Published online Jun 7, 2009. doi: 10.3748/wjg.15.2628
Revised: June 30, 2008
Accepted: July 7, 2008
Published online: June 7, 2009
AIM: To estimate the prevalence of small intestinal bacterial overgrowth (SIBO) in our geographical area (Western Sicily, Italy) by means of an observational study, and to gather information on the use of locally active, non-absorbable antibiotics for treatment of SIBO.
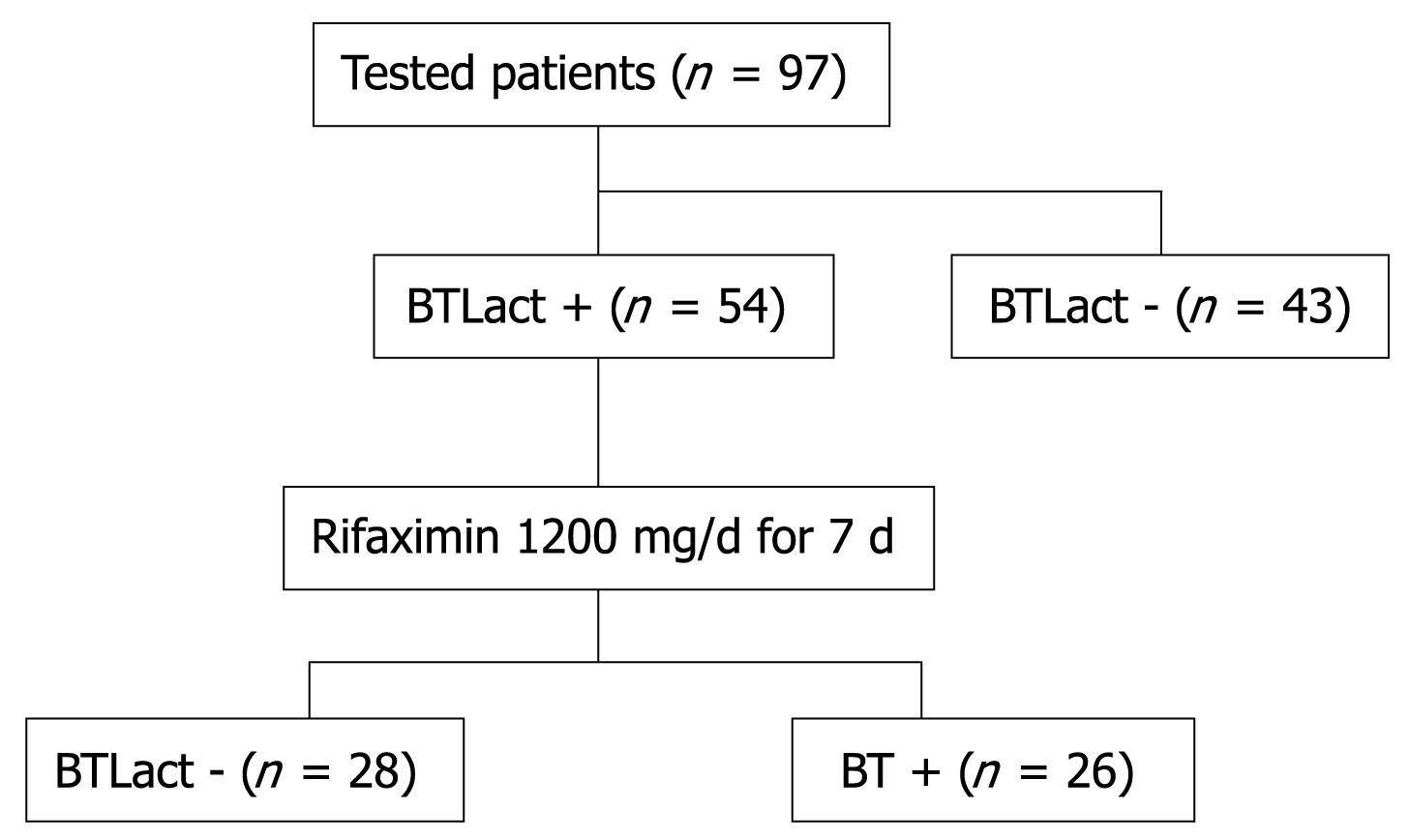
METHODS: Our survey included 115 patients fulfilling the Rome II criteria for diagnosis of irritable bowel syndrome (IBS); a total of 97 patients accepted to perform a breath test with lactulose (BTLact), and those who had a positive test, received Rifaximin (Normix®, Alfa Wassermann) 1200 mg/d for 7 d; 3 wk after the end of treatment, the BTLact was repeated.
