INTRODUCTION
Abdominal pain is very common in the general population[1], and pain is the most prevalent symptom in the gastrointestinal (GI) clinic[2]. Gastroenterologists face a challenge in treating these symptoms. Consequently, characterization of visceral pain is one of the most important issues in the diagnosis and assessment of organ dysfunction, as diseases giving rise to deep pain often are difficult to diagnose. This is partly due to the sparse and diffuse termination of visceral afferents in the spinal dorsal horn that overlap several segments, which is further complicated by convergence with somatic afferents (spinal convergence), autonomic (involvement of vagal nerve and spinal afferents) and enteric nervous (homeostatic and secretory) systems. Hence, activation of peripheral sensory afferents may lead to symptoms related to GI motor function (sweating, vasodilation, nausea and vomiting), which blurs the clinical picture. Consequently, complaints related to the autonomic nervous system or related to referred somatic pain are a clinical challenge. To understand the sensory system and how it can be tested, it is important to understand the basic neurophysiological mechanisms behind GI pain.
In healthy subjects, most visceral afferent activity does not reach higher brain centers, except information regarding filling of the esophagus, stomach, rectum and bladder. However, when the internal organs are potentially in danger - e.g. via inflammation and diseases - symptoms such as discomfort and pain are typically reported. The feeling is mostly vague and difficult to characterize, in contrast to distinct localization and characterization in somatic diseases. The different neuroanatomical structures of the two systems explain to some degree why visceral pain is more challenging to diagnose than its somatic counterpart (Figure 1). Visceral afferents that mediate conscious sensations run predominantly together with sympathetic nerves that reach the central nervous system (CNS), although some afferents join parasympathetic and parallel pathways. However, the upper esophagus and rectum also possess somatic innervation. The importance of this dual innervation is not clear, although the rectum has more complex functions than most other visceral organs and may need a more differentiated innervation. The peritoneum and parietal serous membranes of the lungs and heart possess their own parietal nerve supply, which is organized like the skin[3]. Hence, pain from these structures gives a distinct, intense and localized pain, which is comparable to the pain evoked by skin lesions. Most of the visceral afferents converge with lamina I, II and V spino-thalamic tract (STT) neurons, which receive input from both superficial and deep somatic tissue as well as other visceral organs[4]. Although the neuronal mechanisms are more complex, this convergence leads to referred somatic pain as well as viscero-visceral hyperalgesia. The latter phenomenon may explain several comorbid conditions such as increased number of anginal attacks in patients with gallbladder calcinosis, and increased number painful sensations to normal air and feces in the gut in patients primarily suffering from dysmenorrhea[5–9]. Most visceral organs exhibit spinal representation overlapping multiple segmental levels[10]. This widespread and low-density nature of visceral sensory innervation explains why large areas of the gut appear to be relatively insensitive to pain stimuli. The extensive resulting CNS activation may also explain the diffuse and unpleasant nature of visceral pain. Finally, unlike the somatic system, where prolonged or summated stimuli such as during inflammation are necessary to activate the N-methyl-D-aspartate (NMDA) receptor, it seems as though - in the visceral context - the NMDA receptor can be more easily activated by short-lasting and low intensity stimuli[1112]. The resulting amplification of nociceptive processing explains why the manifestation of visceral pain is so often unpleasant and intense in its clinical presentation.
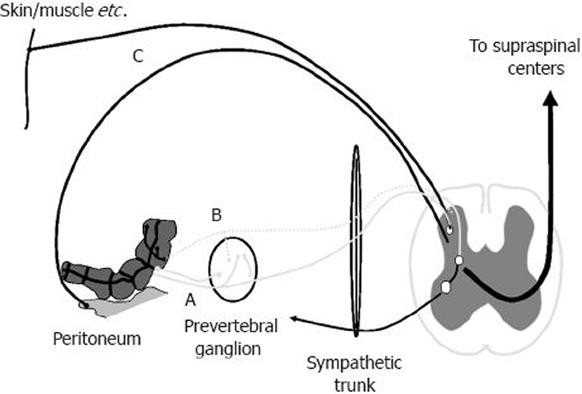
Figure 1 Afferent nerve supply of the gut.
True visceral afferents innervate the gut, and most run temporarily together with either the sympathetic or parasympathetic nerves to enter the spinal cord. During inflammation, silent afferents (dashed line) may become activated and contribute to the sensory response. The peritoneum and parietal serous membranes of the lungs and heart have their own parietal nerve supply, which is organized like that of the somatic structures.
