Published online Jul 14, 2009. doi: 10.3748/wjg.15.3246
Revised: May 23, 2009
Accepted: May 30, 2009
Published online: July 14, 2009
AIM: To investigate the role of insulin-like growth factor binding protein-7 (IGFBP-7) in the activation and transdifferentiation of hepatic stellate cells (HSC) in vitro.
METHODS: Rat HSC-T6 cells were cultured in separate dishes and treated with various concentration of transforming growth factor (TGF)-β1, IGFBP-7 or anti-IGFBP-7 antibody for 24 h. The supernatant or a cytoplasm suspension was obtained from cultured HSC, followed by transfer of cells to form cell-coated dishes. Immunocytochemistry and Western blotting were used to analyze the expression of IGFBP-7 induced by TGF-β1 and the level of fibronectin, collagen I and α-smooth muscle actin (SMA). The pro-apoptotic effect of anti-IGFBP-7 antibody was determined by flow cytometry.
RESULTS: Immunocytochemistry and Western blotting revealed that the expression of IGFBP-7 in TGF-β1 treated HSC was significantly up-regulated compared to that in the control group. In addition, fibronectin, collagen I and α-SMA also showed enhanced expression in accordance with the transdifferentiation process in a dose-dependent manner to some extent. Moreover, flow cytometry suggested that anti-IGFBP-7 antibody induced apoptosis of activated HSC, which is responsible for the development of liver fibrosis, and may represent a novel pathway and target for therapeutic intervention.
CONCLUSION: IGFBP-7 showed increased expression in activated HSC and played an important role in the activation and transdifferentiation process of HSC. Anti-IGFBP-7 antibody may ameliorate liver fibrogenesis.
-
Citation: Liu LX, Huang S, Zhang QQ, Liu Y, Zhang DM, Guo XH, Han DW. Insulin-like growth factor binding protein-7 induces activation and transdifferentiation of hepatic stellate cells
in vitro . World J Gastroenterol 2009; 15(26): 3246-3253 - URL: https://www.wjgnet.com/1007-9327/full/v15/i26/3246.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.3246
Hepatic fibrosis and cirrhosis are characterized by excessive fibrosis attributable to hepatic stellate cell (HSC) proliferation and activation[1], resulting in excessive production of extracellular matrix (ECM), including fibrin-forming collagens I and III, proteoglycans, fibronectins and hyaluronic acid[2]. The occurrence of hepatic fibrosis is also a common response to most chronic liver injuries such as viral hepatitis, parasitic infection, metabolic or autoimmune diseases, congenital abnormalities, and alcohol abuse[13]. However, the pathogenesis of hepatic fibrosis is still unknown[4]. In recent studies, HSC were the primary source of excess ECM assembly in liver fibrosis[5]. During the development of liver fibrosis, HSC undergo a process of activation, resulting in the expression of ECM, reduced retinoid storage capability and transdifferentiation to a myofibroblast-like phenotype which is characterized by expression of α-smooth muscle actin (α-SMA)[6]. Fibrotic changes result in structural changes and lead to reconstruction of hepatic lobules, dysfunction of liver bioactivity, and even death. At present, there is no effective treatment to halt the progression of liver fibrosis and cirrhosis. The only current life-prolonging intervention is liver transplantation. Thus, HSC play an important role in the pathogenesis of liver fibrosis.
HSC activation process is very complicated and is the result of a complex interplay between different hepatic cell types such as inflammatory cells and secreted cytokines, chemokines and growth factors[7]. The cellular transformation that develops gradually in vivo can be mimicked in vitro by short term culture of HSC on plastic, providing a model to study the intra- and extra-cellular determinants that regulate the transformation/activation process. Excessive cellular proliferation and an abundant ECM protein production that is not counteracted by increased ECM protein degradation have been shown to be the specific hallmark of HSC activation[8]. Another major feature of the activation process is the responsiveness of HSC to cytokines, resulting in the up-regulation of the platelet-derived-growth factor (PDGF) and members of the transforming growth factor (TGF)-β family. PDGF appears to a major mitogen for stellate cells, and members of the TGF-β family are the primary fibrogenic cytokines[910]. Boers et al[11] have detected a significant change in expression of insulin-like growth factor binding protein (IGFBP)-7 as a novel marker for hepatic fibrosis.
