Published online Jun 28, 2009. doi: 10.3748/wjg.15.3038
Revised: May 19, 2009
Accepted: May 26, 2009
Published online: June 28, 2009
AIM: To present a series of cases with symptomatic acute extensive portal vein (PV) and superior mesenteric vein (SMV) thrombosis after splenectomy treated by transjugular intrahepatic approach catheter-directed thrombolysis.
METHODS: A total of 6 patients with acute extensive PV-SMV thrombosis after splenectomy were treated by transjugular approach catheter-directed thrombolysis. The mean age of the patients was 41.2 years. After access to the portal system via the transjugular approach, pigtail catheter fragmentation of clots, local urokinase injection, and manual aspiration thrombectomy were used for the initial treatment of PV-SMV thrombosis, followed by continuous thrombolytic therapy via an indwelling infusion catheter in the SMV, which was performed for three to six days. Adequate anticoagulation was given during treatment, throughout hospitalization, and after discharge.
RESULTS: Technical success was achieved in all 6 patients. Clinical improvement was seen in these patients within 12-24 h of the procedure. No complications were observed. The 6 patients were discharged 6-14 d (8 ± 2.5 d) after admission. The mean duration of follow-up after hospital discharge was 40 ± 16.5 mo. Ultrasound and contrast-enhanced computed tomography confirmed patency of the PV and SMV, and no recurrent episodes of PV-SMV thrombosis developed during the follow-up period.
CONCLUSION: Catheter-directed thrombolysis via transjugular intrahepatic access is a safe and effective therapy for the management of patients with symptomatic acute extensive PV-SMV thrombosis.
- Citation: Wang MQ, Lin HY, Guo LP, Liu FY, Duan F, Wang ZJ. Acute extensive portal and mesenteric venous thrombosis after splenectomy: Treated by interventional thrombolysis with transjugular approach. World J Gastroenterol 2009; 15(24): 3038-3045
- URL: https://www.wjgnet.com/1007-9327/full/v15/i24/3038.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.3038
Portal vein (PV) and superior mesenteric vein (SMV) thrombosis is an uncommon but lethal complication occurring after splenectomy[12]. The incidence of this type of complication ranges from 1.6% to 11% in some series[3] and from 6.3% to 10% in others[45]. Although many patients with PV and SMV thrombosis may be asymptomatic, the consequences of these thromboses can be severe, and include mesenteric ischemia and variceal bleeding, with a mortality rate of 5%-37%[2]. There are no uniform protocols for the effective treatment of PV-SMV thrombosis following splenectomy, including duration of anticoagulation therapy and the potential effectiveness of prophylactic perioperative antiplatelet agents[6–9]. However, to avoid lethal complications, appropriate treatment should be performed as soon as possible, especially in patients with SMV involvement[46].
Recently, endovascular interventional techniques have been recognized as promising alternatives for the treatment of PV-SMV thrombosis. Case reports of the successful treatment of PV-SMV thrombosis include intra-arterial infusion of thrombolytics via the superior mesenteric artery (SMA) by the transfemoral artery[1011], thrombolysis via a transjugular intrahepatic approach[12–14], and percutaneous transhepatic mechanical or pharmacologic thrombolysis[15–17]. The aim of the present study is to report the clinical outcome of 6 patients with acute symptomatic PV and SMV thrombosis after splenectomy who were treated with transjugular intrahepatic portal access aspiration thrombectomy and catheter-directed thrombolysis at our hospital.
The study was approved by the institutional review board at our hospital. The potential risks and benefits of the procedure were explained, and informed consent was obtained from each patient.
Between March 2001 and October 2007, using the transjugular intrahepatic approach, we treated 6 patients (1 woman, 5 men) with a mean age of 41.2 years (range 32-52 years) who had symptomatic extensive PV and SMV thrombosis after open splenectomy. All 6 patients had acute abdominal symptoms of less than 3 wk (range, 3-16 d; average, 8.5 d).
Of these patients, four underwent splenectomy due to liver cirrhosis with portal hypertension, gastroesophageal variceal bleeding, and hypersplenism; splenectomy was performed in two patients due to portal hypertension and gastroesophageal variceal bleeding, caused by splenic vein occlusion. These 2 patients had a history of pancreatitis. All 6 patients had marked splenomegaly. The pre-splenectomy platelet count was less than 100 × 103/mm3 (3.5-6.5 × 103/mm3; reference range, 100-300 × 103/mm3), and in the normal range in 2 patients. The interval between the onset of symptoms of PV-SMV thrombosis and splenectomy was 11 to 35 d (median, 20.5 d).