THE RATIONALE FOR EXPERIMENTAL STIMULATION OF THE HUMAN GUT
In clinical practice, several symptoms of underlying diseases confound the characterization of pain. These may include complaints relating to psychological, cognitive and social aspects of the illness, as well as systemic reactions such as fever and general malaise[13]. Furthermore, analgesic treatment and other medications often cause sedation and/or other side effects, which invariably bias the clinical evaluation of pain-related symptoms. Consequently, most studies evaluating drug efficacy in sensory functions of the gut have included a large number of patients. As a result of the above factors, together with the heterogeneity of the material, complicated statistical models have frequently been used - albeit often with equivocal effects. In the clinical situation, this is not a major problem. But, in assessment of analgesics in clinical trials, these confounders can easily invalidate the outcome.
However, in experimental pain models, the confounding factors can often be turned to advantages in the assessment of basic GI functions, mechanisms of disease and treatment efficacy. Under these circumstances, the investigator controls the experimentally induced pain (including the nature, location, intensity, frequency and duration of the stimulus), and provides quantitative measures of the psychophysical, behavioral or neurophysiological responses[13–15]. Different experimental animal models have been used in this context. The advantages of these models are obvious: neuronal activity can be studied directly in anesthetized or spinalized animals with invasive recording techniques or via assessment of behavior[16]. However, as neurobiology of the pain system differs substantially even between animal species, translation from animal studies to human pain studies has some major shortcomings.
Human experimental pain studies have for those reasons gained much interest during recent years. In man, pain is closely related to culture, linguistic terms and expressions, and should be regarded as the net effect of complex multidimensional mechanisms that involve most parts of the CNS including intensity coding, affective, behavioral and cognitive components. This explains some of the difficulties and challenges in quantifying human sensory experiences with simple neurophysiological and/or behavioral methods, and why interest in more advanced human experimental pain studies has increased rapidly during the last decade[1317]. The ultimate goal of advanced human experimental pain research is to obtain a better understanding of pain mechanisms involved in pain transduction, transmission and perception under normal and pathophysiological conditions, such as clinical pain. Obviously, the risk of perforation and other complications during invasive procedures limits the testing possibilities when stimulating the gut. As a result of these difficulties in accessing the GI tract, visceral experimental pain testing is far more resource-intensive and challenging than the more traditional somatic pain stimulations. As a result, most previous visceral studies have relied on relatively simple mechanical or electrical stimuli. These methods are easy to apply. But they have numerous limitations[13]. One should bear in mind that as pain is a multidimensional perception, the response to a single stimulus of a given modality only represents a limited fraction of the entire pain experience. Hence, the possibility of combining different methods to gut stimulation and induction of hyperalgesia will provide the possibility to more closely imitate the clinical situation, and provide extensive and differentiated information on the visceral nociceptive system[1318]. Ideally, experimental stimuli to elicit gut pain in humans should be physiological, minimally invasive, reliable in test-retest experiments, and quantifiable. Preferably, the pain should mimic observations in diseased organs by inducing phenomena such as allodynia and hyperalgesia. Most experimental studies have been completed in functional diseases such as functional chest pain, non-ulcer dyspepsia and irritable bowel syndrome; but, to some degree organic diseases (e.g. ulcers, inflammatory bowel disease and chronic pancreatitis) have also been investigated[18–23]. The different types of stimulation (electrical, mechanical, thermal, chemical and ischemic) that evoke visceral pain in humans, as well as their limitations, have been described in detail previously[1324]. In this review, we focus on novel developments regarding test systems that allow standardized stimulation of the GI tract and their applications.
MECHANICAL STIMULATION OF THE GUT
In the last decade, several studies have addressed the mechanical and sensory function of the GI tract by means of mechanical distension. Simple and physiological gut distension, such as ingestion of well-defined meals, may be useful in clinical studies[25]. Balloon or bag distension is, however, the favored method as the mechanical stimulation intensity is easier to control. Most recent studies have used the Barostat based on volume changes in an air-filled balloon kept at constant pressure levels, and several protocols and stimulation paradigms have been recommended for the Barostat, such as phasic and tonic distension. These stimulation paradigms have been thoroughly discussed, and will not be described here - for review see Whitehead et al[26] and van der Schaar et al[27]. The major advantages of the Barostat system and similar pressure-volume-based methods are the relatively low cost and the documented reproducibility between laboratories[28]. Furthermore, they are reliable and easy to use for routine purposes. Such systems have also been used for assessing sensory and pain thresholds, and under different conditions, attempts have been made to calculate the compliance and tension of the organs[26272930].
