterol 2009; 15(26): 3261-3268
- URL: https://www.wjgnet.com/1007-9327/full/v15/i26/3261.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.3261
Allergic rhinitis and asthma are common chronic conditions, with a recently reported annual symptom prevalence of 15% for both diseases in young teenagers in Western Europe[1]. Allergic rhinitis is often seasonal and caused by exposure to environmental allergens. It has been shown that, in allergic rhinitis, nasal allergen provocation induces an inflammatory response not only in nasal but also in bronchial mucosa[2], and vice versa[3], emphasizing the importance of the airways as a single anatomic-functional unit. Furthermore, chronic allergic rhinitis is known to be an independent risk factor of asthma[4]. Allergic rhinitis is frequently accompanied by allergic conjunctivitis, characterized by decreased conjunctival epithelial integrity and repair mechanisms even outside the pollen season[5].
Probiotics are live microorganisms which, when administered in adequate amounts confer a health benefit on the host[6]. Specific probiotic strains have been shown to be effective in the prevention[7–9] and treatment[10] of atopic eczema, alleviating allergic inflammation both locally and systemically. The evidence of probiotic efficacy against allergic rhinitis and immunological sensitization predisposing to asthma is insufficient and contradictory at present. The previously studied probiotic strains or combinations of these may not have targeted airway allergies, or the populations studied may not have been responsive to immune modulation[9].
We hypothesized that allergic rhinitis can be alleviated by the use of specific probiotics selected to both modify the intestinal microbiota and to confer immune effects, which can be demonstrated in the intestinal tract prior to an allergic reaction. We assumed that the nasal mucosa is the most vulnerable structure of the airways, allowing the greatest inhalational allergen penetration. Thus we chose to study the effects of selected probiotic bacteria in allergic rhinitis in children during the birch pollen season. The specific strains used in this study were selected based on their anti-inflammatory properties and an expected effect in promoting a Th1 type of immune response[1112]. The study population was chosen to cover subjects with confirmed birch pollen allergy. By selecting children, we hypothesized that there would be more opportunity to modify the immune responses.
Forty-seven children with clinically and immunologically documented and physician-verified birch pollen allergy were enrolled in the study. All had previously had symptoms of allergic rhinitis confined to the birch pollen season, and the specific test result to birch pollen had been positive, either with skin prick testing (positive result if the mean wheal diameter for birch pollen allergen was greater than 3 mm) or demonstration of specific IgE antibody in serum (according to the manufacturer’s reference values, the level for positivity was a specific IgE more than 0.35 kU/L). The symptoms of the patients during the previous pollen season included sneezing and runny or blocked nose, and the nasal symptoms were often associated with allergic eye symptoms, such as itching and redness of the conjunctiva. Patients with diagnosed asthma, habitual use of probiotics and/or prebiotics and recent use of antibiotics were excluded from the study.
The study was approved by the Ethics Committee of the Hospital District of South-Western Finland and informed, written consent was received from the participants and their parents. The study was registered at http://www.clinicaltrials.gov under the identifier NCT00746226.
The study material consisted of capsules containing 5 ×109 CFU of a combination of 25% Lactobacillus acidophilus (L. acidophilus) NCFMTM (ATCC 700396) and 75% Bifidobacterium lactis (B. lactis) Bl-04 (ATCC SD5219), Danisco Cultures, Madison, USA. The strains were selected based on their anti-inflammatory properties and Th1-type immune stimulating effects and their ratio was chosen to optimize these functions. The viability of the study material was determined at monthly intervals during the study by Danisco Cultures, Madison. This laboratory was not involved in the study, and no significant reduction in viability was observed (results not shown). The placebo consisted of identical capsules containing microcrystalline cellulose. The study material was randomized at the site of production by a person not involved in the study. The volunteers, researchers and biostatisticians remained blinded for the duration of the study and the analyses. The code was broken after completion of the statistical analyses.
Eligible subjects were assigned to receive one of the individually coded test products. The subjects or their parents were instructed to consume one capsule daily or to suspend its contents in a suitable liquid.