RESULTS: Based on the BTLact results, SIBO was present in about 56% of IBS patients, and it was responsible for some IBS-related symptoms, such as abdominal bloating and discomfort, and diarrhoea. 1-wk treatment with Rifaximin turned the BTLact to negative in about 50% of patients and significantly reduced the symptoms, especially in those patients with an alternated constipation/diarrhoea-variant IBS.
CONCLUSION: SIBO should be always suspected in patients with IBS, and a differential diagnosis is done by means of a “breath test”. Rifaximin may represent a valid approach to the treatment of SIBO.
- Citation: Peralta S, Cottone C, Doveri T, Almasio PL, Craxi A. Small intestine bacterial overgrowth and irritable bowel syndrome-related symptoms: Experience with Rifaximin. World J Gastroenterol 2009; 15(21): 2628-2631
- URL: https://www.wjgnet.com/1007-9327/full/v15/i21/2628.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.2628
Irritable bowel syndrome (IBS) is a common gastrointestinal (GI) disease with a prevalence ranging between 11% and 14% of adult population; it is characterized by altered motility, visceral hypersensitivity, abnormal brain-gut interaction, autonomic dysfunction, and immune activation. Although the physiopathological mechanisms underlying IBS are not fully identified, a unifying explanation of symptoms arises from the observation that 92% of IBS patients share the symptom of bloating regardless of their predominant complaint. Thus, the hypothesis has been envisaged that a small intestinal bacterial overgrowth (SIBO) could explain bloating in IBS, based on the following findings: (1) a significantly higher total hydrogen (H2) excretion after lactulose ingestion in a large percentage (84%) of IBS patients; (2) a 75% improvement of IBS symptoms after eradication of SIBO with locally acting antibiotics; and (3) a tight correlation between the pattern of bowel movement and the type of excreted gas[1].
The prevalence of SIBO among newly diagnosed IBS patients is not exactly known, and the largely variable data reported in literature reflect the different sensitivity and specificity of the methods, either biochemical or microbiological, used to make diagnosis of SIBO. However, an exact estimation of SIBO prevalence should have important therapeutic implications, since SIBO and related symptoms (i.e. abdominal bloating) can be successfully treated by locally active, non-absorbable antibiotics[23].
Analysis of breath specimens for volatile metabolites of orally administered substrates offers a simplified detection method for the presence of an abnormal small-intestinal flora; this technique (“breath test”, BT) is not only simpler and more acceptable to patients than jejune aspiration, but also gives quicker information to the clinician than microbiologic culture of the jejune aspirate[4].
The BT with lactulose (BTLact) is based on the properties of lactulose, which is not absorbed through the small intestine and reaches unchanged the colon, where it is metabolized by the bacterial flora. When the bacteria come in contact with lactulose, they metabolize it and produce intestinal gasses, such as methane and hydrogen, which can be detected and assayed in the expired air. In healthy population, bacteria are not present in the duodenum and, therefore, at least 2 h are needed for lactulose to reach the colon and be metabolized. Thus, an increase of hydrogen concentrations in the expired air within 90-120 min strongly suggests a bacterial overgrowth and contamination of the small intestine. The sensitivity and specificity of BTLact in the diagnosis of SIBO are actually estimated to be 68% and 44%, respectively[5]; its main advantage of the BTLact is the relative simplicity of the method, which can be performed by most of the Gastroenterology Units.
By measuring the increased hydrogen concentrations in the expired air after oral lactulose administration (BTLact), an incidence of 46% has been recently found in an Italian survey among 96 patients with IBS[2], which is in agreement with an Europe study demonstrating a significantly increased GI bacterial flora in 43% of IBS patients compared with 12% of matched-control healthy subjects[6], while a USA-based survey has revealed that this incidence may be even higher than 80% of IBS patients[178].