A major pitfall in early balloon distension studies was the use of latex balloons causing large errors because of latex deformability and lack of control of the stimulation field. Consequently, polyurethane or polyethylene bags are now recommended generally. There are, however, still several limitations and sources of error with systems based exclusively on volume and pressure. These include the fact that the data obtained must be corrected for compressibility of air as well as other major concerns, for further details see Drewes et al[31]. Basically, a common mistake in GI distension studies is to consider the mechanoreceptors as pressure, volume or tension receptors. In fact, the sensory rating may not be strictly related to pressure (or tension) during gut distension.
Circumferential strain or stress are more likely parameters correlating with receptor responses to stimulus intensity[32]. This is partly due to the fact that strain is a non-dimensional parameter independent of the geometry of the organ and directly associated with tissue deformation. In fact, recent studies have clearly demonstrated that circumferential strain is an important determinant of mechanoreceptor-mediated responses[33–36]. Correspondingly, studies providing tension calculations based on Barostat methods have shown conflicting results, e.g. in a recent study of the stomach, the estimated tension seemed to correlate with the sensation[2937], whereas another study showed a high inter-individual variability in the sensation score to the applied tension, which suggests that factors other than wall tension influence the sensation[38]. However, uncertainties in the assumptions given above, and lack of adequate geometric and biomechanical considerations can also explain these findings[39].
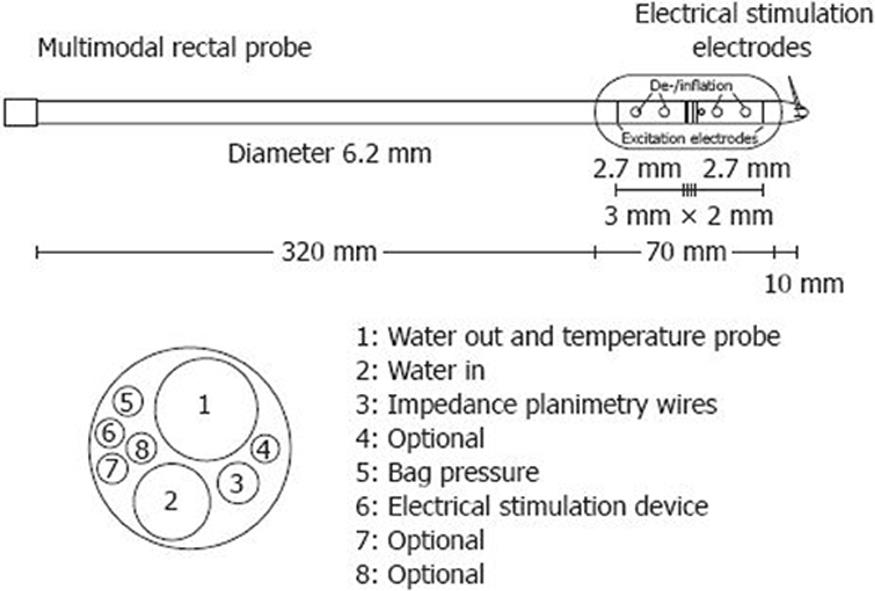
Methods based on impedance planimetry allows recordings of luminal cross-sectional area directly and calculation of the radius in the distended GI segment[33353640–42]. As an example, a schematic drawing of the multimodal rectal probe is shown in Figure 2. Estimates of circumferential wall tension, stretch and strain based on measured radius are more accurate than estimates based on volume exclusively[32]. Findings during rectal distension support this theory, as stretch ratio at pain detection threshold produces an excellent intra-class coefficient of 0.98, both with and without administration of the antimuscarinic drug butylscopolamine[43]. An example of a rectum probe is shown in Figure 3. To reliably compute, e.g. rectal stress and strain, more complex modeling is necessary. Thus, in future studies mechanical distension combined with, for example, ultrasound methods or magnetic resonance imaging may offer the possibility of a better anatomical characterization of the GI tract[4445].