All 6 patients initially presented with abdominal pain of insidious onset associated with nausea; 3 had distension, 3 had diarrhea, 2 had low-grade fevers, 1 had vomiting, and 1 had heme-positive stools. All patients were hemodynamically stable, and no clinical signs of peritonitis were noted at abdominal examination (Table 1).
| No. of patients | Age (yr)/sex | Symptoms | Etiologies of splenectomy | Platelet count at admission (× 103/mm3) | Onset of symptoms postoperative day |
| 1 | 40/M | Fever, abdominal pain, distension, and nausea | Cirrhosis, portal hypertension, and variceal bleeding | 540 | 16 |
| 2 | 43/M | Epigastric pain, diarrhea, fever, and nausea | Cirrhosis, portal hypertension, variceal bleeding, hypersplenism | 280 | 14 |
| 3 | 38/F | Abdominal pain, nausea, distension, and vomiting | Portal hypertension, variceal bleeding | 660 | 11 |
| 4 | 42/M | Abdominal pain, diarrhea, and nausea | Cirrhosis, portal hypertension, variceal bleeding, hypersplenism | 360 | 19 |
| 5 | 32/M | Abdominal pain, distension, nausea, heme-positive stools | Cirrhosis, portal hypertension, variceal bleeding, hypersplenism | 240 | 35 |
| 6 | 52/M | Abdominal pain, nausea, and diarrhea | Portal hypertension, hypertension, variceal bleeding, hypersplenism | 340 | 28 |
On admission, increased platelet count (340-660 × 103/mm3) was observed in 4 patients (Table 1). Increased white blood cell count (12.5 ± 1.5 × 103/mm3; range, 11.5-14.0 × 103/ mm3) was found in 3 patients. Increases in transaminases (AST, 60-80 IU, reference range, 5-40 IU; ALT, 65-110 IU, reference range, 5-40 IU) were seen in 3 patients. Normal values were detected for thrombin time, serum D-dimer, C-reactive protein, serum D-lactate, and amylase. A workup for hypercoagulable states (factor V leiden, protein C and S deficiency, antithrombin III deficiency, and lupus anticoagulant) yielded normal results.
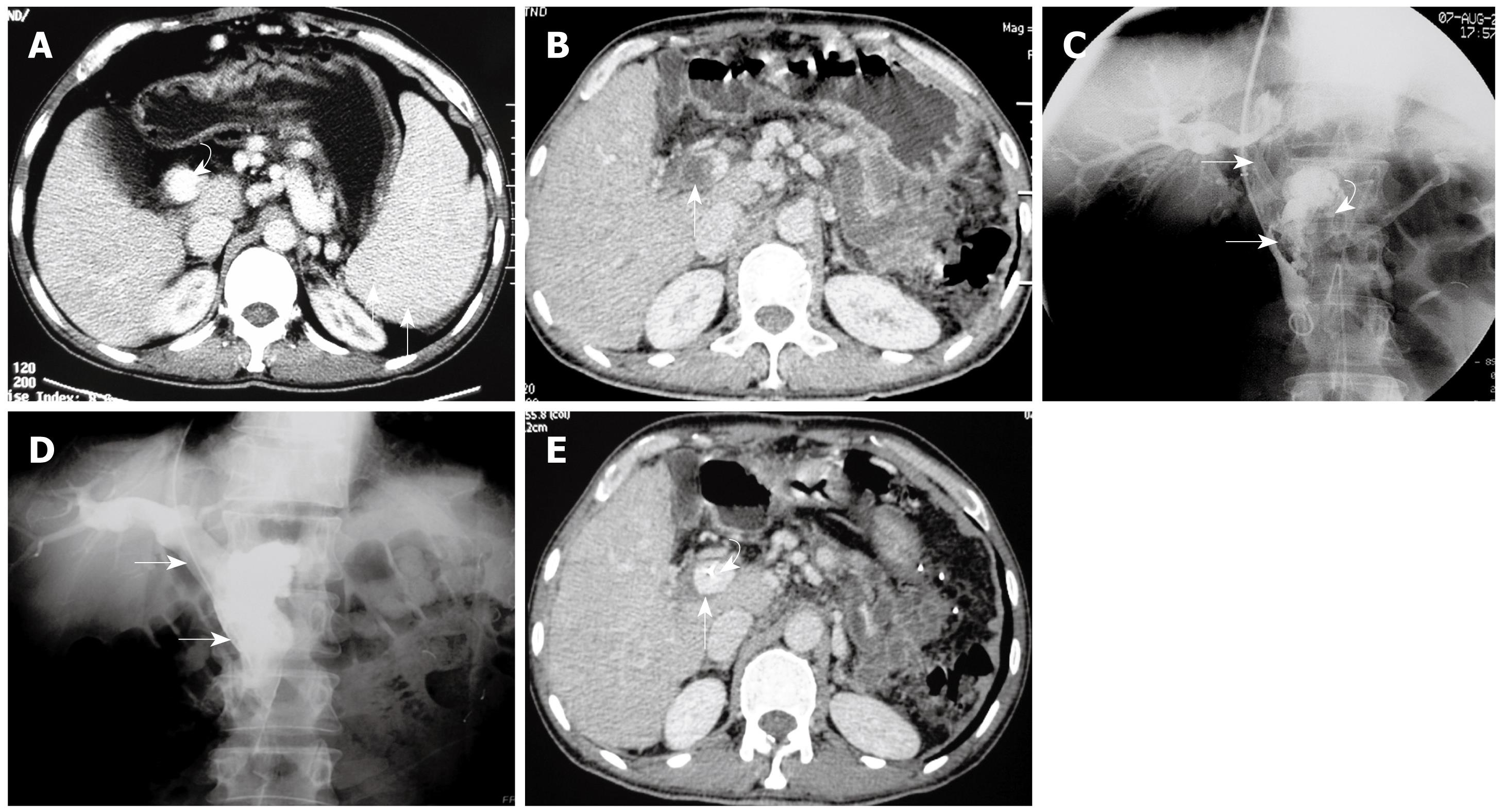
Ultrasonography (US) and computed tomography (CT) were performed in all 6 patients. All patients had SMV thrombosis extending into the main portal vein which was confirmed by the imaging study.