We, therefore, performed an observational analysis on patients with an initial diagnosis of IBS according to the Rome II criteria, in order to estimate the prevalence of SIBO in our geographical area (Western Sicily, Italy) and to gather information on the use of locally active, non-absorbable antibiotics for treatment of concomitant SIBO and IBS.
Our survey included a total of 125 patients who were addressed to our Medical Centre because of abdominal pain and discomfort, in the period ranging between January and December 2006; patients with severe cardiovascular and/or respiratory and/or renal diseases, as well as patients with cancer or under treatment with antibiotics and corticosteroids were excluded.
One hundred and fifteen of these subjects fully complied with Rome II criteria, i.e. (1) 3 mo of continuous or recurrent symptoms of abdominal pain or irritation that may be relieved with a bowel movement or coupled with a change in frequency or related to a change in the consistency of stools; (2) two or more of the following symptoms present at least 25% of time: (a) change in stool frequency (> 3 bowel movement daily or < 3 bowel movements weekly); (b) noticeable difference in stool form (hard, loose and watery stools or poorly formed stools); (c) passage of mucous in stools; (d) bloating or feeling of abdominal distention; (e) altered stool passage (e.g. sensations of incomplete evacuation, straining, or urgency).
These 115 subjects received a symptomatological diagnosis of IBS, while the other ten subjects had a diagnosis of either Crohn’s disease or ulcerative colitis or celiac disease.
Patients with an IBS diagnosis were asked for their informed consent to the management of personal data, in compliance with the “privacy” regulations in force in Italy. According to their intestinal habits, patients were divided into a constipation-variant (20.6%; six male and 14 female), diarrhoea-variant (31.9%; 16 male and 15 female) or alternated alveus-variant (47.5%; 19 male and 27 female) IBS. The severity of the alveus disturbances was scored according to a 5-point semi-quantitative scale (0 = none; 1 = minimum; 2 = mild; 3 = moderate; 4 = severe).
The IBS patients were then asked to undergo a BTLact to check the presence of SIBO; only 97 patients accepted, while the remaining 18 patients refused further investigations. At the evening before the BTLact, the patients were required to eat only boiled rise with no sausage or cheese, and grilled meat, and to drink only no-gas water. If constipation was present, the dietary prescriptions were extended to the 3 d preceding the exam. On the day of test, the patients were completely fasted, and smoking was forbidden to all patients (smokers included). Immediately before the test, a sample of expired air was taken to assay the basal H2 concentrations in the still fasted subjects; then 25 g of lactulose was administered and the expired air was sampled every 30 min in the next three consecutive hours.
A positive test required an early increase of H2 concentration in the expired air higher than 20 ppm over basal values within 90 min of the oral administration of lactulose, followed by a second distinct peak after additional 15 min or more[9].
The patients, who had a positive BTLact, received a diagnosis of SIBO and were treated with Rifaximin (Normix®, Alfa Wassermann) at the daily dose of 1200 mg for 7 d. Three weeks after the end of the treatment, the BTLact was repeated and the alveus disturbances were scored again.
The demographic characteristics of the patients were described as means and standard deviations (min-max ranges), or frequencies when appropriate. The frequencies of symptoms observed in patients with diagnosis of SIBO and IBS were compared using χ2 test; the frequency of positive BTLact was analysed by the Fisher’s exact test.
The 97 patients with diagnosis of IBS, who accepted BTLact, were 41 male and 56 female. The BTLact was found positive in 54 (55.6%) patients; in particular, the test was positive in 61.3% of patients with diarrhoea-, 52.2% of patients with constipation- and 52% of patients with alternated constipation/diarrhoea-variant IBS. There was no significant difference in the frequencies of IBS variants between patients with positive or negative BTLact. The global symptomatological score was not different between the patients with positive or negative BTLact, except for patients with the alternated constipation/diarrhoea-variant IBS; among these patients, the symptomatological score was significantly higher in the BTLact-positive subjects compared to BTLact-negative subjects (Table 1).
