INTRODUCTION
CD74, also known as the invariant chain or Ii, is a non-polymorphic glycoprotein that has diverse immunological functions. The most well-known function of CD74 is regulating the trafficking of class II major histocompatibility complex (MHC) proteins in antigen presenting cells. More recently, CD74 expression has been examined in cell types other than antigen presenting cells (APCs), such as epithelial cells[1]. Some studies also suggest that CD74 might be expressed independently of class II MHC, indicating additional functions[2]. Various studies have indicated that CD74 is highly expressed in inflammatory disorders and cancers. It also acts as a receptor for macrophage migration inhibitory factor (MIF) and facilitates adhesion of Helicobacter pylori (H pylori) to gastric epithelial cells (GECs)[34]. CD74 expression is increased during H pylori infection, chronic inflammatory conditions of the gastrointestinal (GI) tract, and gastric and colon cancers. One critical function it has in carcinogenesis is to act as an accessory signaling molecule for cell proliferation. This molecule is particularly important in the complex immunological mechanisms of the gastrointestinal tract and in the link between chronic inflammation and carcinogenesis in the GI tract.
THE ROLE OF CD74 IN ANTIGEN PRESENTATION
CD74 was initially characterized for its role in regulating class II MHC folding and intracellular sorting and has been studied in most detail in APCs. Newly synthesized CD74 self-assembles as a trimer and this trimer serves as a scaffold onto which class II MHC molecules assemble. CD74 blocks the peptide binding cleft of class II MHC and prevents premature binding of antigenic peptides. Upon endocytosis of antigens, CD74 directs the class II MHC complex to the endosomal pathway using two di-leucine-based signals[5]. Within an endosomal compartment CD74 is digested, leaving just one residual peptide, CLIP (amino acids 91-99), blocking the peptide binding cleft of class II MHC. A class II MHC-like molecule, HLA-DM, then binds to the class II MHC and CLIP is released leaving the peptide binding cleft open for antigenic peptide binding. Class II MHC molecules with bound peptides are then exported to the surface of the antigen presenting cell (APC) for presentation of foreign peptides to T cells. CD74 plays a crucial role in antigen presentation, as class II MHC processing and regulation cannot properly occur in the absence of CD74.
In addition to APCs, other cells of the gastrointestinal tract, such as epithelial cells, express class II MHC proteins and CD74 and act as APCs, which is an unusual trait of the GI tract. We have previously shown that gastric epithelial cells express class II MHC proteins and are capable of processing antigens for presentation to T cells[67]. Expression of class II MHC and the potential for antigen presentation have also been shown in intestinal epithelial cells. In one elaborate study by Hershberg et al, the trafficking of these molecules in epithelial cells was followed in a polarized manner outlining a functional system for antigen presentation[8]. Another important group of cells that express class II MHC proteins are the subepithelial intestinal myofibroblasts (IMF)[910]. These cells are α-smooth actin positive stromal cells that exist in the lamina propria of the gut[11]. They also act as antigen presenting cells and play an important role in inflammatory diseases and carcinogenesis by releasing cytokines and growth factors and interacting with the immune cells of the lamina propria.
In conventional APCs, CD74 surface expression is low as CD74 is proteolytically removed in endosomes, as we and others[1213] have shown. However, gastric epithelial cells express readily detectable levels of CD74 on the surface. When we examined human gastric biopsy sections by immunohistochemistry for epithelial expression of CD74, gastric biopsy samples from 44 random patients stained with anti-CD74 monoclonal antibodies (mAb) showed the expression of CD74 in the epithelium. In the samples that were positive for H pylori or had gastritis, CD74 staining was heavily increased[14]. This was corroborated by confocal microscopy studies of gastric epithelial cells grown as a polarized monolayer where expression was higher on the apical side of the cells[1].
CD74 ISOFORMS
CD74 is post-translationally glycosylated and exists in different isoforms. As evidence for this, our previous studies showed proteins with different mobilities when immunoprecipitated and subjected to gel electrophoresis[1]. After chemical deglycosylation, only the isoforms that result from alternative translation initiation or splicing were observed. The most common isoform is 33 kDa (p33), but p35, p41 and p43 isoforms also exist[15]. The p35 isoform contains a longer cytoplasmic tail due to the use of an alternative translation initiation site, while the p41 isoform results from alternative splicing, and p43 has both. Both the p33 and p35 isoforms appear to function in regulating class II MHC antigen presentation. However, the p41 isoform might also play an important role in T cell selection in the thymus[16]. An important posttranslational modification of CD74 commonly seen is the addition of a glycosaminoglycan side chain, chondroitin sulfate. This isoform has been reported to act as an accessory protein during T cell activation through interaction with CD44 on T cells[17].