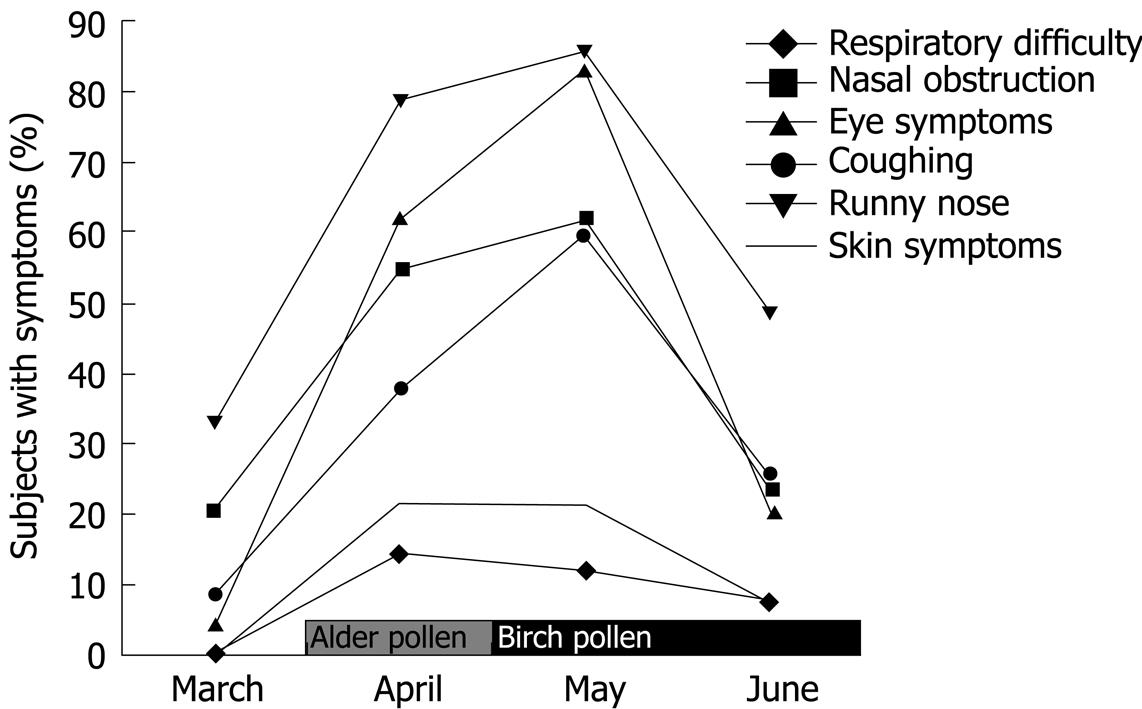
The patients attended the study unit 3 times during spring 2006 (Figure 1). The first visit was in March before the birch pollen season, and consumption of the study material started after this visit. The second visit was at the end of April-beginning of May during the birch pollen season and the third visit was in June at the end of the season, when consumption of the study product was finished.
Clinical examination of the patients was carried out at each study visit. At each visit, nasal smears were taken from both nostrils by gently rubbing the middle or inferior turbinate with a cotton covered stick, followed by fixing and staining with eosin and methylene blue. Eosinophil counts were determined semiquantitatively by microscope as follows: -, no eosinophils in any of the fields of view; +, a few scattered eosinophils at least in some fields of view; ++, eosinophils easily found in every field of view; and +++, every field of view abundant in eosinophils. Venous blood samples were drawn at each study visit. At the first and third visit, the blood eosinophil count was determined by automated blood cell counter (Advia 120, Bayer Health Care, Germany), and allergen-specific IgE antibodies in serum were measured by an immunofluorometric assay (ImmunoCAPTM, Phadia AB, Uppsala, Sweden). All samples were initially screened using a mix of inhalation allergens (Phadiatop ImmunoCAP). The samples which were positive at screening were subsequently tested for birch pollen-specific IgE.
The concentrations of the cytokines interleukin (IL)-4, IL-5, IL-6, IL-10 and tumor necrosis factor (TNF)-α were measured by multiplex flow cytometric assay using a commercial Human Th1/Th2 Cytokine Kit (BD Immunocytometry Systems and BD Biosciences Pharmingen). The concentrations of transforming growth factor (TGF)-β2 were measured at the first and second visit and soluble CD-14 (sCD-14) at the first and third visit using commercial sandwich ELISA specific for these molecules (R&D Systems Europe Ltd., Abingdon, UK).
The presence of blocked or runny nose, respiratory difficulty, coughing, eye symptoms or eczema was recorded by the parents on the diary cards throughout the study. The number of days with each symptom during each of the study months was reported.
The major groups of fecal bacteria were analyzed using the FISH method as previously described[13]. In brief, fecal samples were suspended in PBS and homogenized. Bacteria were fixed with paraformaldehyde and hybridized with Cy3 indocarbocyanin-labeled oligonucleotide probe. Probes included Bac303 (5’-CCAATGTGGGGGACCTT-3’) for the Bacteroides-Prevotella group, Bif164 (5’-CATCCGGCATTACCACCC-3’) for bifidobacteria, His150 [5’-TTATGCGGTATTAATCT (C/T) CCTTT-3’] for clostridia of the C. histolyticum group and MUC-1437 (5’-CCTTGCGGTTGGCTTCAGAT-3’) for Akkermansia muciniphila-like bacteria[14], total cells were enumerated using an EUB338 (5’-GCTGCCTCCCGTAGGAGT-3’)-fluorescein (FITC)-labelled probe[1516].