IGFs play an important role in the regulation of metabolism, development and growth of HSC[12]. The capacity of IGFs to exert their biological effects via interactions with specific cell surface receptors is modulated by the presence of a family of structurally related IGF-binding proteins. So far, 6 distinct IGFBPs have been identified and differ in molecular mass, binding properties for IGFs and posttranslational modifications as well as tissue and development regulated expression[13]. In addition, low affinity binders termed IGFBP-rPs have been found. IGFBP-7, the first protein proven to be functionally related to IGFBPs, called IGBP-rP1, is one of these low affinity binders to IGF-I and is expressed relatively more highly upon HSC activation, especially in the end stage[11].
The aim of the present study was to investigate whether IGFBP-7, at different concentrations, may induce the activation and perpetuation of HSC. Our initial experiments demonstrated that expression of IGFBP-7 was up-regulated in patients with liver fibrosis and cirrhosis. We examined roles of IGFBP-7 in different stages of HSC by exposure of HSC to exogenous cytokines. The involvement of IGFBP-7 was evaluated in activated HSC and increased ECM was detected. IGFBP-7 also induced the differentiation of HSC from a quiescent to an activated phase, whereas the specific antibody of IGFBP-7 can induce apoptosis of activated HSC. The data suggest that IGFBP-7 is sufficient to initiate the activation of HSC in conjunction with or prior to observation, which strongly implicates IGFBP-7 in the pathogenesis of hepatic fibrosis. In addition, overexpression of IGFBP-7 provides a novel cellular model to study the pathogenesis of human hepatic fibrosis.
HSC-T6 lines were a generous gift from Scott L Friedman of the Mount Sinai School of Medicine (NY, USA). Briefly, samples were washed and transferred into 25 cm2 culture dishes (Orange Scientific, Belgium) for culture under standard conditions in a normoxic atmosphere of 16% O2, 5% CO2, and 79% N2 (by volume) in RPMT1640 medium (Gibco, USA) containing 10% fetal calf serum (FCS), 100 U/mL penicillin, 100 g/mL streptomycin and 3.57 g/L HEPES at 37°C. HSC were kept in secondary culture (partially “activated” HSC) and were passaged once every 2 to 3 d. Briefly, for cells in secondary culture, 105 cells/well were seeded into 12-well plates supplemented with RPMT1640 (Gibco) without FCS, or 10 cells/well were transferred to 96-well plates. After allowing HSC to attach overnight, cultures were incubated with TGF-β1 (Peprotech, UK) or IGFBP-7 (R&D, US), or anti-IGFBP-7 antibody (R&D, US), in different concentrations or in combination for 24 h before each experimental manipulation.
After 24 h in cell culture to allow cells to attach, cell-coated dishes were obtained, fixed with 1% paraformaldehyde for 10 min, and washed by PBS. Endogenous peroxidases and biotins were then quenched using an endogenous peroxidase blocking kit and biotin blocking kit, respectively. The sections were blocked with 3% FCS (Roche, US) and incubated with one of the following antibodies: polyclonal anti-α-SMA/monoclonal anti-collagen I (Abcan, UK), and polyclonal anti-fibronectin (Santa Cruz, US). Sections were washed and incubated with biotinylated secondary antibody (Santa Cruz, US and Jackson, US). Bound secondary antibody was detected using the UltraSensitive™ SP kit (DAKO, US) according to the manufacturer’s instructions. For modeling negative controls, the primary antibodies were substituted with PBS. The reaction products were visualized by diaminobenzidine tetrahydrochloride (DAB) (DAKO, US). Stained sections were viewed under a Nikon Eclipse 800 microscope and IOD (integrated optical density) or IA (integrated absorption) of the positive brown particles determined semiquantitatively by examining 5 fields randomly at × 200 magnification in each slice.
Culture supernatant and cellular lysates were obtained from a cultured HSC-T6 line according to the standard protocol. In brief, 2 × 105/mL active cells were cultured in 12-well plates in the absence or presence of TGF-β1 at different concentrations. Cultures were harvested after 12 h of incubation, supernatants were extensively dialyzed, and the remaining cells were trypsinized, then centrifuged, lysed, and finally cellular lysates were attained. Thirty microliter of cellular lysates were separated on 15% sodium dodecyl polyacrylamide gels (SDS-PAGE) under reducing conditions and then transferred onto PVDF membranes (SABC, US). After blocking with TBST (TBS and 0.1% Tween) for 3 h, the membranes were incubated with anti-IGFBP-7 antibody as the primary antibody overnight then washed with PBS. Secondary antibodies were conjugated with horseradish peroxidase for 3 h. The signals were visualized by DAB. The relative signal intensities of the bands were quantitated using Molecular Analyst software, and the results were normalized to levels of β-actins (Santa Cruz, US) in each sample. In selected experiments, the secretion of collagen I to the media was determined by ELISA (R&D, US) following incubation of confluent cultures of HSC-T6 with TGF-β1 (Peprotech, UK) or anti-IGFBP-7 antibody in different concentrations and combinations for up to 24 h, in accordance with the manufacturer’s instructions.