All patients were treated initially with bowel rest and nasogastric suction, intravenous fluid administration, broad-spectrum prophylactic antibiotics (including ampicillin, gentamycin, and metronidazole), and intravenous heparin adjusted to maintain the activated partial thromboplastin time ratio between 2.0 and 2.5 times the control.
Systemic anticoagulation after the diagnosis of PV and SMV thrombosis was assessed for 2 d in 4 cases and 3 d in 2 cases, however, the symptoms continued in 4 patients with worsening abdominal pain in 2 patients. After discussions with the surgery and medicine departments, and given the lack of clinical and radiographic suspicion for ischemic bowel, these patients were referred to the interventional radiology department for catheter-directed thrombolysis to achieve rapid restoration of PV-SMV flow. The mean time from admission to our institute to treatment with catheter-directed thrombolysis was 3.5 d, with a range of 2.5-5 d.
In our hospital, catheter-directed thrombolysis was employed in patients with acute and subacute PV and SMV thrombosis, with severe symptoms, and with persistent symptoms or worsening of symptoms despite anticoagulation.
Contraindications to interventional thrombolysis included mesenteric infarction, recent gastrointestinal bleeding, recent stroke, and primary or metastatic central nervous system malignancies.
Before the transjugular approach was attempted, the portal system was studied with indirect portography obtained during the venous phase following iodinated contrast medium injections into the SMA and the splenic artery. Angiography revealed patent superior mesenteric and splenic arteries. Venous phase confirmed complete thrombosis of the SMV and PV.
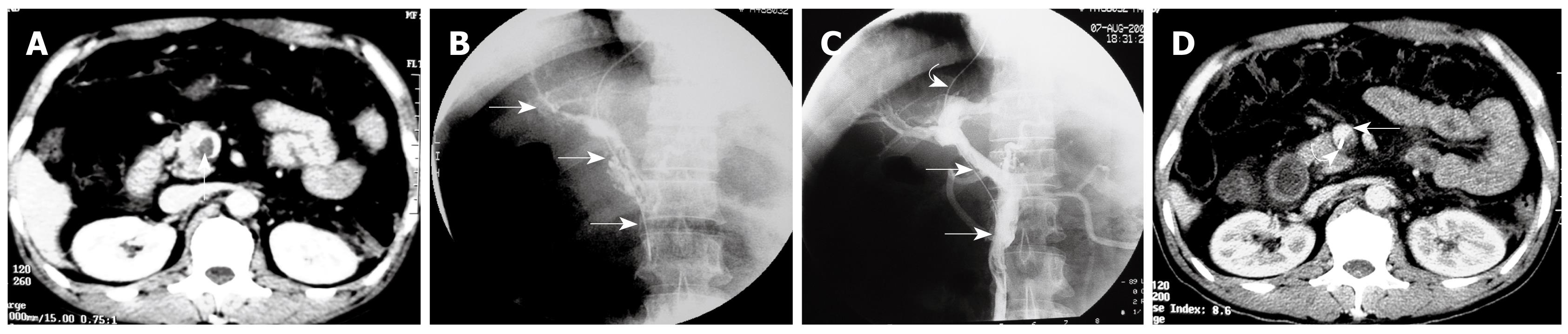
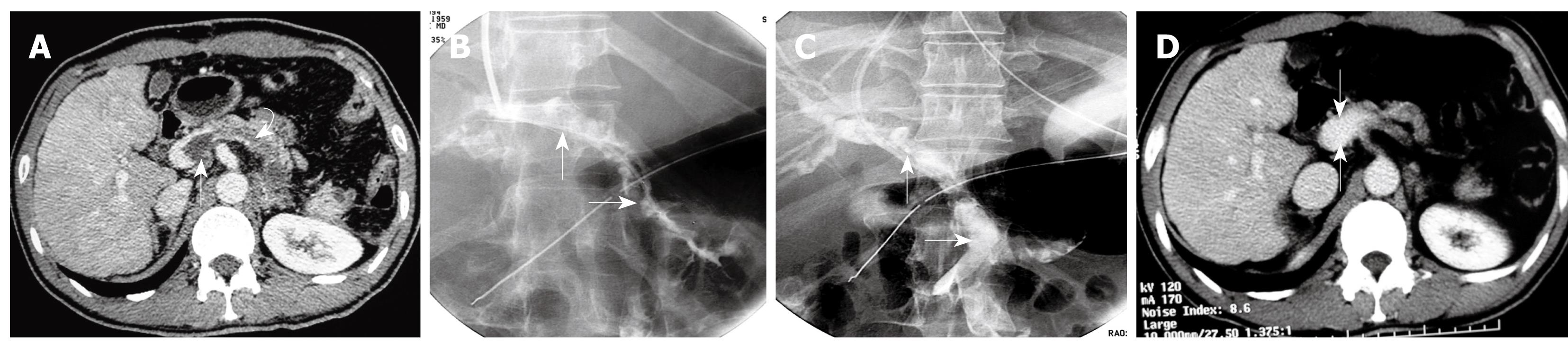
The transjugular approach was carried out according to the technique previously described[12–14] by using US and fluoroscopic guidance of the portal vein puncture. Following infiltration of local anesthesia, a Rosch-Uchida set (Cook, Bloomington, IN, USA) was used to gain access to the portal vein branch. Once the catheter was placed inside a portal branch, the thrombus could be traversed with the aid of a 4 Fr Cobra catheter (Cordis, the Netherlands) and a hydrophilic guidewire (Terumo, Japan). After reaching distal branches of the SMV, the Cobra catheter was exchanged for an 8 mm diameter angioplasty balloon catheter (Boston Scientific, MA, USA), to open up a channel, and then a 10-Fr Rosch-Uchida sheath (Cook) was put into the portal trunk. A bolus of 3000 IU of heparin was injected via a peripheral venous catheter.