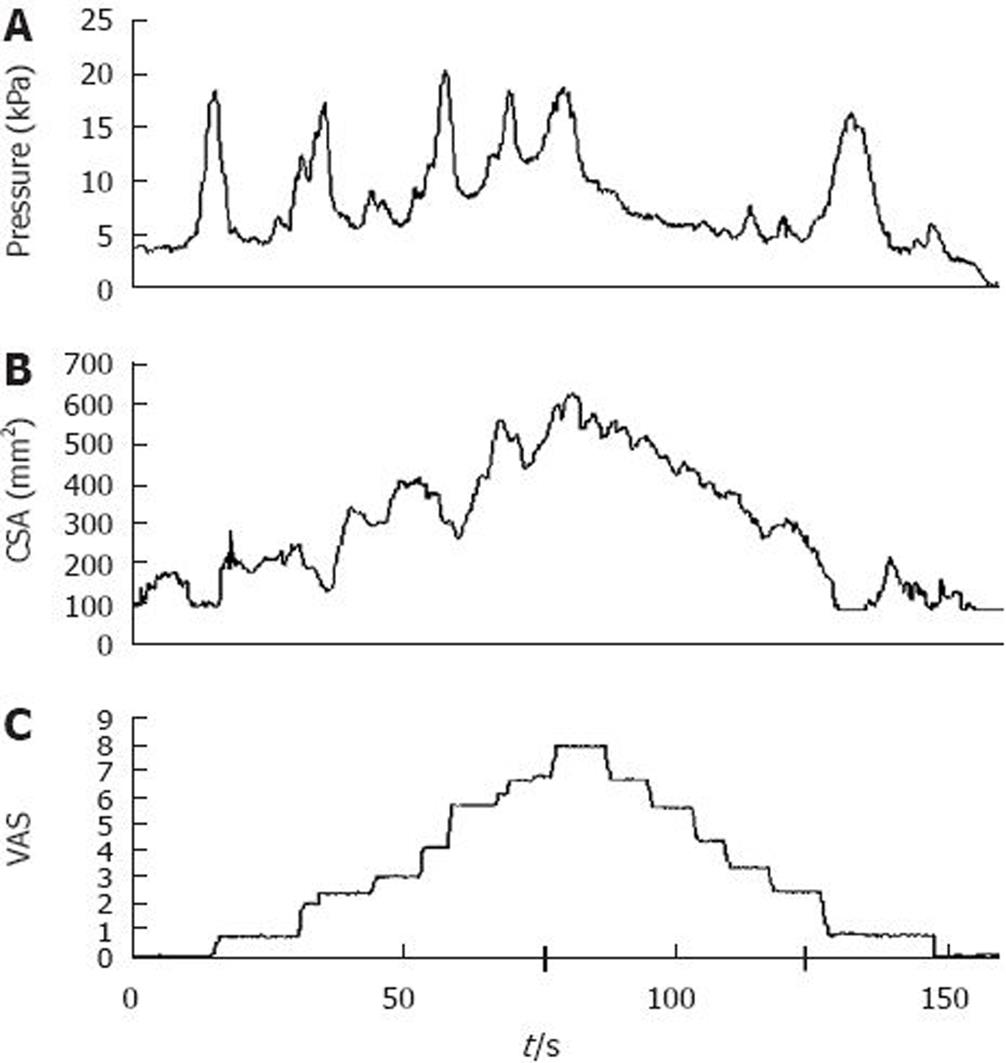
Figure 2 Illustration of mechanical stimulation in the esophagus.
The bag was filled at an infusion speed of 25 mL/min. During distension, (A) pressure, (B) cross-sectional area (CSA) and (C) pain intensity was recorded on line. The increase in CSA corresponded with increasing stimulus intensity after the bag was filled with water, whereas there was little relation between the pressure waves and the sensation. The pain intensity was rated on a visual analogue scale (VAS), with 5 as the pain threshold. An intensity of 8 on the VAS resulted in reversal of the pump. For details see Drewes et al[35].
Figure 3 Probe used for measuring rectal CSA during distension.
ELECTRICAL STIMULATION OF THE GUT
Electrically induced depolarization of sensory afferents has been widely used as an experimental stimulus in humans. Electrical stimuli have proved to be safe in all parts of the GI system; however, it is recommended to monitor the heart during esophageal stimulation, as previous experiments have documented that atrial capture may occur[46].
Electrical stimulation of the GI tract has been used to study, for example, basic pain mechanisms[74247–49] via evoked brain potentials to gut stimuli[50–52], and the effect of analgesics in both healthy volunteers and patients[53]. The main advantage of electrical stimulation is its reproducibility[4354]. Also, its dynamic range (i.e. the range from sensation to pain threshold) is relatively high, which allows more robust assessment of sensory thresholds. A further advantage is that electrodes are easily implemented on different GI probes[134354]. The well defined on- and offset of the stimulus makes it suitable to study pain mechanisms related to time, such as temporal summation[4855] and cerebral evoked potentials.