HSC-T6 lines were maintained under standard conditions as mentioned before, and then were transferred to 12-well dishes. After 24 h in culture, serum-free media was added containing anti-IGFBP-7 antibody in concentrations of 0.25 mg/L, 0.50 mg/L and 1.00 mg/L. Following another 14 h in culture, supernatants were discarded, and 500 g of cells were centrifuged. The remaining cells were washed with PBS, followed by centrifugation again for 5 min at 500 g at 4°C. Then, cell pellets were resuspended in ice-cold binding buffer to 1 × 106 cells/mL, with tubes on ice. Both 5 &mgr;L of annexin-V and 2.5 &mgr;L of propidium iodide (PI) (Beckman Coulter, US) were added to 100 &mgr;L of cell suspensions. Tubes were kept on ice for 10 min in the dark, and then another 100 &mgr;L of binding buffer was added to the preparations and mixed gently. Quantitative analysis of the preparations was performed after 30 min by flow cytometry. Meanwhile, following the 14 h culture, 20 &mgr;L MTT and 100 &mgr;L PBS were added to each well at 37°C in 5% CO2 atmosphere (by volume) for 4 h. Supernatants were discarded, and 150 &mgr;L DMSO was added and mixed gently. After incubation with chromogenic substance for 10 min, absorbance (A) at 490 nm was measured in samples to measure the value of A under different concentrations of anti-IGFBP-7 antibody.
Data are presented as the mean ± SD, and all calculations were made using SPSS11.5. Statistical comparisons were performed using an ANOVA correlation analysis. Paired comparisons were done using the SNK-q test. Significance was set at a value of P < 0.05.
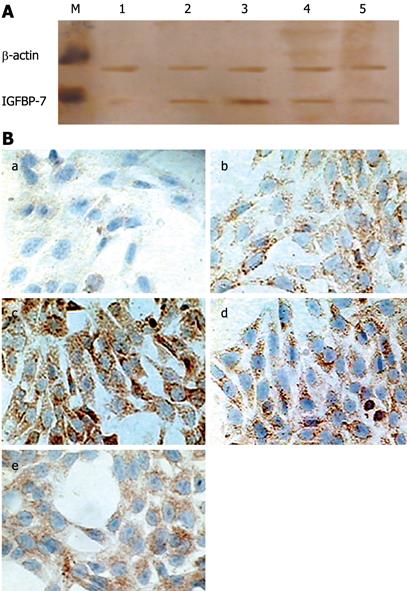
Differences in absorbance between TGF-β1-treated cells and controls are shown in Table 1. Expression of IGFBP-7 was up-regulated by TGF-β1 of 2, 4, 8 and 16 &mgr;L/L, as detected in the cytoplasm of HSC by Western blotting analysis of cellular lysates (Figure 1A). Immunohistochemistry also demonstrated an increased expression of IGFP-7 in TGF-β1-treated HSC on cell-coated dishes. As shown in Figure 1B, in the TGF-β1-treated groups, the amount of positive brown staining was abundant and the value of IOD of positively stained particles increased significantly (Table 1), as compared with the control group in which only a few cells were positively stained. The expression in cells treated with 4 &mgr;L/L of TGF-β1 was stronger than most other groups. Perhaps this effect is the reason that the autocrine expression of TGF-β1 is strengthened, and exogenous TGF-β1 expression decreases the secretion of IGFBP-7.
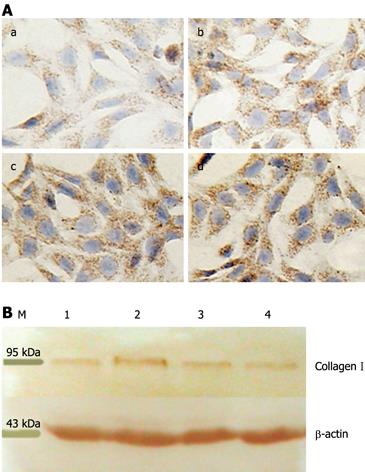
To analyze the in vitro effects of IGFBP-7 on HSC secretion of collagen I, supernatant was collected and the amount of collagen I was analyzed by ELISA following a standard protocol according to the manufacturer’s instructions. As shown in Table 2, the secretion of collagen I was prominent in the TGF-β1-treated groups, compared with the control group and the anti-IGFBP-7-treated groups. However in the wells treated with 2 or 4 ng/mL TGF-β1 and 0.1, 1 or 2 &mgr;g/mL anti-IGFBP-7 antibody, the amount of collagen I was significantly decreased, as compared with the groups treated with TGF-β1 alone. We further performed immunohistochemistry to determine the expression of collagen I in HSC. As shown in Figure 2A, positive brown collagen I staining in IGFBP-7-treated HSC obviously increased compared with the controls in a dose-dependent manner to some extent. Western blotting analysis was also used to characterize the amount of collagen I protein in the cytoplasm of HSC. As shown in Figure 2B, the protein was prominently synthesized in accordance with HSC activation. The differences in absorbance between IGFBP-7-treated groups at different concentrations and the control group in sequence were 0.7663 ± 0.0412, 0.7439 ± 0.0720, 0.7039 ± 0.0889, and 0.6402 ± 0.0475, respectively (P < 0.05).