CD74 AS A RECEPTOR
CD74 has begun to emerge as a more versatile molecule beyond its well-known function of regulating class II MHC trafficking. Multiple studies have revealed that cell surface expression of CD74 is not always dependent on class II MHC[218]. This was found to be true in studies of colorectal mucosa and different types of lymphocytes by immunohistochemistry, immunoprecipitation, and a mutant cell line that did not express class II MHC products. The expression of cell surface CD74 in the absence of class II MHC suggests alternative functions for CD74 apart from antigen presentation.
CD74 as a cytokine receptor
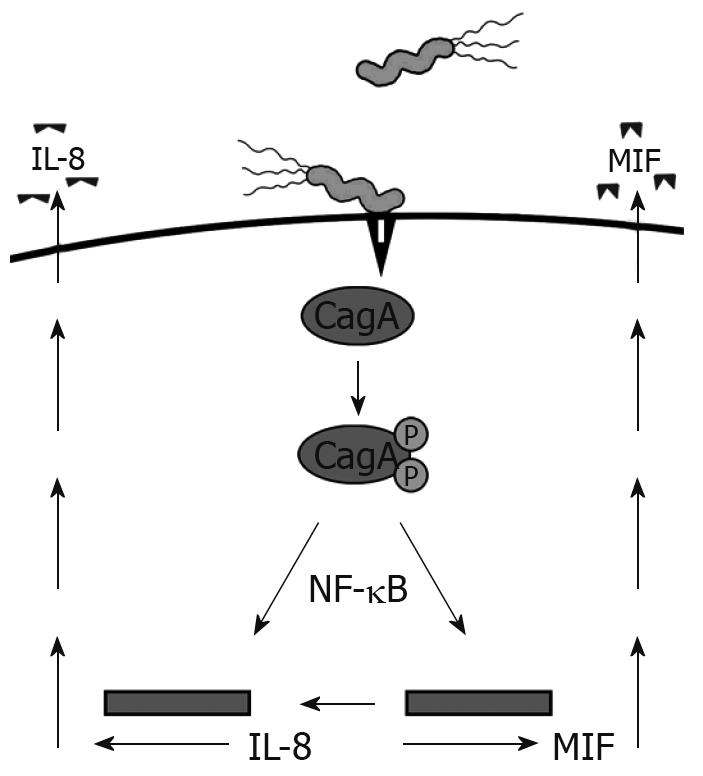
Recently, CD74 has emerged as an integral component of a receptor complex for macrophage MIF[3]. MIF is a versatile cytokine-like molecule that mediates both innate and adaptive immunity, plays a role in chronic inflammation, and has also been linked to carcinogenesis. MIF is expressed by the GI tract during inflammatory conditions, H pylori infection, and cancers, suggesting the importance of this interaction[19–21]. H pylori utilizes a type IV secretion system to inject the CagA protein into GECs, which we have shown induces MIF production[19] (Figure 1). CagA has also been shown to induce NF-κB activation and IL-8 production[22]. This protein is not only important in inflammation by increasing proinflammatory cytokine production, such as IL-8, but is also associated with gastric cancer[23]. The current model of this receptor complex suggests that CD44 is required in order for MIF to induce signaling events[24]. This model might require the chondroitin sulfate modified isoform of CD74, since CD44 has been shown to bind only to this isoform thus far.
Figure 1 H pylori induces MIF and IL-8 production by injecting CagA into GECs via a type IV secretion system.
Another study has suggested that CD74 complexes with CXCR2, an interleukin-8 (IL-8) receptor, which is commonly expressed on macrophages and functions to recruit leukocytes to sites of infection[25]. CXCR2 is also expressed by the gastric epithelium[26]. Since gastric epithelial cells are central players in the inflammatory response, IL-8 may act via the gastric epithelium in various processes associated with gastric inflammation linked to H pylori infection. The role of CXCR2 on the CD74 receptor complex has only recently been suggested and should be further investigated.
