INTRODUCTION
The ampulla of Vater includes a common channel derived from the conjunction of the common bile duct and the pancreatic duct, the intra-duodenal parts of these two ducts and the duodenal mucosa surrounding the opening[1]. Peri-ampullary carcinomas arise from the ampulla of Vater, distal bile duct, pancreas or duodenal epithelium and are managed with surgical excision when resectable. Regional and other abdominal lymph nodes and the liver are the primary metastatic sites. Other sites of metastasis are less common but can be encountered especially in long term survivors.
We present the case of a patient with ampullary carcinoma and local recurrence developing both biopsy-proven bone metastases in unusual locations and brain metastases.
CASE REPORT
A 62-year-old woman presented to the orthopedic clinic complaining of painful localized edema involving the right ankle and shin. Two years earlier, she had undergone surgery for a stage IIA (pT3N0M0) low grade ampullary adenocarcinoma. She had developed mesenteric and retroperitoneal lymph node metastases 4 mo postoperatively. At that time the patient had refused chemotherapy since she was asymptomatic.
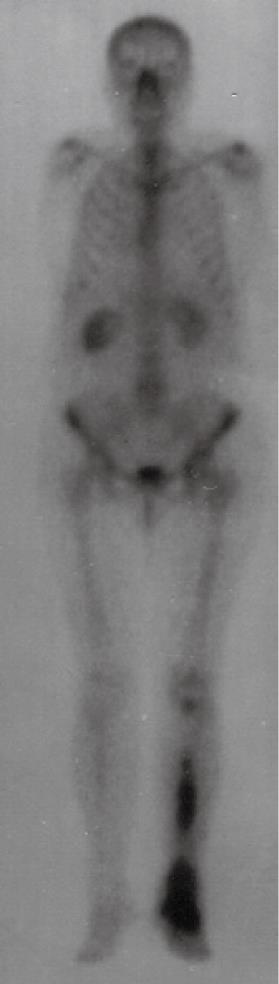
Diagnostic work-up of leg symptoms included an X-ray of the right foot and tibia, which revealed an ill-defined radio-opaque lesion of the right cuneiform bone and a radiolucent lesion of the tibia (Figure 1). An MRI scan of the right lower extremity disclosed multiple confluent bone erosions at the diaphysis of the tibia, with significant extention both proximally and distally. A technetium bone scan showed increased uptake in the right foot and tibia but no other skeletal lesions (Figure 2). In view of the unusual location of the metastasis, a bone biopsy was performed and revealed metastatic adenocarcinoma similar to the primary ampullary tumor. External beam irradiation was given locally (2000 cGy total in five fractions) for pain palliation.
Figure 1 X-ray.
A: The right foot showing the radio-opaque metastatic lesion in the cuneiform bone; B: The right tibia showing a radiolucent metastatic lesion.
Figure 2 A whole body bone scan showing increased uptake in the right foot and tibia.
A follow-up staging CT of the chest, abdomen and pelvis revealed two liver lesions, disseminated bilateral metastatic pulmonary nodules as well as borderline enlarged lymph nodes across the aortic arch and trachea bifurcation. At this time the patient consented to palliative chemotherapy and gemcitabine 1000 mg/m2 iv weekly was administered.
Six months later the patient developed changes in mood, and behavior. A brain CT scan demonstrated ring enhancing lesions affecting the right frontal and left temporal lobes as well as the left cerebellar hemisphere. Palliative whole brain radiation followed by palliative targeted therapy with erlotinib 150 mg/d po was administered. The patient died with progressive disease 7 mo later and about 3 years after the diagnosis of her disease.
DISCUSSION
Carcinoma of the ampulla of Vater is a rare tumor accounting for approximately 0.2% of all gastrointestinal malignancies with an estimated incidence of less than 6 cases per million people per year. However, it is the second most common peri-ampullary neoplasm following pancreatic carcinoma in incidence. The peak incidence is in the 7th and 8th decades of life. There is a slight male to female preponderance[2].