| n | Collagen I (ng/mL) | |
| Control | 6 | 36.61 ± 1.28 |
| TGFβ1 2 ng/mL | 6 | 41.93 ± 2.95c |
| TGFβ1 4 ng/mL | 6 | 49.44 ± 3.21c |
| Anti-IGFBPrP1 antibody 0.1 &mgr;g/mL | 6 | 35.46 ± 5.80a |
| Anti-IGFBPrP1 antibody 1 &mgr;g/mL | 6 | 36.38 ± 2.59a |
| Anti-IGFBPrP1 antibody 2 &mgr;g/mL | 6 | 37.26 ± 2.12a |
| TGFβ1 2 ng/mL + Anti-IGFBPrP1 | 6 | 38.24 ± 2.76c |
| antibody 0.1 &mgr;g/mL | ||
| TGFβ1 2 ng/mL + Anti-IGFBPrP1 | 6 | 33.95 ± 3.27c |
| antibody 1 &mgr;g/mL | ||
| TGFβ1 2 ng/mL + Anti-IGFBPrP1 | 6 | 34.59 ± 3.97c |
| antibody 2 &mgr;g/mL | ||
| TGFβ1 4 ng/mL + Anti-IGFBPrP1 | 6 | 40.24 ± 3.46e |
| antibody 0.1 &mgr;g/mL | ||
| TGFβ1 4 ng/mL + Anti-IGFBPrP1 | 6 | 32.61 ± 4.86e |
| antibody 1 &mgr;g/mL |
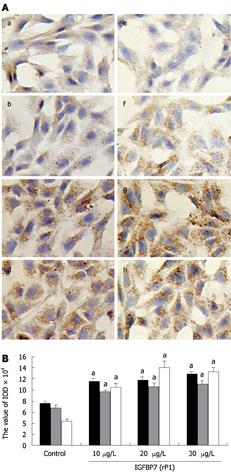
The deposition of fibronectin was examined by immunohistochemistry. As shown in Figure 3A (a-d), fibronectin was detected in the cytoplasm of HSC. In the control group, only a few brown particles were detected although the amount of staining was higher in the IGFBP-7-treated groups, also in a dose-dependent manner. To characterize the HSC phenotype, α-SMA expression was examined. As shown in Figure 3A (e-h), α-SMA-positive HSC were more abundant in IGFBP-7-treated cells than in the control group, and the increase was in a dose-dependent manner. The comparison and analysis of IOD of fibronectin and α-SMA was performed and presented in Figure 3B. A significant difference was seen between each IGFBP-7-treated group at different concentrations and the control group. Thus, IGFBP-7 induced an increased expression and deposition of fibronectin, which led to the transformation of HSC into a myofibroblastic phenotype.