Flow-cytometric analyses were performed using a BDTM LSR II flow cytometer (Becton, Dickinson and Co., USA) equipped with 4 lasers (355, 405, 488 and 635 nm). We used the 488 nm laser at 15 mW. This standard instrument is equipped with 2 light scatter detectors which measure forward (FSC) and side scatter (SSC) and 4 fluorescence detectors detecting appropriately filtered light at green (FL1, 525 nm), yellow (FL2, 575 nm), orange (FL3, 620 nm), and red (FL4, 675 nm) wavelengths. To avoid cell coincidence, the flow rate was kept at the lowest setting (data rate 200-300 events per second). At least 30 000 events were recorded for each sample and all experiments were conducted in duplicate. Data were stored as list-mode files and analyzed off-line using the BD FACSDiva™ software version 4.1.1 (Becton, Dickinson and Co., USA).
Immediately prior to analysis, Flow-Count fluorospheres (Beckman Coulter, USA) were added to each sample. Absolute bacterial cell counts were determined following the manufacturer’s instructions, using the ratio of positive bacteria to fluorospheres counted using the following formula: cells/&mgr;L = [(cells counted)/(fluorospheres counted)] × fluorospheres/&mgr;L. To avoid loss of the signal intensity of hybridized cells, they were kept in the dark on ice at 4°C until the flow cytometry assay. Results were expressed as the numbers of cells hybridizing with the specific group-Cy3 probe and total bacteria EUB 338-FITC probe.
DNA extractions from pure cultures of the different microorganisms and fecal samples were extracted using the QIAamp DNA stool Mini kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. qPCRs were conducted as previously described[1011]. For characterization of the fecal microbiota PCR primers were designed targeting B. lactis according to Gueimonde and co-workers[17]. L. acidophilus NCFMTM was quantified using primers as described by Ouwehand and co-workers[18]. The oligonucleotides were purchased from the Thermo Electron Corporation (Thermo Biosciences, Ulm, Germany). Briefly, PCR amplification and detection were performed with an ABI PRISM 7300-PCR sequence detection system (Applied Biosystems, UK). Each reaction mixture of 25 &mgr;L was composed of SYBR® Green PCR Master Mix (Applied Biosystems, UK.), 1 &mgr;L of each of the specific primers at a concentration of 0.25 &mgr;mol/L, and 1 &mgr;L of template DNA. The fluorescent products were detected at the last step of each cycle. A melting curve analysis was made after amplification to distinguish the targeted PCR product from the non-targeted PCR product.
Microbial activity was determined by measurement of lactic acid, short chain fatty acids: acetic, butyric, lactic and propionic acids, and branched chain fatty acids: valeric, isobutyric, 2-methylbutyric and isovaleric acid. These were determined by gas chromatography as described previously by Holben et al[19]. The results were expressed in mmol/kg.
Changes in the immunological status of the intestine were monitored by measuring the concentrations of IgA and calprotectin from the soluble fraction of feces. For IgA measurements the frozen samples were thawed and extracted with BSA as described previously and stored at -20°C before analysis[20]. Concentrations of IgA were then determined with the ELISA according to the manufacturer’s instructions (E80-102, Bethyl Laboratories, Inc., Montgomery, TX, USA). The concentrations of calprotectin were determined with ELISA following the manufacturer’s instructions (Calpro AS, Oslo, Norway). The results were expressed as &mgr;g per g fresh weight.
Differences between the treatment groups were analysed using the Mann-Whitney U test. Differences within the treatment groups between the different time points were analyzed using the Wilcoxon signed rank test. Correlations between the level of fecal colonization of B. lactis or L. acidophilus NCFMTM and immune or microbiological variables in the probiotic group during the second visit were determined with the Spearman rank correlation test. To determine differences between the groups and over time, the data was analyzed with a linear mixed effects model, having effects for time, group, and their joint effect. Individual baseline differences were taken into account by including the first time point as a covariate in the model. P-values below 0.05 were considered significant.
Baseline clinical characteristics of the 2 groups are presented in Table 1. Twenty patients in the probiotic and 21 in the placebo group completed the study. Three patients, 2 in the probiotic group and 1 in the placebo group, were excluded during the study because of newly developed asthma. Three patients, 2 in the probiotic group and 1 in the placebo group, decided to drop out of the study. The mean duration of breast-feeding was 7.5 mo (range 0-18 mo), there being 2 patients whom were not breast-fed.
| Probiotic | Placebo | P-value | |
| N | 24 | 23 | |
| Drop-outs | 2 | 1 | P > 0.99 |
| Age (yr) | 9.0 (4.2-11.9) | 8.9 (6.1-12.9) | P = 0.845 |
| Gender (M/F) | 11 (13) | 8 (15) | P = 0.24 |
| Length (cm) | 135.2 (107.3-156.5) | 133.6 (113.8-152.5) | P = 0.50 |
| Weight (kg) | 31.0 (11.9-47.4) | 32.4 (20.2-45.3) | P = 0.80 |
The impact of the onset of the birch pollen season on allergy symptoms in the two study groups is depicted in Figure 1. The peak period of birch pollen exposure, April and May, resulted in specific symptoms such as nasal obstruction and runny nose. All the recorded symptoms increased during April and May (the height of the pollen season in Turku, Finland), and decreased again in June (P < 0.001).