CD74 as a bacterial receptor
CD74 is an interesting example of a host cell receptor usurped by a pathogen because H pylori uses it to adhere to gastric epithelial cells (GECs). H pylori is a gram-negative spiral bacterium that colonizes the human gastroduodenal mucosa. Infection with H pylori usually begins in childhood and persists for decades if untreated. H pylori is recognized as a major contributor to chronic gastritis and peptic ulcer formation and is strongly associated with gastric carcinoma and lymphoma[2728]. Due to the strength of the evidence supporting an association between adenocarcinomas of the gastric mucosa and H pylori infection, H pylori was classified as a class I carcinogen by the International Agency for Research on Cancer in affiliation with the World Health Organization[29]. Gastric cancer remains among the most common forms of cancer and is the second deadliest cancer worldwide. Gastric cancer accounts for approximately 700 000 deaths annually worldwide and in the US there are 24 000 new cases and 14 000 deaths annually[30]. The prevalence rates of H pylori seropositivity and the incidence of gastric cancer are highly associated within several populations from various countries. For instance, seropositivity can be as high as 80%-100% in some age groups in some countries or in minorities with lower incomes in the United States[31]. These groups have the highest risk of developing gastric cancer and/or gastric ulcers. Thus, H pylori-associated diseases represent a significant global and domestic problem and result in considerable morbidity, mortality, and societal costs.
H pylori adhesion to the gastric epithelium is important in successful colonization of the gastric mucosa. Adherent strains survive in the gastric mucosa, reach high bacterial densities, and can re-colonize, whereas non-adherent strains are cleared[32]. These observations support the notion that adhesion is essential in H pylori persistence and disease induction. An assortment of molecules on epithelial cells have been proposed as receptors for H pylori adherence including carbohydrate moieties [such as Lewisb (Leb) blood group antigen and sialyl-dimeric-Lewisx (Lex)], lysophospholipid, and other structures[33–35]. Our studies have indicated that H pylori also utilizes CD74 to attach to gastric epithelial cells (GEC)[4]. The binding of H pylori to CD74 on gastric epithelial cells was confirmed by a series of independent approaches. For instance, blocking of CD74 with antibodies significantly reduced the binding of H pylori to gastric epithelial cells. Immunoprecipitation revealed that H pylori predominantly binds to the 33 kDa isoform of CD74, but further investigation is needed to test for attachment to the CS modified isoform. As H pylori has been reported to bind to various glycoconjugates, including glycosaminoglycans[36], this isoform of CD74 might contribute to the overall interaction of H pylori with the host gastric epithelium. We also revealed that urease is the protein on H pylori that binds to CD74[37]. This interaction is particularly interesting because many bacteria express urease, so the possibility exists that there might be wider applications of this type of interaction with CD74 depending on urease sequence variation between bacteria.
After adhesion of H pylori to GEC, the expression of cell surface CD74 is increased[14]. We further showed that CD74 expression increases in gastric epithelial cells of infected humans and a recent study confirmed that this increase in CD74 expression also occurred in a mouse model of H pylori infection[38]. Upon H pylori binding to CD74, NF-κB activation occurs resulting in the production of proinflammatory cytokines, including IL-8. IL-8 plays a major role in the proinflammatory immune response to H pylori infection, therefore, the interaction of H pylori with the gastric epithelial cells might be of critical importance in the immune response to infection.
THE ROLE OF CD74 IN SIGNALING EVENTS
The role of CD74 in signal transduction was initially hypothesized when it was found to be phosphorylated and associated with proteins that coordinate various signal transduction pathways[39–41]. Interestingly, the observation that CD74 deficient mice have a defect in B cell development that results in decreased levels of follicular B cells provided insights into the important role of signals delivered through CD74 in B cell development. The cytosolic domain of CD74 alone was noted to induce B cell maturation by activation of NF-κB[42]. CD74 appears to promote B cell survival; therefore, it has been implicated in B cell neoplasms such as gastric mucosa-associated lymphoid tissue (MALT) lymphomas associated with H pylori infection.