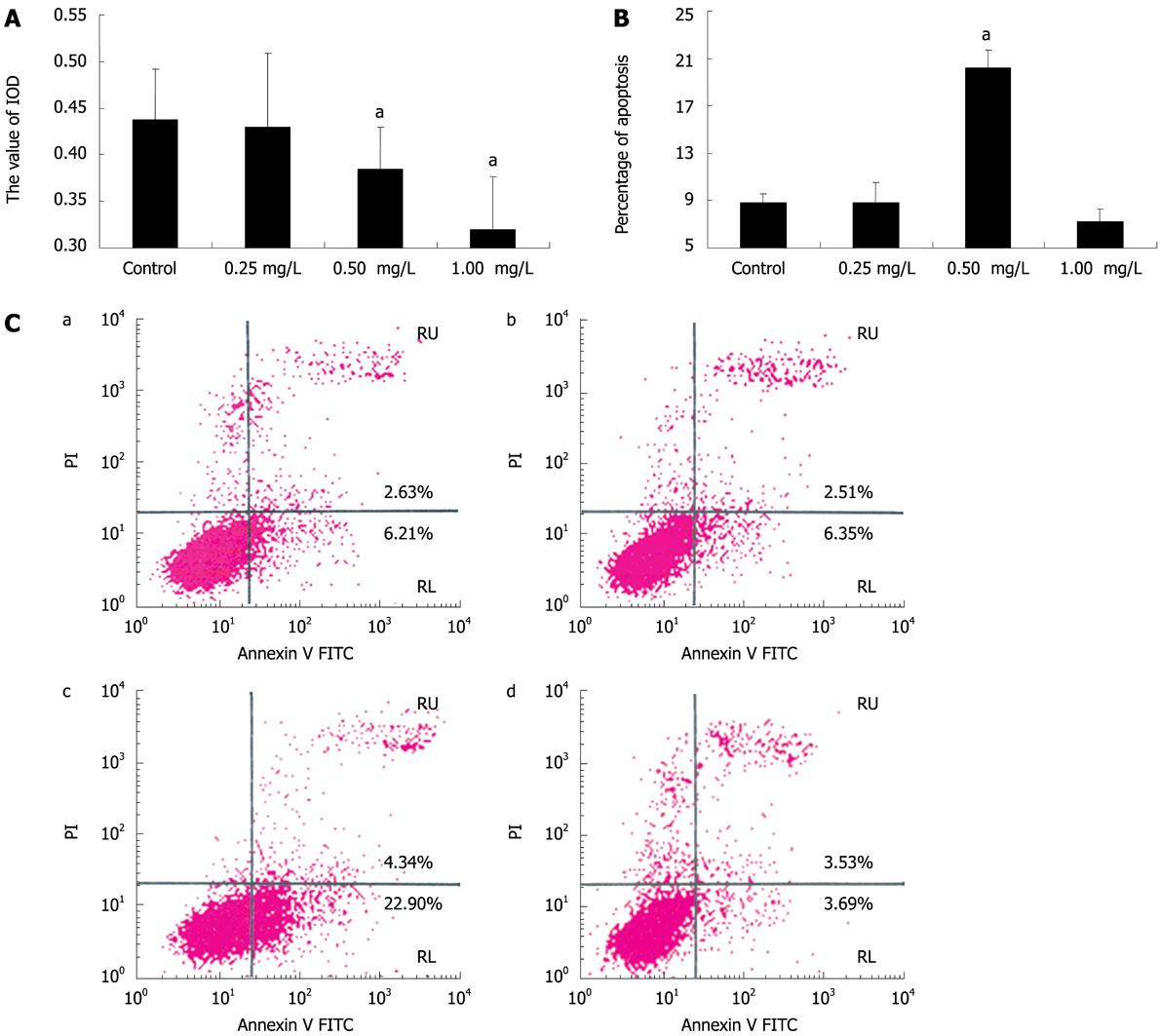
We further examined the effect of anti-IGFBP-7 antibody on activated HSC. To characterize the inhibitory effect of anti-IGFBP-7 antibody on activated HSC, MTT assays were performed. As shown in Figure 4A, A decreased after anti-IGFBP-7 antibody treatment, compared with the control group. Also, treatment with 0.50 mg/L and 1.00 mg/L of antibody could significantly reduce the value of A. To determine the proportion of apoptotic cells to all activated cells, flow cytometry with annexin/PI was used. As shown in Figure 4B, the ratio of apoptotic cells was prominent in the 0.50 mg/L anti-IGFBP-7 antibody treatment group, as compared with the control group. The difference between 0.25 mg/L, 1.00 mg/L and the control was not significant (Figure 4). Statistical analysis of these results is shown in Figure 4C.
HSC are the main source of ECM, which contributes to the occurrence and development of liver fibrosis[14]. We previously reported increased IGFBP-7 protein levels in patients with liver fibrosis and cirrhosis. In this study, expression and function of IGFBP-7 were studied in cultured HSC which were administered different reagents. The object was to define the bioactivity and function of IGFBP-7 in such a model of HSC in different stages of activation and transdifferentiation. For this purpose, we used TGF-β1 as the profibrotic cytokine to activate HSC from quiescent to partially activated and ultimately completely transdifferentiated HSC. Then, the expression of IGFBP-7 was analyzed in cell-coated dishes. The resulting stained-cell dishes revealed that the expression of IGFBP-7 was significantly increased in TGF-β1-treated HSC (“activated” HSC). It has been demonstrated that the IGFBP-7 showed enhanced expression and could work as a novel marker in the pathogenesis of HSC[11]. Our observations further confirmed this statement. HSC could be considered to be one of the principal effectors involved in fibrogenesis. In response to liver injury as well as other cytokines, HSC are considered to undergo a phenotypic transformation from a quiescent to a myofibroblast-like phenotype, characterized by DNA synthesis, motility, contractility and synthesis of ECM[1516]. TGF-β1 is the best-known primary fibrogenic cytokine[910], whereas it is not clear which specific gene is responsible for the fibrotic response.