Through the 10-Fr sheath, an angled 8-Fr guiding catheter (Cordis) was used to aspirate as much of the thrombus as possible from the SMV and PV with a Luer-Lok 60-mL syringe. The aspiration procedure was performed from distal to proximal clots in 8-12 cycles (10 ± 2). Simultaneously, a 5-Fr pigtail catheter (Cordis) was used to fragment the thrombus with “spinning technique”[16] and an injection of urokinase (TIANJIN Biochemical Pharmaceutical Co., LTD, China) 200 000- 300 000 IU using a hand-pulse spray technique.
Following the mechanical aspiration procedure, a 4-Fr multiple side-hole catheter (Angiodynamics, Queensbury, NY, USA) was placed with the tip in the SMV, and then continuous thrombolytic therapy was started with urokinase 50 000 IU/h. Heparin infusion was given simultaneously via a peripheral venous catheter, at a dose of 1000 IU/h. The adequacy of anticoagulation was adjusted to maintain the activated partial thromboplastin time between 2.0 and 2.5 times the control, during treatment and throughout hospitalization. During the prolonged infusion of thrombolytics, patients had PV-SMV venographic follow-up via the infusion catheter every 24 h. Color Doppler ultrasound scan (CDUS) assessment of PV and SMV patency was performed at 24, 48, and 72 h, 1 wk following the procedure, and at discharge.
Termination of the infusion of thrombolytics was based on clinical and radiographic findings. The catheter infusion of thrombolytics was discontinued after the patients’ symptoms (i.e. abdominal pain, distention, and diarrhea) had improved sufficiently that they were able to begin oral intake, and the repeated venography demonstrated good flow from the SMV into the portal vein, and no relapse of the thrombosis. The patients were then placed on chronic anticoagulation with warfarin adjusted to maintain an International Normalized Ratio of 2-3 after discharge.
Follow-up US was performed at patient discharge, every 2-3 mo in the first year, and every 4-6 mo in the second year. Follow-up CT was carried out at patient discharge, and then every 3 mo for 1 year and then every 6-12 mo thereafter.
Technical success was defined as successful catheterization of the portal vein, removal of the majority clots, and restoration of flow in the trunk of PV and SMV. Clinical success was defined as relief of symptoms and bowel resection was not required after the procedure. Minor complications were defined as no therapy or normal therapy without consequence.
Technical success was achieved in all 6 patients. No complications, such as hemorrhage or contrast extravasation were observed during the procedures. Direct venography of PV-SMV after access to the portal vein confirmed extensive thrombosis in the PV and SMV with poorly formed collateral drainage (Figures 1 and 2).
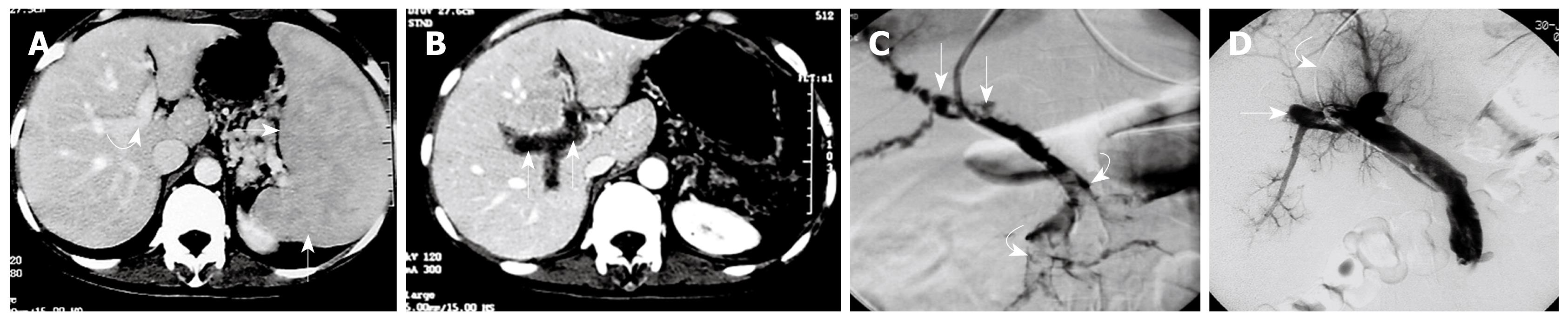
Using pigtail catheter fragmentation, local urokinase injection, and manual aspiration thrombectomy of the PV-SMV thrombosis resulted in removal of clots ≥ 60% (60%-80%) in all 6 patients (Figures 3 and 4). Restoration of partial flow in the main PV and SMV was documented on immediate follow-up direct portal venography.