| Demographic characteristics | n | Breath test at screening | |
| Positive (n = 54) | Negative (n = 43) | ||
| Sex (M/F, mean ± SD) | 40/57 (40.7 ± 16.2) | ||
| IBS variants n (%) | |||
| Chronic diarrhoea | 31 (31.9) | 19 (35.2) | 12 (27.9) |
| Stipsis | 20 (20.6) | 11 (20.4) | 9 (20.9) |
| Alternated stipsis/diarrhoea | 46 (47.5) | 24 (44.4) | 22 (51.2) |
| Symptoms score | |||
| Chronic diarrhoea | 2.2 ± 0.8 | 2.2 ± 0.7 | 2.1 ± 0.6 |
| Stipsis | 2.3 ± 0.9 | 2.4 ± 0.8 | 2.1 ± 0.9 |
| Alternated stipsis/diarrhoea | 2.0 ± 1.2 | 2.3 ± 0.9 | 1.7 ± 0.6a |
The 54 BTLact-positive patients were treated with Rifaximin, and the BTLact was repeated 3 wk after end of the treatment. In 28 patients, the BTLact turned to be negative, thus showing that the antibiotic was able to significantly control the bacterial overgrowth in the small intestine; in these patients a statistically significant reduction of the symptomatological score from 2.3 ± 0.6 to 0.9 ± 0.8 was also achieved (P = 0.003). On the contrary, the remaining 26 patients still had a positive BTLact, and no change in the symptomatological score was observed (2.3 ± 0.6 vs 2.3 ± 0.6). No treatment-related adverse effect was observed. A flow-chart of the study is shown in Figure 1.
Bacterial flora consisting of Gram-positive and Gram-negative germs, aerobes and anaerobes, is distributed along the GI tract in varying quantities from zero to a maximum of 1012/mL of endoluminal aspirate. This bacterial ecosystem counterbalances with the ecological niche of the host organism and harmonizes with the various digestive, secretory, motor, absorption and sensitivity functions of the entire intestine.
This dynamic equilibrium between environment, bacterial flora and host may be interrupted due to a variety of complex reasons, leading to quantitative and qualitative modifications of the normal intestinal microbial flora that can cause SIBO[10]. SIBO thus represents an invasion of the small intestine, from the upper part by pathogenic strains of oro-alimentary origin, and from the lower part by colo-fecal germs through an incontinent Bauhin’s valve.
The SIBO has various clinical and biological presentations: chronic diarrhea, malabsorption syndrome and exudative enteropathy are the main criteria of diagnosis[11]; the syndrome is characterized by an increase of overall bacterial burden in biotope > 105 CFU/mL in adults and > 104 CFU/mL in children, emergence of different species of enterobacteria, bacteroides, clostridia and fusobacteria in small intestine. Microecological changes are accompanied by B12 vitamin deficiency anemia, hypovitaminosis, protein deficiency, translocation of bacteria and their toxins from intestine in blood, emergence of endotoxinemia and possible generalization of infection[12].
Our survey suggested that, based on the results of a BTLact, SIBO was present in about 56% of newly diagnosed IBS patients in our geographical area. Our study has significant limitations, since it was open label; moreover, some clinicians do not consider the BTLact as a gold standard for diagnosis of SIBO and they recommend indirect parameters, like serum vitamine B12 levels and folate levels, as main indicator of SIBO. Notwithstanding, our observations find a confirmation in the prevalence of abnormal BTLact recently reported by other authors[13].
The treatment of SIBO must be firstly focused on the correction of wrong food and dietary habits that usually underlying the disorder (e.g. excessive use of fast-food), and then to the reduction of bacterial colonization of small intestine by means of antibiotics[14–16]. In this regard, the use of locally acting, non-absorbable antibiotics would be particularly useful in reducing immediately the bacterial count waiting for the slow-acting beneficial effects of dietary measures. Decontamination of the small intestine is more successful when probiotics are prescribed (both after antibiotics and independently), which suppress the opportunistic flora, protect the mucous coat, improve digestion and arrest diarrhea[17].