IGFBPs showed high expression together with other markers of HSC activation, especially in the end stage. The liver is the major producer of IGFBPs, mainly IGFBP-1 and -3[17]. The present study mainly focused on the function and distribution of IGFBP superfamily members among different organs and tissues. IGFBPs are the binding proteins of IGF-1 and -2; they regulate the interaction of IGF-1 and -2 with their receptors, and modulate the growth-promoting and metabolic activities of IGF-1 and -2 by affecting the downstream signal transduction of IGFR[18]. In vitro, the proliferation of HSC is enhanced by IGF-1[1920]. In vivo, however, production of IGF-1 by HSC has recently been reported to attenuate hepatic cirrhosis[17]. Furthermore, IGF-1 replacement therapy seems to benefit patients with hepatic cirrhosis[21]. Because of the binding activity of IGFBPs, the induction of these binding proteins may affect hepatic cirrhosis. An in vitro study revealed that IGFBPs could display intrinsic bioactivities that are dependent on or independent of binding of IGF-1 and -2, in the process of hepatocyte growth, differentiation and activity[22]. So far, 6 distinct IGFBPs have been identified, which differ in molecular mass and binding affinities for IGF-1 and -2[13]. Moreover, low affinity IGF binders termed IGFBP-rPs have been found. IGFBP-7, also called IGFBP-rP1, is one of these low affinity binders of IGF-1. In the transcription of activated human HSC, expression of 5 IGFBP family members was detected. The most prominent was the extremely high expression of IGFBP-5, which is known to be the most abundantly expressed protein in activated HSC. In addition, IGFBP-7 is obviously detected in these cells, whereas a smaller induction is seen for IGFBP-4, -6 and -3[6]. The growth-promoting and metabolic activities of IGF-1 and -2 are modulated by the family of IGFBPs[23]. In certain pathological conditions, IGFBP-1 through -6 all may inhibit the growth of cells. IGFBP-1 and -3 may destroy glucose equilibrium in humans; IGFBP-1, -5, and -6 may also disturb this capability[24], whereas IGFBP-7 (rP1) could modulate proliferation, cohesion of cells, the regeneration of blood vessel and biosynthesis of prostacyclin[25]. IGFBP-8 (rP2) is considered to be a key cytokine in the fibrogenesis of tissues and organs[13]. Since modulation of IGF activity is very complex and varied, their differences may be important factors in determining the local autocrine and paracrine activities of IGFs. The striking up-regulation of IGFBP-7 predicted that it is a good marker of HSC activation and is also a promising target for therapeutic intervention in hepatic fibrosis.
Increased collagen and fibronectin deposition were detected in IGFBP-7-treated HSC, demonstrating that IGFBP-7 can activate and induce ECM production and fibrosis in vitro. Moreover, α-SMA-expressing cells increased among TGF-β1-treated cells, suggesting that they acquired a myofibroblastic phenotype[26]. Taken together, our findings indicate that superexpression of IGFBP-7 induces the deposition of ECM through the transformation of HSC into myofibroblastic cells which then contribute to the resulting fibrosis. We have shown that myofibroblastic changes are induced in vitro by IGFBP-7. In patients with liver fibrosis and cirrhosis, HSC are mainly responsible for the excessive deposition of ECM, many of which may have the phenotypic characteristics of myofibroblasts. A myofibroblast is strictly defined by electromicroscopic findings as a cell that is vimentin- and/or α-SMA-positive, with prominent rough endoplasmic reticulum, a modestly developed periphery, with focal densities, and producing collagen, granules, gap, and actin-filament-based junctions[27]. However, most of the present studies are restricted to an immunohistochemical determination without electron microscopy, such as spindle-shaped cells expressing α-SMA[28]. Thus, α-SMA-expressing cells in our model undergo a myofibroblastic change, and this in vitro phenomenon can also be triggered by exogenous IGFBP-7 applied to human stellate cells. In humans, HSC are mainly located at the sinusoid within the subendothelial space of Disse in close contact with the hepatocytes. They comprise roughly one-third of the nonparenchymal cell population or about 5%-8% of total liver cells[29]. During the development of liver fibrosis, HSC undergo a process of activation, resulting in a reduced retinoid storage capability and transformation to a myofibroblastic phenotype that is characterized by expression of α-SMA. In fact, the myofibroblast-like phenotype is not limited to liver, but also is a prominent feature of fibrosis in other tissues including pancreas, kidney, lung and skin[30].