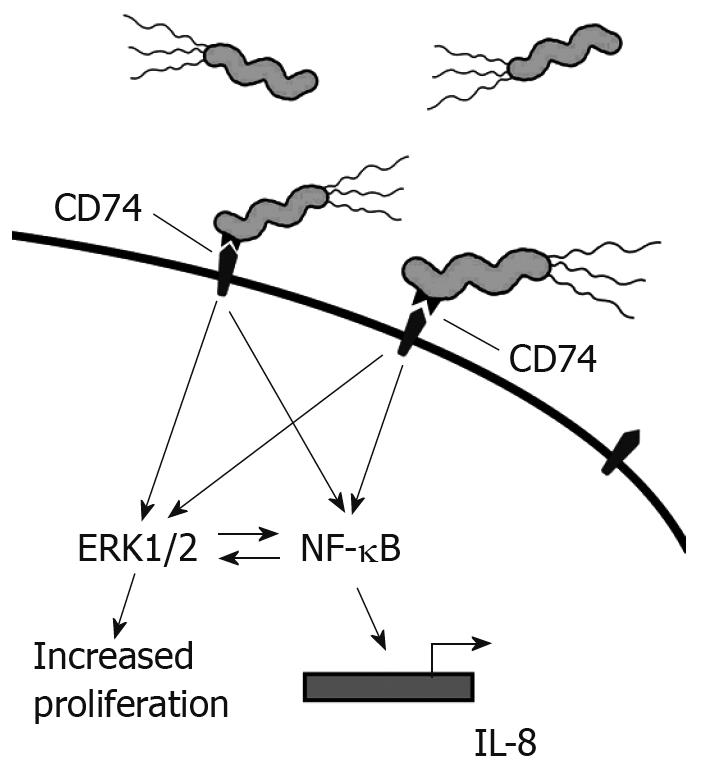
Our studies have shown that the interaction of H pylori with CD74 on GECs induces NF-κB activation and IL-8 production, as shown in Figure 2. MIF has also been shown to bind to CD74[3]. One study outlines a role for CD44 in CD74 signaling. CD44 was required to initiate ERK1 and ERK2 signaling after MIF binding to CD74. Cells deficient in CD44 or transfected with truncated CD44 were not able to induce ERK signaling[24]. In B cells, MIF binding to CD74 led to AKT, Syk, and NF-κB activation, and proliferation in a CD44-dependant manner[43]. CXCR2 has also been shown to complex with CD74 on monocytes[25]. This study also illustrated that MIF bound directly to CXCR2 and induced monocyte arrest. This study further showed that MIF might interact with CXCR4 on T cells and induce effector T cell arrest. However, it is not yet clear how CD44 is involved in the complexing of CD74 with CXCR2 and what signaling may be induced through CXCR4 since CXCR2 and CXCR4 are G protein-coupled receptors.
Figure 2 H pylori binds to CD74 on gastric epithelial cells and induces Nf-κB and Erk1/2 activation and IL-8 production.
Other studies suggest that MIF signaling may also occur by non-receptor mediated endocytosis in addition to the above described receptors[44]. In this proposed mechanism, endocytosed or endogenous MIF interacts with the Jun activation domain-binding protein (Jab1), which is a transcriptional activator for AP-1[45]. Activation of AP-1 might affect cell cycle events by inducing degradation of the cyclin dependent kinase inhibitor, which is a tumor suppresser gene.
THE ROLE OF CD74 IN GI INFLAMMATION
CD74 is highly expressed in inflammatory disorders. We have shown it to be expressed on the gastric epithelium and up-regulated during H pylori infection[14]. Others have shown expression to be increased in ulcerative colitis, where overexpression was shown in DNA microarray profiles[46]. Additionally, CD74 is increased during inflammation associated cancers, such as gastric and colon[4748]. Concurrently, MIF is highly expressed during many inflammatory conditions of the GI tract. We and others have shown that production is increased during H pylori infection[1949]. MIF is also highly expressed during inflammatory bowel disorders (IBD), such as ulcerative colitis and Crohn’s disease, where it is induced by intestinal bacteria[50]. In one study, elevated MIF levels were found in Crohn’s disease patients at approximately six times higher levels than in healthy controls. Crohn’s disease is an inflammatory bowel disorder where the immune system attacks part of the GI tract, and is accompanied by chronic inflammation. Also, this group went on to study MIF in murine colitis where they found colitis to be dependent on continuous MIF production, as evidenced by the protection from colitis by MIF-deficient mice or blocking MIF with monoclonal antibodies in mice with established colitis leading to reduced inflammation.