Our study demonstrated that a 7-d treatment with Rifaximin determined the negativization of BTLact in about 50% of treated patients. Although the treatment was very short (7 d) and no long-term follow-up available, our data seem to confirm other experiences reported in the most recent literature by Majewski et al[3] that a daily dose of 800 mg Rifaximin for 4 wk significantly reduced the symptoms in 20 patients with IBS and led to a negative BT in almost half of patients. Moreover, in another series of 23 patients with SIBO and positive BT, administration of Rifaximin 1200 mg/d for 7 d followed by treatment with probiotics led to a negative BT in 19 (82.6%) cases and significantly reduced the peak in hydrogen concentrations in the expired air from 40.9 ± 20.4 to 4.78 ± 8.42 ppm[2]; Rifaximin was also more effective than chlortetracycline in improving symptoms in patients with SIBO and IBS[18]. More evidences on the efficacy of Rifaximin have been reported in patients with SIBO and acute diverticulitis of colon[19], and patients with SIBO and celiac disease[20].
In this study, 48% of patients treated with Rifaximin failed to achieve a clinical benefit and turn the BTLact to negative. On the other hand, the difficulty in identifying the specific bacterial population and the affected part of the digestive tract by SIBO prevents the possibility of using targeted antibiotics; the recommendation is to use a broad-spectrum antibiotic therapy, capable of eradicating aerobes and anaerobes, preferably with a topical rather than a general action. Valuable alternatives to Rifaximin that have been proven to be effective in the treatment of SIBO are norfloxacin and amoxicillin-clavulanic acid[21], levofloxacin and/or metronidazole[22], gentamycin[23], trimethoprim/sulfamerazine and polymyxin[24], and chlortetracycline[18].
The symptoms of irritable bowel syndrome (alternated stipsis and diarrhoea) may be frequently mimicked by an overgrowth of the bacteria that normally reside in the intestine.
The authors aimed to establish which is the actual incidence of such a bacterial overgrowth among patients with symptoms of IBS, and to get more information on how to treat this disturbance.
The investigation has shown that more than 50% of patients with an earlier diagnosis of IBS, suffering indeed of an intestinal bacterial overgrowth, can be treated by means of an appropriate treatment.
The intestinal bacterial overgrowth can be detected by means of a specific test (“hydrogen breath test”), which is encouraged to be performed by gastroenterologists.
The paper provides an interesting information on the frequency of the intestinal bacterial contamination and overgrowth, which is due to the modern alimentary habits and produces symptoms that may be confused with other gastrointestinal diseases; a simple test, such as the measurement of hydrogen in the expired air, can identify the disorder and allow for the right pharmacological treatment.
| 1. | Lin HC. Small intestinal bacterial overgrowth: a framework for understanding irritable bowel syndrome. JAMA. 2004;292:852-858. |
| 2. | Cuoco L, Salvagnini M. Small intestine bacterial overgrowth in irritable bowel syndrome: a retrospective study with Rifaximin. Minerva Gastroenterol Dietol. 2006;52:89-95. |
| 3. | Majewski M, Reddymasu SC, Sostarich S, Foran P, McCallum RW. Efficacy of Rifaximin, a nonabsorbed oral antibiotic, in the treatment of small intestinal bacterial overgrowth. Am J Med Sci. 2007;333:266-270. |
| 4. | King CE, Toskes PP. Breath tests in the diagnosis of small intestine bacterial overgrowth. Crit Rev Clin Lab Sci. 1984;21:269-281. |
| 5. | Ghoshal UC, Ghoshal U, Das K, Misra A. Utility of hydrogen breath tests in diagnosis of small intestinal bacterial overgrowth in malabsorption syndrome and its relationship with oro-cecal transit time. Indian J Gastroenterol. 2006;25:6-10. |