The adenoma to adenocarcinoma carcinogenesis sequence model seems to be applicable to these tumors, given that the incidence of adenoma surrounding carcinoma of the papilla of Vater ranges from 82% to 91%[3]. Intestinal and pancreaticobiliary types represent the most common histological subtypes, indicating that peri-ampullary carcinomas emanate from the corresponding epithelia covering the distal parts of pancreatic and common bile duct and peri-ampullary duodenum, respectively[2]. A more favorable prognosis of the intestinal sub-type compared with the pancreatico-biliary type has been observed in some series[145]. Other histologic types less commonly seen in an ampullary location include mucinous, signet-ring cell, neuroendocrine and undifferentiated carcinomas[26–9].
Compared to other upper GI tract sites, as well as its periampullary counterparts, ampullary cancer has a favorable prognosis possibly due to the relatively early presentation with jaundice, rendering it resectable in 80%-90% of cases[1011]. Moreover molecular lesions of ampullary carcinoma are different from other peri-ampullary cancers, a fact that may also contribute to the different prognosis[12]. For example k-ras, which is mutated in 90% of pancreatic cancers, is mutated in only half of the ampullary carcinomas. Overall the 5-year survival rate ranges from 33% to 67.7% in resected cases[1012–14]. Tumor size and grade, lymph node status and number of positive nodes, perineural infiltration and surgical margins are the most crucial predictors of survival in localized disease[15–18]. Vascular invasion, ulcerative tumor, pancreatic invasion, age and peri-operative blood transfusions are additional prognostic factors seen in some series[18–21]. Nodal involvement is the most robust predictor of survival and forms the basis for recommending adjuvant radiotherapy and chemotherapy to decrease the incidence of locoregional recurrence and distal metastases respectively. Expression of specific proteins by immunohistochemistry has also been linked to prognosis. Expression of transcription factor CDX2 (Caudal-type homeodomain transcription factor), a regulator of normal intestinal and colonic epithelial differentiation, has been linked to improved prognosis compared with ampullary tumors that do not express the transcription factor[22]. In contrast high expression of the transporter protein hENT1 (human Equilibrative nucleoside transporter 1) has been shown to correlate with a shorter survival compared with ampullary carcinomas that displayed low expression of the transporter[23].
Ampullary cancer usually metastasizes to regional nodes, liver, adjacent organs and lungs. In contrast skeletal and brain metastases are common with other primary tumor locations such as lung and breast. We present a patient with an ampullary tumor that developed both bone metastases in unusual locations and brain metastases during the course of her disease. Bone and brain metastases from ampullary carcinoma are not very commonly seen in practice and are rarely reported in the literature. In a series of 135 patients with ampullary cancer who had previously undergone pancreaticoduodenectomy, bone metastases were seen in 5% and brain metastases in less than 4%[13]. In a smaller series of 14 cases of carcinomas of the ampulla of Vater, two had brain metastases but they concerned patients with carcinomas with neuroendocrine differentiation[8], while bone metastases were reported in three cases of another series of 24 patients (13%)[24].
Other isolated cases of bone metastases and unusual metastatic locations, such as the ureter, ovaries, testes, bronchus, umbilicus and distal lymph nodes have also been reported[25–30]. The specific location of bone metastases from ampullary carcinoma are not in general reported and, thus, it is unknown if the common sites seen in several malignancies such as the spine, pelvis and proximal lower extremities are also the most frequent sites of bone metastases in this malignancy.
Although no randomized trials have been performed to support adjuvant treatment, local radiation therapy concomitant with 5-fluoropyrimidine-based chemotherapy has been advocated for resected ampullary carcinoma with adverse prognostic features such as size greater than 2 cm, positive lymph nodes, positive surgical margins, poor differentiation and neurovascular invasion[3132]. In one of these series with 12 patients using protracted infusion of 5-FU concomitantly with radiation treatment, a 2 years survival rate of 89% and a median survival time of 34 mo was reported[32]. In another series a statistically significant improvement in 3 years survival was observed with adjuvant chemoradiotherapy compared with no adjuvant treatment only in patients with high risk characteristics but not in the whole group[31]. In metastatic disease palliative chemotherapy is based on pancreatic cancer type regimens with fluoro-pyrimidines, gemcitabine and platinum derivatives. Resection of solitary liver metastases in an attempt at palliation and prolongation of survival has been done and should be considered in appropriately selected patients[33].
In conclusion, despite their infrequency, bone metastases in the extremities and brain metastases should be included in the differential diagnosis of patients with a history of ampullary carcinoma who present with symptoms referring to such locations in order to expedite the diagnosis and facilitate treatment.