At the start of the study, there was no difference in the observed symptoms between the 2 study groups. Runny nose tended (P = 0.078) to be reported in May by fewer subjects in the probiotic group (76.2%) than in the placebo group (95.2%). During June, fewer subjects reported nasal blocking in the probiotics group (11.1%) than in the placebo group (33.3%). This did not, however, reach statistical significance (P = 0.101). Concomitantly with the differences in nasal symptoms, the number of subjects with infiltration of eosinophils in the nasal mucosa increased in the placebo group during the April/May visit but an increase was not observed in the probiotic group (P = 0.013), where the fraction of subjects with nasal eosinophil infiltration remained unchanged (Table 2).
| Month | Placebo | Probiotic |
| March | 14 (63.6) | 17 (70.8) |
| April/May | 19 (95.0) | 12 (57.1)1 |
| June | 12 (60.0) | 12 (60.0) |
During May, the subjects in the probiotic group reported more days with eye symptoms, 12.5 d (interquartile range (IQR) 6-18 d), than subjects in the placebo group, 7.5 d (IQR 0-11.5 d), P = 0.066. In June, this difference was no longer detectable.
In addition to the increase in symptoms over the season, concentrations of birch pollen-specific IgE also increased in both groups from March to June, P = 0.0015 and P = 0.0002 for placebo and probiotic respectively (Table 3). Likewise, blood eosinophil numbers increased in both placebo (P = 0.03) and probiotic groups (P = 0.002), from March to June (Table 3). Concentrations of IL-6 decreased in both groups from March to April/May, P = 0.0004 and P = 0.0002 in placebo and probiotic groups, respectively (Table 3). Concentrations of IL-10 were reduced in the placebo group from March to April/May, P = 0.0495. For both groups, concentrations of TNF-α decreased from March to April/May, P = 0.0007 and P = 0.0005 in placebo and probiotic groups, respectively. Concentrations of TNF-α were higher in the placebo group at the start of the study, P = 0.0062, however, at the April/May visit no difference was observed, P = 0.17. Concentrations of interferon-γ and IL-2 were below the detection limit for both groups at all 3 time points tested. No differences in the concentrations of TGF-β2, IL-4, IL-5 and CD14 were detected (Table 3).
| March | April/May | June | ||||
| Placebo | Probiotic | Placebo | Probiotic | Placebo | Probiotic | |
| Specific IgE birch pollen (kU/L) | 26.0 (18.0-45.8) | 25.5 (15.5-54.0) | - | - | 150.00 (36.0-150.0)1 | 150.00 (150.0-150.0)1 |
| Blood eosinophils (109/L) | 0.30 (0.24-0.44) | 0.18 (0.12-0.34) | - | - | 0.40 (0.25-0.72)1 | 0.42 (0.28-069)1 |
| IL-4 (pg/mL) | 2.20 (1.5-2.8) | 1.70 (0.0-2.2) | 1.70 (0.8-2.2) | 1.7 (1.3-2.1) | - | - |
| IL-5 (pg/mL) | 19.6 (0.0-69.7) | 9.10 (0.0-24.2) | 14.70 (0.0-28.3) | 18.4 (0.0-60.2) | - | - |
| IL-6 (pg/mL) | 16.6 (5.7-38.3) | 4.70 (3.0-11.4)3 | 4.20 (2.3-7.3)1 | 3.0 (2.0-3.9)1 | - | - |
| IL-10 (pg/mL) | 1.60 (1.4-1.9) | 1.50 (0.0-2.1) | 1.40 (0.0-1.8)2 | 0.0 (0.0-1.5) | - | - |
| TNF-α (pg/mL) | 7.70 (3.9-13.5) | 3.50 (2.5-5.2)3 | 2.90 (2.3-4.8)1 | 2.5 (2.1-3.2)1 | - | - |
| TGF-β2 (pg/mL) | 242 (0.0-342) | 247 (0.0-369) | 213.6 (0.0-314.3) | 267.9 (0.0-445.9) | - | - |
| CD-14 (&mgr;g/mL) | 1.20 (1.1-1.3) | 1.20 (1.1-1.3) | - | - | 1.20 (1.1-1.3) | 1.10 (1.1-1.3) |
Concentrations of fecal IgA and calprotectin exhibited great individual variance (P < 0.0001 and P = 0.015, respectively). Because of this significant variation, the data were analyzed taking the baseline level into account. Neither the probiotic treatment, nor pollen season had any significant effects on the fecal calprotectin concentrations. However, fecal IgA concentrations were increased in the placebo group during April/May (P = 0.028), which was prevented by the probiotic intervention. In the latter group, the concentration remained stable during the entire study period, Table 4.