MIF or H pylori binding to CD74 induces NF-κB and subsequent cellular responses, such as the secretion of proinflammatory cytokines. MIF also increases inflammatory responses by overriding glucocortocoid suppression of inflammatory immune responses, including cytokines such as IL-1, IL-6, IL-8, and TNF-α[51]. One of the major proinflammatory cytokines produced after engagement of CD74 and receptor complexing is IL-8. IL-8 is a chemoattractant for neutrophils to a site of inflammation or infection. Upon arrival, they endocytose the antigen and form a phagosome in which reactive oxygen species and hydrolytic enzymes are released. While this process is crucial in fighting infections, it might also exacerbate inflammation in H pylori infection and inflammatory bowel disease[52–54]. In another study of glucocortocoid resistant ulcerative colitis, MIF was found to increase IL-8 production through the p38 MAPK pathway with isolated lamina propria mononuclear cells from patient biopsies[55]. When MIF was blocked with monoclonal antibodies after prednisolone treatment, activation of the p38 pathway and IL-8 decreased.
MIF might also contribute to inflammation by regulating Toll Like Receptor 4 (TLR4) expression on immune cells. TLR4 engagement by ligands such as bacterial LPS leads to proinflammatory cytokine production. This mechanism might be especially important in IBD, in which intestinal bacteria are a major contributor to the induction of the strong inflammatory response. In an in vivo study in mice, TLR4 expression in colonic tissue was not seen in MIF knockout mice, although it was in wild-type mice[56]. When the MIF knockout mice were administered rMIF, TLR4 expression was restored and further increased in colonic mice. In a human study of macrophages, neutralizing MIF or deleting the MIF gene resulted in decreased expression of TLR4 and a decreased response to LPS and gram negative bacteria; in broader experiments a decreased response to staphylococcal toxic shock and septic shock was demonstrated[57]. In addition, these cells did not respond well to LPS or gram negative bacteria and had a decreased expression of TLR4. The role of this receptor in the inflammation seen during H pylori infection is not clear because although H pylori LPS has been suggested to induce only weak responses, there are some studies suggesting it might contribute to the overall immune response to H pylori[5859].
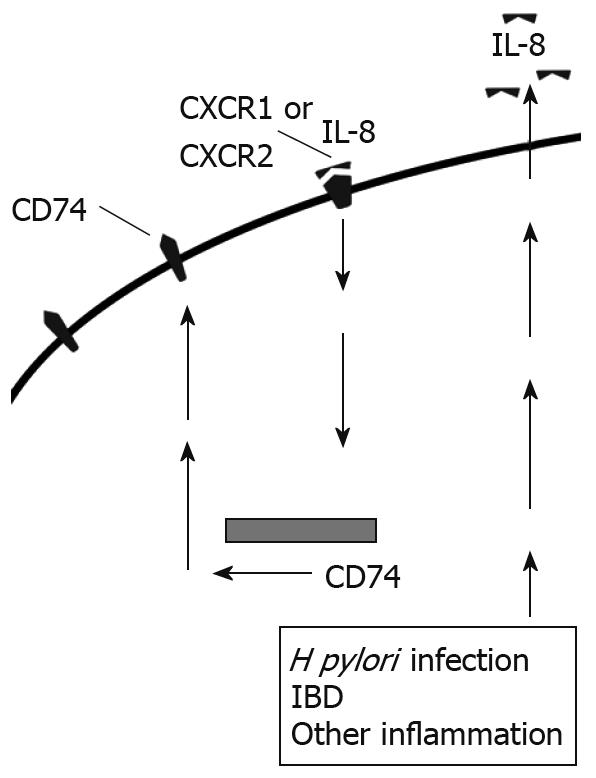
In addition to the role IL-8 plays in inflammation, we have previously shown that IL-8 increases expression of CD74 by gastric epithelial cells, both at the cell surface and mRNA levels[14] (Figure 3). Similarly, we found increased CD74 expression in vivo. Most of the H pylori infected samples and the samples with gastritis for reasons other than H pylori infection had much higher expression of CD74 than uninfected samples not exhibiting signs of gastritis. Other studies have further shown the expression of CD74 increased in ulcerative colitis and Crohn’s Disease[46]. Increased CD74 expression could then go on to intensify inflammation by providing more free receptors for MIF or H pylori attachment.