| 6. | Posserud I, Stotzer PO, Bjornsson ES, Abrahamsson H, Simren M. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut. 2007;56:802-808. |
| 7. | Pimentel M, Wallace D, Hallegua D, Chow E, Kong Y, Park S, Lin HC. A link between irritable bowel syndrome and fibromyalgia may be related to findings on lactulose breath testing. Ann Rheum Dis. 2004;63:450-452. |
| 8. | Van Citters GW, Lin HC. Management of small intestinal bacterial overgrowth. Curr Gastroenterol Rep. 2005;7:317-320. |
| 9. | Walters B, Vanner SJ. Detection of bacterial overgrowth in IBS using the lactulose H2 breath test: comparison with 14C-D-xylose and healthy controls. Am J Gastroenterol. 2005;100:1566-1570. |
| 10. | Pimentel M, Kong Y, Park S. Breath testing to evaluate lactose intolerance in irritable bowel syndrome correlates with lactulose testing and may not reflect true lactose malabsorption. Am J Gastroenterol. 2003;98:2700-2704. |
| 11. | Karsenti D, Bechade D, Fallik D, Bili H, Desrame J, Coutant G, Algayres JP, Daly JP. [Small intestine bacterial overgrowth: six case reports and literature review]. Rev Med Interne. 2001;22:20-29. |
| 12. | Bondarenko VM, Lykova EA, Matsulevich TV. [Microecological aspects of small intestinal bacterial overgrowth syndrome]. Zh Mikrobiol Epidemiol Immunobiol. 2006;22:57-63. |
| 13. | Bayeli PF, Mariottini M, Lisi L, Ferrari P, Tedone F. [Guidelines on intestinal dysmicrobism (SIBO Small Intestine Bacterial Overgrowth)]. Minerva Gastroenterol Dietol. 1999;45:297-308. |
| 14. | Di Stefano M, Miceli E, Missanelli A, Corazza GR. Treatment of small intestine bacterial overgrowth. Eur Rev Med Pharmacol Sci. 2005;9:217-222. |
| 15. | Corazza GR, Sorge M, Strocchi A, Benati G, Di Sario A, Treggiari EA, Brusco G, Gasbarrini G. Non-absorbable antibiotics and small bowel bacterial overgrowth. Ital J Gastroenterol. 1992;24:4-9. |
| 16. | Polter DE, Boyle JD, Miller LG, Finegold SM. Anaerobic bacteria as cause of the blind loop syndrome. A case report with observations on response to antibacterial agents. Gastroenterology. 1968;54:1148-1154. |
| 17. | Lykova EA, Bondarenko VM, Parfenov AI, Matsulevich TV. [Bacterial overgrowth syndrome in the small intestine: pathogenesis, clinical significance and therapy tactics]. Eksp Klin Gastroenterol. 2005;54:51-57, 113. |
| 18. | Di Stefano M, Malservisi S, Veneto G, Ferrieri A, Corazza GR. Rifaximin versus chlortetracycline in the short-term treatment of small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2000;14:551-556. |
| 19. | Tursi A, Brandimarte G, Giorgetti GM, Elisei W. Assessment of small intestinal bacterial overgrowth in uncomplicated acute diverticulitis of the colon. World J Gastroenterol. 2005;11:2773-2776. |
| 20. | Tursi A, Brandimarte G, Giorgetti G. High prevalence of small intestinal bacterial overgrowth in celiac patients with persistence of gastrointestinal symptoms after gluten withdrawal. Am J Gastroenterol. 2003;98:839-843. |
| 21. | Attar A, Flourie B, Rambaud JC, Franchisseur C, Ruszniewski P, Bouhnik Y. Antibiotic efficacy in small intestinal bacterial overgrowth-related chronic diarrhea: a crossover, randomized trial. Gastroenterology. 1999;117:794-797. |
| 22. | Castiglione F, Rispo A, Di Girolamo E, Cozzolino A, Manguso F, Grassia R, Mazzacca G. Antibiotic treatment of small bowel bacterial overgrowth in patients with Crohn's disease. Aliment Pharmacol Ther. 2003;18:1107-1112. |
| 23. | Bhatnagar S, Bhan MK, Sazawal S, Gupta U, George C, Arora NK, Kashyap DK. Efficacy of massive dose oral gentamicin therapy in nonbloody persistent diarrhea with associated malnutrition. J Pediatr Gastroenterol Nutr. 1992;15:117-124. |
| 24. | Knoke M, Bernhardt H, Mollmann R, Bootz T. [Therapeutic study of the effect of selective decontamination on microbial overgrowth syndrome of the small intestine]. Gastroenterol J. 1989;49:59-62. |