| Visit 1 (March) | Visit 2 (April/May) | Visit 3 (June) | ||||
| Placebo | Probiotic | Placebo | Probiotic | Placebo | Probiotic | |
| Fecal IgA (&mgr;g/g) | 157.0 (105.0-278.8) | 157.0 (114.0-371.0) | 233.0 (136.0-288.8)1 | 155.0 (44.5-336.5)1 | 203.0 (99.0-388.0)1 | 135.0 (63.4-325.3)1 |
| Fecal calprotectin (&mgr;g/g) | 21.6 (9.5-58.6) | 14.3 (10.7-30.1) | 21.1 (12.6-46.7) | 16.2 (13.1-31.8) | 22.1 (10.9-53.2) | 15.5 (12.0-31.5) |
Administration of B. lactis Bl-04 and L. acidophilus NCFMTM led to a significant increase in the fecal numbers of B. lactis (P = 0.0032) and L. acidophilus NCFMTM (P = 0.0036), from March to April/May and numbers remained high until the end of the intervention in June, indicating good compliance by the subjects (Table 5). The differences were also significant compared to the placebo group during April/May; B. lactis (P < 0.0001) and L. acidophilus NCFMTM (P = 0.0002), and June; B. lactis (P < 0.0001) and L. acidophilus NCFMTM (P < 0.0001), Table 5.
| March | April/May | June | ||||
| Placebo | Probiotic | Placebo | Probiotic | Placebo | Probiotic | |
| Eubacterium4 | 2.7 × 109 (0.3 × 109) | 2.5 × 109 (0.4 × 109) | 1.8 × 109 (1.0 × 109) | 1.6 × 109 (0.2 × 109)2 | 2.00 × 109 (0.2 × 109) | 1.9 × 109 (0.3 × 109) |
| Bifidobacterium4 | 5.0 × 108 (5.0 × 108) | 3.5 × 108 (3.3 × 108) | 2.1 × 108 (0.4 × 108)2 | 1.6 × 108 (0.3 × 108)1 | 2.50 × 108 (0.4 × 108) | 2.0 × 108 (0.2 × 108) |
| Bacteroides4 | 4.9 × 108 (3.5 × 108) | 5.4 × 108 (3.2 × 108) | 2.3 × 108 (0.4 × 108)2 | 1.7 × 108 (0.3 × 108)1 | 1.30 × 108 (0.2 × 108) | 1.5 × 108 (0.4 × 108) |
| Clostridium4 | 1.4 × 108 (1.0 × 108) | 1.5 × 108 (0.3 × 108) | 0.7 × 108 (0.2 × 108)2 | 0.8 × 108 (0.2 × 108)1 | 1.30 × 108 (0.3 × 108)2 | 1.6 × 108 (0.2 × 108)1 |
| Akkermansia4 | 1.3 × 108 (0.2 × 108) | 1.3 × 108 (0.2 × 108) | 1.2 × 108 (0.2 × 108) | 1.1 × 108 (0.1 × 108) | 0.90 × 108 (0.2 × 108) | 1.1 × 108 (0.2 × 108) |
| B. lactis5 | 2.6 × 107 (1.7 × 107) | 1.5 × 107 (1.3 × 107) | 0.1 × 107 (0.1 × 107) | 11.2 × 107 (4.2 × 107)13 | 0.01 × 107 (0.05 × 107) | 2.6 × 107 (1.0 × 107)13 |
| L. acidophilus NCFM5 | 1.0 × 106 (0.8 × 106) | 0.7 × 106 (0.5 × 106) | 0.2 × 106 (0.1 × 106)1 | 3.5 × 106 (1.3 × 106)3 | 0.02 × 106 (0.1 × 106) | 4.2 × 106 (2.4 × 106)3 |
A general decrease in numbers of the major bacterial groups Eubacterium, Bifidobacterium, Bacteroides and Clostridium accompanied the birch pollen season in both study groups (Table 5). This may not be explained by the increase in fecal numbers of B. lactis and L. acidophilus as the decrease in fecal numbers was manifested in both probiotic and placebo groups. In the placebo group, concentrations of 2-methylbutyric acid were reduced from April/May to June, P = 0.0501. However, no differences in concentrations of other measured fecal organic acids were observed between the 2 groups for any of the time points (Table 6).