Apoptosis of activated HSC is significantly detected when anti-IGFBP-7 antibody is administered to the medium. Flow cytometric analysis is a novel, specific and exact way to detect and measure apoptosis, based on the new parameters appearing on the surface of cells such as phospholipids that become exposed at the cell surface and form one of the specific signals for recognition and removal of apoptotic cells by macrophages. The anti-IGFBP-7 antibody acts as a proapoptotic factor, having great impact on the survival and proliferation of HSC. We predict that the anti-IGFBP-7 antibody also performs this function in vivo, which will be investigated in subsequent studies.
In summary, we have shown that IGFBP-7 plays a pivotal role in the pathogenesis of HSC activation and has profibrotic activities in vitro. IGFBP-7 induces the production of ECM via activation of HSC and the development of a myofibroblastic phenotype in HSC. The over-expression of IGFBP-7 in vitro also provides a new model to study the pathogenesis of HSC activation. The inhibitory effect of anti-IGFBP-7 antibody on activated HSC provides a promising intervention for liver fibrosis.
Hepatic stellate cells (HSC) are the main source of extracellular matrix (ECM), and thus play an important role in the occurrence and development of liver fibrosis. Insulin-like growth factor binding protein (IGFBP)-7 is a novel protein, which may be relevant to the activation of HSC.
IGFBP-7 expression has been detected significantly during fibrogenesis, but how this protein is related to liver fibrosis is largely unknown. In this study, the authors demonstrated that IGFBP-7 could activate HSC in accordance with the transdifferentiation process in a dose-dependent manner to some extent.
Recent studies have highlighted the importance of IGF-I and IGFBP-3 in the liver tissues and the roles they play in fibrogenesis. This is the first study to report that expression of IGFBP-7 is also enhanced in this process and IGFBP-7 could activate HSC in vitro. Furthermore, this protein may be the main mediator of HSC bioactivity.
By understanding how IGFBP-7 is relevant to liver fibrosis, this study may represent a new strategy for therapeutic intervention in the treatment of patients with liver fibrosis.
HSC are specific cells located in the hepatic lobules, and are the primary source of excess ECM assembly during liver fibrosis. The activation of HSC is regulated by known and unknown cytokines. IGFBP-7 is one member of the IGFBP superfamily, which is thought to work as a potential activator of HSC.
The paper by Liu et al is an interesting one, not only because of its findings, but also because of the quality of the presented data.
| 2. | Gressner AM. Liver fibrosis: perspectives in pathobiochemical research and clinical outlook. Eur J Clin Chem Clin Biochem. 1991;29:293-311. |
| 3. | Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247-2250. |
| 4. | Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524-529. |
| 5. | Parsons CJ, Takashima M, Rippe RA. Molecular mechanisms of hepatic fibrogenesis. J Gastroenterol Hepatol. 2007;22 Suppl 1:S79-S84. |
| 6. | Yumei F, Zhou Y, Zheng S, Chen A. The antifibrogenic effect of (-)-epigallocatechin gallate results from the induction of de novo synthesis of glutathione in passaged rat hepatic stellate cells. Lab Invest. 2006;86:697-709. |
| 7. | Gressner AM. Cytokines and cellular crosstalk involved in the activation of fat-storing cells. J Hepatol. 1995;22:28-36. |
| 8. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. |
| 9. | Casini A, Pinzani M, Milani S, Grappone C, Galli G, Jezequel AM, Schuppan D, Rotella CM, Surrenti C. Regulation of extracellular matrix synthesis by transforming growth factor beta 1 in human fat-storing cells. Gastroenterology. 1993;105:245-253. |
| 10. | Tiggelman AM, Boers W, Linthorst C, Sala M, Chamuleau RA. Collagen synthesis by human liver (myo)fibroblasts in culture: evidence for a regulatory role of IL-1 beta, IL-4, TGF beta and IFN gamma. J Hepatol. 1995;23:307-317. |