Figure 3 IL-8 produced by epithelial cells binds to CXCR1 and CXCR2 and up-regulates CD74 expression in an autocrine manner.
THE ROLE OF CD74 IN GI CANCERS
CD74 has a strong link to carcinogenesis, as does MIF. This ligand/receptor combination might be an important link between chronic inflammation and carcinogenesis. CD74 expression and MIF production have been shown to be increased during H pylori infection and gastric cancer[1447]. High expression has also been noted in colon cancer along with highly increased serum concentrations of MIF in patients with colorectal cancers[6061]. The contribution of CD74 to carcinogenesis is multifaceted. High levels of CD74 expression associated with class II MHC expression might prevent tumor antigen presentation by blocking the peptide binding cleft and preventing antigenic peptide binding for presentation to T cells, rendering tumors less immunogenic. One study suggested this to be the case with colon neoplasms where expression was even increased from low to high grade neoplasms[48]. Chronic inflammation and IL-8 production leads to a prolonged increase in CD74 expression, which might not only decrease antigen presentation, but also exacerbate IL-8 production upon engagement by MIF or H pylori. MIF binding to the CD74 receptor complex also promotes proliferation of epithelial cells[1962]. Long term increased proliferation overtakes natural cell cycle events and sets the stage for carcinogenesis.
MIF binding to CD74 might contribute to carcinogenesis in chronic conditions through the up-regulation of proinflammatory cytokines, including IL-8, which up-regulates CD74 and has its own mechanisms leading to increased proliferation, tumor growth, and angiogenesis. MIF or IL-8 binding to their receptors on epithelial cell surfaces induces the shedding of EGFR ligands in a mellatoprotease-dependent manner, and activation of EGFR through engagement of these ligands[63]. We and others have shown that this pathway is activated during H pylori infection[626465]. Additionally, we found that EGFR expression is up-regulated in gastric epithelial cells during H pylori infection by MIF and IL-8" after the word infection. The EGFR is highly expressed in various cancers and is involved in pro-inflammatory responses and pro-carcinogenic events, including cell proliferation, migration, and invasion. Expression and activation of this receptor is well-documented in gastric and intestinal cancers[6667]. One study suggests a correlation between EGFR expression on tumor cells, proliferation, and prognosis in gastric cancer[68]. Another study showed that treatment with antisense RNA for EGFR inhibited gastric tumor growth in a mouse model[69]. Blocking EGFR or its ligands is being studied in order to develop more effective treatments for GI cancers.
MIF also increases epithelial cell proliferation after binding to CD74. One way MIF might affect proliferation and cell cycle events is by regulating p53 tumor suppressor. Numerous cell cycle and apoptosis genes are controlled by p53. We have shown that phosphorylation of the p53 is decreased after MIF binding to CD74[19]. Others have shown that MIF can interact directly with p53 and prevent translocation to the nucleus where it becomes activated and acts as a transcription factor for apoptotic genes[70]. When p53 is blocked from transport to the nucleus, apoptotic pathways are decreased and proliferation increases. Suppression of p53 in macrophages results in a more robust inflammatory response, implying a further link between p53 and inflammation[71].
CONCLUSION
CD74 is a much more versatile molecule than originally thought, playing many important roles in the immune system. Of crucial importance is the role it plays in class II MHC processing and the regulation of antigen presentation. This is important in the GI tract because epithelial cells and subepithelial myofibroblasts express CD74 and act as antigen presenting cells. Furthermore, the expression of CD74 on the cell surface might increase chronic inflammatory responses important in both H pylori infection and IBD. As a receptor for MIF, CD74 also plays and crucial role in chronic inflammation and might represent a major link between chronic inflammation and carcinogenesis in gastric and intestinal cancers. Development of therapeutics for cancer involving blocking CD74 might provide effective treatments for GI cancers.
Supported by The National Institutes of Health Grant K22AI068712, the Texas Board of Higher Education, and the John Sealy Memorial Endowment Fund for Biomedical Research