| March | April/May | June | ||||
| Placebo | Probiotic | Placebo | Probiotic | Placebo | Probiotic | |
| Acetic acid | 64.6 (6.0) | 74.50 (7.2) | 59.20 (5.6) | 65.50 (5.8) | 66.90 (5.1) | 58.20 (4.5) |
| Propionic acid | 15.5 (1.3) | 17.10 (1.6) | 15.20 (1.2) | 16.10 (2.1) | 16.20 (1.9) | 14.60 (1.5) |
| Isobutyric acid | 2.30 (0.3) | 2.20 (0.2) | 2.00 (0.2) | 1.90 (0.1) | 1.80 (0.2) | 1.90 (0.2) |
| Butyric acid | 11.9 (2.0) | 13.40 (2.5) | 12.50 (1.2) | 11.70 (1.7) | 14.00 (1.8) | 11.50 (1.7) |
| 2-methylbutyric acid | 1.60 (0.2) | 1.30 (0.2) | 1.60 (0.2) | 1.20 (0.1) | 1.10 (0.1)1 | 1.20 (0.2) |
| Isovaleric acid | 1.80 (0.2) | 1.70 (0.1) | 1.60 (0.2) | 1.50 (0.1) | 1.50 (0.2) | 1.50 (0.2) |
| Lactic acid | 0.14 (0.14) | 0.83 (0.56) | 0.00 (0.00) | 0.14 (0.14) | 0.36 (0.25) | 0.50 (0.38) |
| Valeric acid | 2.70 (0.3) | 2.60 (0.3) | 2.70 (0.2) | 2.50 (0.2) | 2.60 (0.3) | 2.50 (0.3) |
| Capronic acid | 0.06 (0.06) | 0.31 (0.20) | 0.17 (0.12) | 0.27 (0.16) | 0.03 (0.03) | 0.22 (0.09) |
Probiotics have been used to reduce the risk of atopic eczema, frequently the first allergic symptom to manifest itself. Previous intervention studies showing risk reduction for eczema have failed to reduce the risk of respiratory allergies. This may be due to strain and host specific characteristics. However, a recent study with a combination of Lactobacillus rhamnosus (L. rhamnosus) GG and B. lactis Bb-12, achieved for the first time, a reduction in risk of both eczema and later sensitization, but only in a high-risk population[21]. Nevertheless, we need to acknowledge that a more profound understanding of the complex nature of atopy and atopic disease is needed, as it is likely that there are distinct etiological factors and pathogenetic mechanisms underlying the heterogeneous manifestations of the disorder. Thus, one mode of prevention or treatment may not suffice to target the plethora of allergic disease.
In the present study, we observed in the probiotic group a reduction of nasal eosinophil infiltration, an objective marker of allergic rhinitis. The degree of eosinophil infiltration of the respiratory mucosa is known to directly correlate with the intensity of the disease[22]. Indeed, trends for less runny nose during the peak of the pollen season and reduced nasal blocking towards the end of the season were observed in the current study. Earlier trials with probiotics and pollen allergy have shown that L. rhamnosus GG was not effective in relieving birch pollen allergy symptoms[23]. Likewise, Lactobacillus casei Shirota, was not found to be effective in reducing symptoms of Japanese cedar pollen allergy[24], although the strain did reduce serum concentrations of IL-5, IL-6, interferon-γ and specific IgE in subjects with allergic rhinitis[25]. A recent study showed, however, that B. longum BB536 was able to relieve eye symptoms in subjects suffering from Japanese cedar pollen allergy[2627], likewise L. rhamnosus GG and L. gasseri TMC0356 reduced nasal symptoms of Japanese cedar pollen allergy[28].
Interestingly, our results indicate that gut microbiota are involved in regulating the inflammatory processes also in airway allergies. The fecal levels of bifidobacteria, clostridia and Bacteroides were reduced at the peak of the birch pollen season. As the change occurred in both treatment groups, it is not likely to be a consequence of the intervention, but may rather relate to the birch pollen season or possibly, but unlikely, to the concomitant antihistamine medication. Changes in fecal microbiota composition, a decrease in Bacteroides fragilis levels, in response to cedar pollen challenge have previously been reported[29]. The underlying reasons for the temporary reduction of the numbers of the major phyla of the microbiota can only be speculated. Despite the change in the composition of the fecal microbiota, only a small change in 2-methylbutyric acid concentrations was observed, and no other changes in the determined intestinal microbial metabolites were detected.
The probiotic strains used in the current study were selected on the basis that they had either anti-inflammatory properties or could be expected to have a Th1 response-promoting effect[1112]. Their ratio was chosen to optimize these 2 effects. The probiotic intake was reflected in temporary colonization of the gut by the study probiotics and in their enhanced numbers in fecal samples from the study children. This indicates that the study subjects had good compliance to the treatment and the study procedure. Furthermore, fecal L. acidophilus NCFMTM numbers correlated positively with fecal acetic acid concentrations (R = 0.612, P = 0.0116), fecal propionic acid concentrations (R = 0.449, P = 0.0642) and fecal butyric acid concentrations (R = 0.519, P = 0.0323). These observations may suggest that the presence of, in particular, L. acidophilus NCFMTM increases microbial fermentation in the colon. However, the consumption of the probiotic strains did not prevent changes in the colonization pattern when compared to the placebo group.