After mechanical thrombolysis, continuous thrombolytic therapy via the indwelling infusion catheter in the SMV was performed for three to six days (4.5 ± 1.5 d). The mean total dose of urokinase via the catheter infusion was 5.8 million IU (range, 3.6-7.2 million). At completion of indwelling catheter infusion of thrombolytics a near complete lysis of clots (removal of clots greater than 90%) was observed in 4 patients and a partial lysis with a degree of residual thrombus less than 20% was observed in 2 patients, which were confirmed by repeated venography via the infusion catheter at the SMV.
Sufficient clinical improvement was seen in all 6 patients after 12-24 h of the mechanical thrombolysis procedure, characterized by a progressive reduction in abdominal pain, nausea, diarrhea, and distention. The patients continued to improve clinically during thrombolysis via the indwelling infusion catheter in the SMV. Oral intake was started at 3-5 d (4 ± 1.0 d) after abdominal pain, nausea, distention, and diarrhea was completely resolved. No patient required bowel resection after the procedures. No thrombotic, hemorrhagic, or infectious complications were noted during hospitalization. All 6 patients were discharged within 6-14 d (8 ± 2.5 d) of admission.
Contrast-enhanced CT was obtained in all 6 patients before discharge. The images demonstrated almost complete disappearance of PV-SMV thrombosis in 4 patients, and partial recanalization of the PV and SMV with residual thrombus (less than 20% compared to pre-treatment) in 2 patients. Three patients had abnormal AST and ALT values at admission, which returned to normal at discharge.
The mean duration of follow-up after hospital discharge was 40 ± 16.5 mo (range, 15-62 mo). All 6 patients are alive at writing, and no recurrent episodes of PV and SMV thrombosis developed during the follow-up period. Chronic anticoagulation with oral warfarin was initiated in all 6 patients at least 6 mo (6-12 mo) after hospital discharge. During the 15-62 mo follow-up period, US and contrast-enhanced CT confirmed the patency of the PV-SMV, without cavernous transformation of the PV or extrahepatic collaterals.
The platelet count returned to the normal range in four patients. Long term oral aspirin was given at a dose of 100 mg/d to 2 patients because their platelet count was > 300 × 103/mm3 (380 × 103/mm3, 460 × 103/mm3, respectively, at the last examination).
Although the exact mechanisms of PV-SMV thrombosis formation after splenectomy remain unclear, altered platelet function as well as transient thrombocytosis after splenectomy, a decrease in portal blood flow and pressure, and stasis of blood in the stump of the splenic vein appear to predispose to PV-SMV thrombosis[618]. Ikeda et al[4] reported that patients with PV-SMV thrombosis after splenectomy had a significantly heavier splenic weight than those without PV-SMV thrombosis, suggesting that a large splenic mass is a possible risk factor for post-splenectomy PV-SMV thrombosis. Stamou et al[7] reported that a platelet count of more than 650 × 103/mm3 and greater spleen weight (> 650 g) was associated with the development of portal system thrombosis. In our cases, marked splenomegaly was present in all 6 patients. In addition, a significant increase in platelet count was found in 4 patients at admission. However, we can not draw any conclusions due to our very small group of patients and lack of a control group.
Medical and surgical options are of limited value in extensive PV-SMV thrombosis[12]. In symptomatic PV-SMV thrombosis patients, treatment depends on the presence or absence of clinical and CT peritoneal signs. An emergency laparotomy with resection of necrotic bowel is necessary in the former condition and anticoagulant and/or thrombolytic therapy in the latter condition. The advantage of surgical embolectomy is that it allows for direct inspection of the bowel at the time of embolectomy and resection of necrotic bowel, if necessary. However, it is often difficult to remove all the thrombus from the small branches of the mesenteric veins. Often, adjuvant thrombolytic therapy is necessary and can be associated with a high risk of bleeding in the postoperative patient[1920]. Systemic anticoagulant and/or thrombolytic therapy is of limited value in extensive PV-SMV thrombosis, as it has low efficacy and is time consuming[2].
In patients with acute or subacute symptomatic PV-SMV thrombosis, endovascular interventional treatment has been reported with encouraging initial results[10–17]. With this approach, PV-SMV thrombosis can be managed by pharmacologic thrombolysis and/or mechanical thrombectomy. For pharmacologic thrombolysis, possible routes of treatment include indirect intra-arterial infusion of thrombolytic agents via the SMA[1011] and direct access to the portal vein, by the transjugular[12–14] or transhepatic routes[15–17]. Mechanical thrombectomy techniques include balloon angioplasty, thrombectomy devices, aspiration thrombectomy, stent placement, and TIPS creation[1415].