| 11. | Boers W, Aarrass S, Linthorst C, Pinzani M, Elferink RO, Bosma P. Transcriptional profiling reveals novel markers of liver fibrogenesis: gremlin and insulin-like growth factor-binding proteins. J Biol Chem. 2006;281:16289-16295. |
| 12. | Novosyadlyy R, Dargel R, Scharf JG. Expression of insulin-like growth factor-I and insulin-like growth factor binding proteins during thioacetamide-induced liver cirrhosis in rats. Growth Horm IGF Res. 2005;15:313-323. |
| 13. | Hwa V, Oh Y, Rosenfeld RG. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr Rev. 1999;20:761-787. |
| 14. | Anan A, Baskin-Bey ES, Bronk SF, Werneburg NW, Shah VH, Gores GJ. Proteasome inhibition induces hepatic stellate cell apoptosis. Hepatology. 2006;43:335-344. |
| 15. | Ramadori G, Veit T, Schwogler S, Dienes HP, Knittel T, Rieder H, Meyer zum Buschenfelde KH. Expression of the gene of the alpha-smooth muscle-actin isoform in rat liver and in rat fat-storing (ITO) cells. Virchows Arch B Cell Pathol Incl Mol Pathol. 1990;59:349-357. |
| 16. | Rockey DC, Boyles JK, Gabbiani G, Friedman SL. Rat hepatic lipocytes express smooth muscle actin upon activation in vivo and in culture. J Submicrosc Cytol Pathol. 1992;24:193-203. |
| 17. | Conchillo M, de Knegt RJ, Payeras M, Quiroga J, Sangro B, Herrero JI, Castilla-Cortazar I, Frystyk J, Flyvbjerg A, Yoshizawa C. Insulin-like growth factor I (IGF-I) replacement therapy increases albumin concentration in liver cirrhosis: results of a pilot randomized controlled clinical trial. J Hepatol. 2005;43:630-636. |
| 18. | Wolf E, Schneider MR, Zhou R, Fisch TM, Herbach N, Dahlhoff M, Wanke R, Hoeflich A. Functional consequences of IGFBP excess-lessons from transgenic mice. Pediatr Nephrol. 2005;20:269-278. |
| 19. | Skrtic S, Wallenius K, Gressner AM, Jansson JO. Insulin-like growth factor signaling pathways in rat hepatic stellate cells: importance for deoxyribonucleic acid synthesis and hepatocyte growth factor production. Endocrinology. 1999;140:5729-5735. |
| 20. | Svegliati-Baroni G, Ridolfi F, Di Sario A, Casini A, Marucci L, Gaggiotti G, Orlandoni P, Macarri G, Perego L, Benedetti A. Insulin and insulin-like growth factor-1 stimulate proliferation and type I collagen accumulation by human hepatic stellate cells: differential effects on signal transduction pathways. Hepatology. 1999;29:1743-1751. |
| 21. | Sanz S, Pucilowska JB, Liu S, Rodriguez-Ortigosa CM, Lund PK, Brenner DA, Fuller CR, Simmons JG, Pardo A, Martinez-Chantar ML. Expression of insulin-like growth factor I by activated hepatic stellate cells reduces fibrogenesis and enhances regeneration after liver injury. Gut. 2005;54:134-141. |
| 22. | Clemmons DR. Insulin-like growth factor binding proteins and their role in controlling IGF actions. Cytokine Growth Factor Rev. 1997;8:45-62. |
| 23. | Clemmons DR. Use of mutagenesis to probe IGF-binding protein structure/function relationships. Endocr Rev. 2001;22:800-817. |
| 24. | Kelley KM, Schmidt KE, Berg L, Sak K, Galima MM, Gillespie C, Balogh L, Hawayek A, Reyes JA, Jamison M. Comparative endocrinology of the insulin-like growth factor-binding protein. J Endocrinol. 2002;175:3-18. |
| 25. | Rajaram S, Baylink DJ, Mohan S. Insulin-like growth factor-binding proteins in serum and other biological fluids: regulation and functions. Endocr Rev. 1997;18:801-831. |
| 26. | Yasuoka H, Zhou Z, Pilewski JM, Oury TD, Choi AM, Feghali-Bostwick CA. Insulin-like growth factor-binding protein-5 induces pulmonary fibrosis and triggers mononuclear cellular infiltration. Am J Pathol. 2006;169:1633-1642. |
| 27. | Eyden B. The myofibroblast: an assessment of controversial issues and a definition useful in diagnosis and research. Ultrastruct Pathol. 2001;25:39-50. |
| 28. | Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, Borok Z. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol. 2005;166:1321-1332. |
| 29. | Hautekeete ML, Geerts A. The hepatic stellate (Ito) cell: its role in human liver disease. Virchows Arch. 1997;430:195-207. |
| 30. | Serini G, Gabbiani G. Mechanisms of myofibroblast activity and phenotypic modulation. Exp Cell Res. 1999;250:273-283. |