The study population was chosen to cover subjects with confirmed birch pollen allergy. The subjects selected suffered from significant symptoms of birch pollen allergy; this may have exceeded the potential effect of the intervention. Furthermore, all subjects were allowed to use oral antihistamines prophylactically. Also this has undoubtedly reduced the possibility of observing a treatment effect. Notwithstanding these limitations, positive influences could be observed on established clinical markers of pollen allergy, particularly in mucosal responses, where the increases in nasal eosinophil infiltration and increases in fecal IgA were prevented. Inhibition of increased Th2 type reactivity in the gut during the birch pollen season in the probiotic group may indicate that the shift to Th2 responses which has been associated with the development of allergy can be relieved by supplementation with this particular probiotic combination.
In conclusion, our study showed that consumption of a combination of L. acidophilus NCFMTM and B. lactis Bl-04 could positively influence markers of respiratory allergy, especially in the mucosae, and also resulted in a tendency for a reduction in reported nasal symptoms.
Pollen allergies are increasing in affluent societies. This is thought to be related to a reduced microbial exposure. Probiotics could provide a safe form of microbial exposure possibly through modulation of the intestinal microbiota.
Studies have shown that selected probiotics may modulate the immune response and contribute to the relief or primary prevention of atopic dermatitis. Few studies have, however, investigated the potential of probiotics on pollen allergies. The present study indicates a potential for the specific combination of Lactobacillus acidophilus (L. acidophilus) NCFM and Bifidobacterium lactis (B. lactis) Bl-04 in the alleviation of birch pollen allergy in children.
The present randomized, placebo-controlled, double-blind study showed that the intestinal microbiota changes during the birch pollen season, and the combination of L. acidophilus NCFM and B. lactis Bl-04 was not able to counteract this change. Despite the prophylactic use of antihistamines, the probiotic combination did reduce an objective marker of pollen allergy (nasal eosinophilia) and indicated a trend for a reduction in subjective markers, nasal blocking and runny nose.
Probiotics may provide an alternative or complementary treatment for pollen allergies. A future study could investigate whether this would lead to a reduced use of antihistamines.
Probiotics are live microorganisms which, when administered in adequate amounts confer a health benefit on the host. Specific strains of probiotics have been observed to modulate the immune system and the composition and activity of the intestinal microbiota.
The study addresses an interesting and relevant issue on which data is scarce, and the findings may contribute to our understanding of the role of probiotics in allergic rhinitis.
| 1. | Asher MI, Montefort S, Björkstén B, Lai CK, Strachan DP, Weiland SK, Williams H. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733-743. |
| 2. | Braunstahl GJ, Overbeek SE, Kleinjan A, Prins JB, Hoogsteden HC, Fokkens WJ. Nasal allergen provocation induces adhesion molecule expression and tissue eosinophilia in upper and lower airways. J Allergy Clin Immunol. 2001;107:469-476. |
| 3. | Braunstahl GJ, Overbeek SE, Fokkens WJ, Kleinjan A, McEuen AR, Walls AF, Hoogsteden HC, Prins JB. Segmental bronchoprovocation in allergic rhinitis patients affects mast cell and basophil numbers in nasal and bronchial mucosa. Am J Respir Crit Care Med. 2001;164:858-865. |
| 4. | Settipane RJ, Hagy GW, Settipane GA. Long-term risk factors for developing asthma and allergic rhinitis: a 23-year follow-up study of college students. Allergy Proc. 1994;15:21-25. |
| 5. | Hughes JL, Lackie PM, Wilson SJ, Church MK, McGill JI. Reduced structural proteins in the conjunctival epithelium in allergic eye disease. Allergy. 2006;61:1268-1274. |
| 6. | FAO/WHO. Guidelines for the evaluation of probiotics in food. Available from: URL: http://www.who.int/foodsafety/publications/fs_management/probiotics2/en/. |
| 7. | Kalliomäki M, Salminen S, Poussa T, Isolauri E. Probiotics during the first 7 years of life: a cumulative risk reduction of eczema in a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2007;119:1019-1021. |
| 8. | Kalliomäki M, Salminen S, Poussa T, Arvilommi H, Isolauri E. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet. 2003;361:1869-1871. |
| 9. | Kalliomäki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357:1076-1079. |