Indirect thrombolytic therapy via the SMA is less technically demanding and has been described for its potential benefits in infusing thrombolytic agents into small mesenteric venous branches[1011]. However, this approach does not allow direct infusion into the thrombus, may result in lytic agents diverting through patent branches and collaterals, and possible prolongation of the total infusion time via the SMA[12], which may result in an increased risk of bleeding. Direct access to the portal vein by a transjugular or transhepatic route directly targets the PV-SMV thrombosis, leading to fast removal of the thrombus and flow improvement, and an improvement in the patient’s symptoms[1415].
Percutaneous transhepatic access is technically relatively easy and allows the maneuver of mechanical devices compared with transjugular intrahepatic access. Usually, this approach is suitable for the removal of larger clots within the trunk of the PV and SMV. The drawbacks of the percutaneous transhepatic route include the development of intraperitoneal or subcapsular hepatic hemorrhage[1621]. This is likely to occur given that the transhepatic route for mechanical thrombectomy of splanchnic venous thrombosis requires traversing the hepatic capsule and is followed by thrombolysis and possibly systemic anticoagulation.
Our 6 patients with acute extensive PV-SMV thrombosis after splenectomy treated with the transjugular intrahepatic approach demonstrates the feasibility of this route to the management of this challenging illness. The transjugular approach access to the portal vein is generally performed with the creation of a transjugular intrahepatic portosystemic shunt; this approach is usually indicated for patients with cirrhosis with portal hypertension caused by portal vein thrombosis[1314]. Compared to the percutaneous transhepatic approach, the transjugular intrahepatic approach does not require traversing the hepatic capsule, and thus would eliminate the risk of subcapsular hemorrhage[1214]. Furthermore, the transjugular intrahepatic approach is safer in patients with anticoagulation. In addition, based on our experience with the TIPS procedure, we opted for this approach to treat an extensive acute PV-SMV thrombosis. Although we did not observe any complications in our 6 patients with the transjugular approach, significant intra-abdominal bleeding is a potential serious complication[22].
Mechanical thrombectomy devices and aspiration thrombectomy are feasible and effective in the re-establishment of portal and mesenteric circulation in patients with acute extensive thrombosis[1221]. Good results have been obtained with thrombectomy devices such as the Arrow-Trerotola, Oasis, Amplatz thrombectomy, and AngioJet, although the clinical experience with these thrombectomy devices in PV-SMV thrombosis is limited[122324]. We did not use mechanical thrombectomy devices in our small series because these devices were unavailable at that time in our angiographic laboratory. Aspiration of the fresh thrombus with a large lumen catheter has also been reported[1425]. The advantages of this technique are that a large-lumen catheter is generally available in standard angiography laboratories, is of low cost compared with the various thrombectomy devices, and is an easy device which can be as effective as the various thrombectomy devices in removing thrombus from a vessel[1625].
In patients with acute extensive thrombosis of the PV-SMV, mechanical thrombectomy could initially be used to debulk the thrombus, and pharmacologic thrombolysis would probably still be necessary in most cases to treat residual thrombosis and to treat thrombus in the small and peripheral veins[1623]. The combination of aspiration and local pharmacological thrombolysis via a direct access to the portal system is more effective and significantly decreases the treatment time in patients with extensive PV-SMV thrombosis compared to indirect and direct thrombolysis infusion alone[122324]. In our 6 cases, aspiration thrombectomy associated with indwelling catheter infusion of thrombolytics into the SMV was effective, resulted in a rapid improvement in symptoms, recanalization of the SMV, resolution of symptoms, and resumption of oral nutrition.
Combining thrombolytic infusion with anticoagulation would appear to increase the risk of bleeding and hemorrhage[2627]. A study by Ouriel et al[26] described the complication rates for patients with lower-extremity arterial or venous occlusions treated with local urokinase or rt-PA. Overall, 15% required transfusion and 1.2% developed intracranial hemorrhage, which was fatal in 8 of 9 cases. In our series, no bleeding complications occurred. This may have resulted from the relatively low dose infusion of urokinase via the catheter in the SMV, no simultaneous peripheral venous infusion of urokinase, and careful monitoring of the coagulation status during treatment. As for thrombolytic agents, we prefer urokinase to rt-PA (recombinant tissue plasminogen activator), because it is similarly active on thrombus dissolution but appears to be safer, being associated with a lower incidence of hemorrhagic complications[26]. In addition, urokinase is generally available in our institution, and is less costly than rt-PA.
Interventional endovascular thrombectomy and direct thrombolysis can offer a non-surgical alternative for the treatment of extensive PV-SMV thrombosis[10–17]. However, this can only be performed in a select group of patients who do not present with bowel ischemia and infarction, or who are not at risk for bleeding, and who have persistent symptoms or worsening of symptoms despite anticoagulation[12]. Minimally symptomatic or asymptomatic patients with PV-SMV thrombosis may best be treated with systemic anticoagulation only. Prompt surgical intervention should be undertaken if the patient’s condition deteriorates or clinical signs of peritonitis develop during the interventional treatment[2]. In this small group, we chose interventional procedures to treat these patients because systemic anticoagulation was ineffective and because the SMV was involved. The results obtained in our series of patients can be considered satisfactory. All 6 patients showed a patent SMV and PV, without recurrent episodes, during a mean follow-up of 40 mo.