| 10. | Isolauri E, Arvola T, Sütas Y, Moilanen E, Salminen S. Probiotics in the management of atopic eczema. Clin Exp Allergy. 2000;30:1604-1610. |
| 11. | Daniel C, Poiret S, Goudercourt D, Dennin V, Leyer G, Pot B. Selecting lactic acid bacteria for their safety and functionality by use of a mouse colitis model. Appl Environ Microbiol. 2006;72:5799-5805. |
| 12. | Foligne B, Nutten S, Grangette C, Dennin V, Goudercourt D, Poiret S, Dewulf J, Brassart D, Mercenier A, Pot B. Correlation between in vitro and in vivo immunomodulatory properties of lactic acid bacteria. World J Gastroenterol. 2007;13:236-243. |
| 13. | Kalliomäki M, Kirjavainen P, Eerola E, Kero P, Salminen S, Isolauri E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol. 2001;107:129-134. |
| 14. | Collado MC, Derrien M, Isolauri E, de Vos WM, Salminen S. Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Appl Environ Microbiol. 2007;73:7767-7770. |
| 15. | Harmsen HJ, Wildeboer-Veloo AC, Raangs GC, Wagendorp AA, Klijn N, Bindels JG, Welling GW. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastroenterol Nutr. 2000;30:61-67. |
| 16. | Harmsen HJ, Gibson GR, Elfferich P, Raangs GC, Wildeboer-Veloo AC, Argaiz A, Roberfroid MB, Welling GW. Comparison of viable cell counts and fluorescence in situ hybridization using specific rRNA-based probes for the quantification of human fecal bacteria. FEMS Microbiol Lett. 2000;183:125-129. |
| 17. | Gueimonde M, Tölkkö S, Korpimäki T, Salminen S. New real-time quantitative PCR procedure for quantification of bifidobacteria in human fecal samples. Appl Environ Microbiol. 2004;70:4165-4169. |
| 18. | Ouwehand AC, Tiihonen K, Saarinen M, Putaala H, Rautonen N. Influence of a combination of Lactobacillus acidophilus NCFM and lactitol on healthy elderly: intestinal and immune parameters. Br J Nutr. 2009;101:367-375. |
| 19. | Holben WE, Williams P, Gilbert MA, Saarinen M, Särkilahti LK, Apajalahti JH. Phylogenetic analysis of intestinal microflora indicates a novel Mycoplasma phylotype in farmed and wild salmon. Microb Ecol. 2002;44:175-185. |
| 20. | Peuranen S, Tiihonen K, Apajalahti J, Kettunen A, Saarinen M, Rautonen N. Combination of polydextrose and lactitol affects microbial ecosystem and immune responses in rat gastrointestinal tract. Br J Nutr. 2004;91:905-914. |
| 21. | Huurre A, Laitinen K, Rautava S, Korkeamäki M, Isolauri E. Impact of maternal atopy and probiotic supplementation during pregnancy on infant sensitization: a double-blind placebo-controlled study. Clin Exp Allergy. 2008;38:1342-1348. |
| 22. | Kramer MF, Jordan TR, Klemens C, Hilgert E, Hempel JM, Pfrogner E, Rasp G. Factors contributing to nasal allergic late phase eosinophilia. Am J Otolaryngol. 2006;27:190-199. |
| 23. | Helin T, Haahtela S, Haahtela T. No effect of oral treatment with an intestinal bacterial strain, Lactobacillus rhamnosus (ATCC 53103), on birch-pollen allergy: a placebo-controlled double-blind study. Allergy. 2002;57:243-246. |
| 24. | Tamura M, Shikina T, Morihana T, Hayama M, Kajimoto O, Sakamoto A, Kajimoto Y, Watanabe O, Nonaka C, Shida K. Effects of probiotics on allergic rhinitis induced by Japanese cedar pollen: randomized double-blind, placebo-controlled clinical trial. Int Arch Allergy Immunol. 2007;143:75-82. |
| 25. | Ivory K, Chambers SJ, Pin C, Prieto E, Arqués JL, Nicoletti C. Oral delivery of Lactobacillus casei Shirota modifies allergen-induced immune responses in allergic rhinitis. Clin Exp Allergy. 2008;38:1282-1289. |
| 26. | Ishida Y, Nakamura F, Kanzato H, Sawada D, Yamamoto N, Kagata H, Oh-Ida M, Takeuchi H, Fujiwara S. Effect of milk fermented with Lactobacillus acidophilus strain L-92 on symptoms of Japanese cedar pollen allergy: a randomized placebo-controlled trial. Biosci Biotechnol Biochem. 2005;69:1652-1660. |
| 27. | Xiao JZ, Kondo S, Yanagisawa N, Miyaji K, Enomoto K, Sakoda T, Iwatsuki K, Enomoto T. Clinical efficacy of probiotic Bifidobacterium longum for the treatment of symptoms of Japanese cedar pollen allergy in subjects evaluated in an environmental exposure unit. Allergol Int. 2007;56:67-75. |
| 28. | Kawase M, He F, Kubota A, Hiramatsu M, Saito H, Ishii T, Yasueda H, Akiyama K. Effect of fermented milk prepared with two probiotic strains on Japanese cedar pollinosis in a double-blind placebo-controlled clinical study. Int J Food Microbiol. 2009;128:429-434. |
| 29. | Odamaki T, Xiao JZ, Iwabuchi N, Sakamoto M, Takahashi N, Kondo S, Iwatsuki K, Kokubo S, Togashi H, Enomoto T. Fluctuation of fecal microbiota in individuals with Japanese cedar pollinosis during the pollen season and influence of probiotic intake. J Investig Allergol Clin Immunol. 2007;17:92-100. |