The limitations of this study include the lack of a control group, randomization, and uniformity of evaluation and treatment. Because of the small sample size, no statistically significant conclusions could be drawn regarding treatment with respect to dosages of thrombolytic agent or heparin, techniques, or underlying risk factors. This is partly related to the low incidence of the illness as well as the natural evolution of therapeutic techniques during the last 10 years.
In summary, the combination of catheter fragmentation of clots, aspiration thrombectomy, and indwelling catheter infusion of thrombolytics via transjugular intrahepatic access to the portal system, is a safe and effective therapy for the management of patients with acute extensive PV-SMV thrombosis. Because of the small size of the study and other factors, the ability to generalize the results is limited.
Thrombosis of the portal vein (PV) and superior mesenteric vein (SMV) is considered a possible cause of death after splenectomy. The reported incidence of PV-SMV thrombosis after elective open splenectomy ranges from 6.3% to 11%. Although many patients with PV-SMV thrombosis may be asymptomatic, the consequences of these thromboses can be severe, including mesenteric ischemia and variceal bleeding, with a mortality rate of 5%-37%. There are no uniform protocols for the effective treatment of PV-SMV thrombosis following splenectomy.
The treatment of symptomatic acute thrombosis of the PV and SMV is controversial. Due to unsatisfactory results obtained in some cases with medical treatment, as well as the difficulty in performing surgical procedures in some cases, new possibilities, such as percutaneous techniques, have been investigated.
The authors report 6 patients with acute extensive PV-SMV thrombosis after splenectomy treated with transjugular approach catheter-directed thrombolysis, which demonstrated the feasibility of this route in the management of this challenging illness. Compared to the percutaneous transhepatic approach, the transjugular approach does not require traversing the hepatic capsule, and thus eliminates the risk of subcapsular hemorrhage. In addition, the transjugular approach is safer in patients with anticoagulation. Secondly, this is the first study to report on the efficacy of the combination of aspiration thrombectomy with an indwelling catheter infusion of thrombolytics into the SMV, which resulted in a rapid improvement in symptoms, recanalization of the SMV, and resolution of symptoms. Finally, the combination of thrombolytic infusion with anticoagulation can be associated with a high risk of bleeding. In the authors’ series, no bleeding complications occurred. This may have resulted from the relatively low dose infusion of urokinase via the catheter in the SMV, no simultaneous peripheral venous infusion of urokinase, and careful monitoring of the coagulation status during treatment.
Interventional endovascular thrombectomy and direct thrombolysis can offer a non-surgical alternative for the treatment of extensive PV-SMV thrombosis. This technique can be performed in patients who do not present with bowel ischemia and infarction, or who are not at risk for bleeding, and have persistent symptoms or worsening of symptoms despite anticoagulation.
Endovascular interventional techniques for the treatment of thrombosis include intra-vascular pharmacologic thrombolysis and mechanical thrombectomy. Catheter-directed intra-vascular thrombolysis can directly target the thrombus, leading to fast removal of the thrombus and flow improvement, and an improvement in symptoms. Interventional mechanical thrombectomy techniques include balloon angioplasty, thrombectomy devices, aspiration thrombectomy, stent placement, and TIPS creation.
This study reports 6 patients that were treated with techniques of interventional radiology to restore patency of portal venous thrombosis (extending to the mesenteric vein) that developed after being submitted to surgical splenectomy. The authors report a 100% success rate of the technique without side effects. Although the size of the series is small, the results can be considered satisfactory and is encouraged.
| 1. | Sobhonslidsuk A, Reddy KR. Portal vein thrombosis: a concise review. Am J Gastroenterol. 2002;97:535-541. |
| 2. | Kumar S, Sarr MG, Kamath PS. Mesenteric venous thrombosis. N Engl J Med. 2001;345:1683-1688. |
| 3. | Brink JS, Brown AK, Palmer BA, Moir C, Rodeberg DR. Portal vein thrombosis after laparoscopy-assisted splenectomy and cholecystectomy. J Pediatr Surg. 2003;38:644-647. |
| 4. | Ikeda M, Sekimoto M, Takiguchi S, Kubota M, Ikenaga M, Yamamoto H, Fujiwara Y, Ohue M, Yasuda T, Imamura H. High incidence of thrombosis of the portal venous system after laparoscopic splenectomy: a prospective study with contrast-enhanced CT scan. Ann Surg. 2005;241:208-216. |
| 5. | Hassn AM, Al-Fallouji MA, Ouf TI, Saad R. Portal vein thrombosis following splenectomy. Br J Surg. 2000;87:362-373. |
| 6. | Soyer T, Ciftci AO, Tanyel FC, Senocak ME, Büyükpamukçu N. Portal vein thrombosis after splenectomy in pediatric hematologic disease: risk factors, clinical features, and outcome. J Pediatr Surg. 2006;41:1899-1902. |
| 7. | Stamou KM, Toutouzas KG, Kekis PB, Nakos S, Gafou A, Manouras A, Krespis E, Katsaragakis S, Bramis J. Prospective study of the incidence and risk factors of postsplenectomy thrombosis of the portal, mesenteric, and splenic veins. Arch Surg. 2006;141:663-669. |
| 8. | Fujita F, Lyass S, Otsuka K, Giordano L, Rosenbaum DL, Khalili TM, Phillips EH. Portal vein thrombosis following splenectomy: identification of risk factors. Am Surg. 2003;69:951-956. |
| 9. | Rossi E, Michelini ME, Pignatti CB, Zanotti F, Franchella A. A case of portal vein thrombosis after laparoscopy-assisted splenectomy and cholecystectomy in a child. J Pediatr Surg. 2007;42:1449-1451. |
| 10. | Antoch G, Taleb N, Hansen O, Stock W. Transarterial thrombolysis of portal and mesenteric vein thrombosis: a promising alternative to common therapy. Eur J Vasc Endovasc Surg. 2001;21:471-472. |
| 11. | Safieddine N, Mamazza J, Common A, Prabhudesai V. Splenic and superior mesenteric artery thrombolytic infusion therapy for acute portal and mesenteric vein thrombosis. Can J Surg. 2007;50:68-69. |
| 12. | Sze DY, O'Sullivan GJ, Johnson DL, Dake MD. Mesenteric and portal venous thrombosis treated by transjugular mechanical thrombolysis. AJR Am J Roentgenol. 2000;175:732-734. |
| 13. | Aytekin C, Boyvat F, Kurt A, Yologlu Z, Coskun M. Catheter-directed thrombolysis with transjugular access in portal vein thrombosis secondary to pancreatitis. Eur J Radiol. 2001;39:80-82. |
| 14. | Ferro C, Rossi UG, Bovio G, Dahamane M, Centanaro M. Transjugular intrahepatic portosystemic shunt, mechanical aspiration thrombectomy, and direct thrombolysis in the treatment of acute portal and superior mesenteric vein thrombosis. Cardiovasc Intervent Radiol. 2007;30:1070-1074. |
| 15. | Hollingshead M, Burke CT, Mauro MA, Weeks SM, Dixon RG, Jaques PF. Transcatheter thrombolytic therapy for acute mesenteric and portal vein thrombosis. J Vasc Interv Radiol. 2005;16:651-661. |
| 16. | Ozkan U, Oğuzkurt L, Tercan F, Tokmak N. Percutaneous transhepatic thrombolysis in the treatment of acute portal venous thrombosis. Diagn Interv Radiol. 2006;12:105-107. |
| 17. | Guglielmi A, Fior F, Halmos O, Veraldi GF, Rossaro L, Ruzzenente A, Cordiano C. Transhepatic fibrinolysis of mesenteric and portal vein thrombosis in a patient with ulcerative colitis: a case report. World J Gastroenterol. 2005;11:2035-2038. |
| 18. | van't Riet M, Burger JW, van Muiswinkel JM, Kazemier G, Schipperus MR, Bonjer HJ. Diagnosis and treatment of portal vein thrombosis following splenectomy. Br J Surg. 2000;87:1229-1233. |
| 19. | Brunaud L, Antunes L, Collinet-Adler S, Marchal F, Ayav A, Bresler L, Boissel P. Acute mesenteric venous thrombosis: case for nonoperative management. J Vasc Surg. 2001;34:673-679. |
| 20. | Shah SR, Deshmukh HL, Mathur SK. Extensive portal and splenic vein thrombosis: differences in hemodynamics and management. Hepatogastroenterology. 2003;50:1085-1089. |
| 21. | Hechelhammer L, Crook DW, Widmer U, Wildermuth S, Pfammatter T. Thrombosis of a superior mesenteric vein aneurysm: transarterial thrombolysis and transhepatic aspiration thrombectomy. Cardiovasc Intervent Radiol. 2004;27:551-555. |
| 22. | Brountzos EN, Alexopoulou E, Koskinas I, Thanos L, Papathanasiou MA, Kelekis DA. Intraperitoneal portal vein bleeding during transjugular intrahepatic portosystemic shunt: treatment with stent-graft placement. AJR Am J Roentgenol. 2000;174:132-134. |
| 23. | Lopera JE, Correa G, Brazzini A, Ustunsoz B, Patel S, Janchai A, Castaneda-Zuniga W. Percutaneous transhepatic treatment of symptomatic mesenteric venous thrombosis. J Vasc Surg. 2002;36:1058-1061. |
| 24. | Kim HS, Patra A, Khan J, Arepally A, Streiff MB. Transhepatic catheter-directed thrombectomy and thrombolysis of acute superior mesenteric venous thrombosis. J Vasc Interv Radiol. 2005;16:1685-1691. |
| 25. | Eid-Lidt G, Gaspar J, Sandoval J, de los Santos FD, Pulido T, González Pacheco H, Martínez-Sánchez C. Combined clot fragmentation and aspiration in patients with acute pulmonary embolism. Chest. 2008;134:54-60. |
| 26. | Ouriel K, Gray B, Clair DG, Olin J. Complications associated with the use of urokinase and recombinant tissue plasminogen activator for catheter-directed peripheral arterial and venous thrombolysis. J Vasc Interv Radiol. 2000;11:295-298. |
| 27. | Schäfer C, Zundler J, Bode JC. Thrombolytic therapy in patients with portal vein thrombosis: case report and review of the literature. Eur J Gastroenterol Hepatol. 2000;12:1141-1145. |