There are, however, also limitations and drawbacks. Depending on the probe design and the electrodes, it may be difficult to obtain optimal mucosal contact between the electrodes and the GI tract because of, for example, longitudinal esophageal mucosal folds. Hence, it is necessary to measure and control the impedance between electrodes during stimulation, preferably at different frequencies. Further, electrical stimuli are neither natural nor specific for any sensory modality, and the electrical stimulation bypasses receptors, which stimulates all afferent nerves directly, including silent fibers. Consequently, electrical stimulation reflects the central nervous response rather than peripheral afferents. However, as most gut afferents are polymodal[56] and respond to a wide range of stimuli, specificity may be of minor importance. The electrical stimulation creates an electrical field, and the action potential is partly determined by the extracellular electrical potential, and partly by the nerve properties, including myelin and ion-channel configuration. Thus, there may be some selectivity relating to fiber type as the non-myelinated afferents (C-fibers) possess a higher activation threshold than myelinated fibers[175758]. Hence, increasing stimulation intensity may depolarize myelinated fibers first, followed by C-fiber recruitment at higher stimulation intensities.
Electrodes can be either unipolar with a reference placed on the skin or bipolar with a set of electrodes. As a result of safety considerations, bipolar stimulation is recommended because the electrical field is more localized. Cardiac arrhythmia may theoretically be evoked by stimulation of nearby organs. Normally, atrial captures can be seen; but, this has no clinical significance. Arrhythmia can be avoided by either turning patch-electrodes away from the dorsal side of the heart, or by using bipolar ring electrodes that exhibit good mucosal contact[42]. Impedance should preferably be less than 3 kΩ before stimulation is initiated. Numerous stimulation paradigms have been recommended and no general consensus exists with respect to the configuration of the optimal electrical pulse. In fact, the stimulus should reflect the purpose, e.g. it is crucial to use single pulses in electrophysiological studies, where early peaks of evoked brain potentials are wanted. On the other hand, a single stimulus in the gut demands rather high intensity to evoke pain, and trains or continuous series of pulses can be used in order to investigate temporal summation to a repeated series of stimuli (termed “wind-up” in animal experiments). Based on this experience, we use either: (1) single square pulses (duration 0.2-2 ms) in electrophysiological studies assessing evoked brain responses; (2) trains of five constant current pulses (rectangular with a duration of 1 ms applied at 200 Hz termed “single burst stimuli”), as such stimuli demand less current to evoke sensory responses; (3) “repeated burst stimuli” given as a series of single burst stimuli to investigate, for example, the central amplification of the repeated stimuli[485559]; or (4) tetanic stimulation to be used for sensory thresholds of temporal summation[60]. This is applied as a train of pulses (0.2 ms, 100 Hz) that is linearly increased from zero. The advantage of this stimulation is that it is precise and less time-consuming.
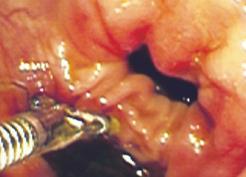
Blind, untargeted stimulation is avoided by integrating electrodes onto endoscopic biopsy forceps, which makes stimulation of well-defined areas in the esophagus, stomach, duodenum, terminal ileum and colon possible[4855]. An example of targeted colonic stimulation is shown in Figure 4. The major advantage of this modification is that electrode position is controllable and can be altered in case of stimulation in the vicinity of somatic structures and nerves. Further, mucosal contact is secured and evoked motor phenomena such as secondary contractions can be studied directly. Hence, the method allows characterization of local and referred pain to stimuli in most areas of the intestine relevant to localized pathology. However, the subjects have to cope with the rather thick endoscopes during experiments, which may be unpleasant especially in the upper GI tract, and which may cause bias in the pain assessment.
Figure 4 An example of targeted colonic stimulation is shown, which demonstrates the electrode position.
The controlled position, which can be altered in case of stimulation in vicinity of somatic structures and nerves, is a major advantage.
As gut segments exhibit differences in anatomy and innervation, a general consensus regarding stimulation location is warranted. Consequently, as described above, stimulation paradigms have a major influence on pain assessment. Thus, future studies should include standardized and validated optimal parameters such as stimulus duration, shape, polarity, frequency and intensity, which allows comparison between different laboratories.
THERMAL STIMULATION
In contrast to mechanical and electrical stimuli, thermal stimuli activate selectively, for example, being either mucosal heat-responsive TRPV1 receptors with temperatures above 43°C, or mucosal cold-responsive TRPA1 with temperatures less than 17°C[61]. Thermal stimulation has been used to study basic pain mechanisms[42434849546162], functional[63] and organic gut disorders[22] and analgesic efficacy in healthy volunteers and patients[53].
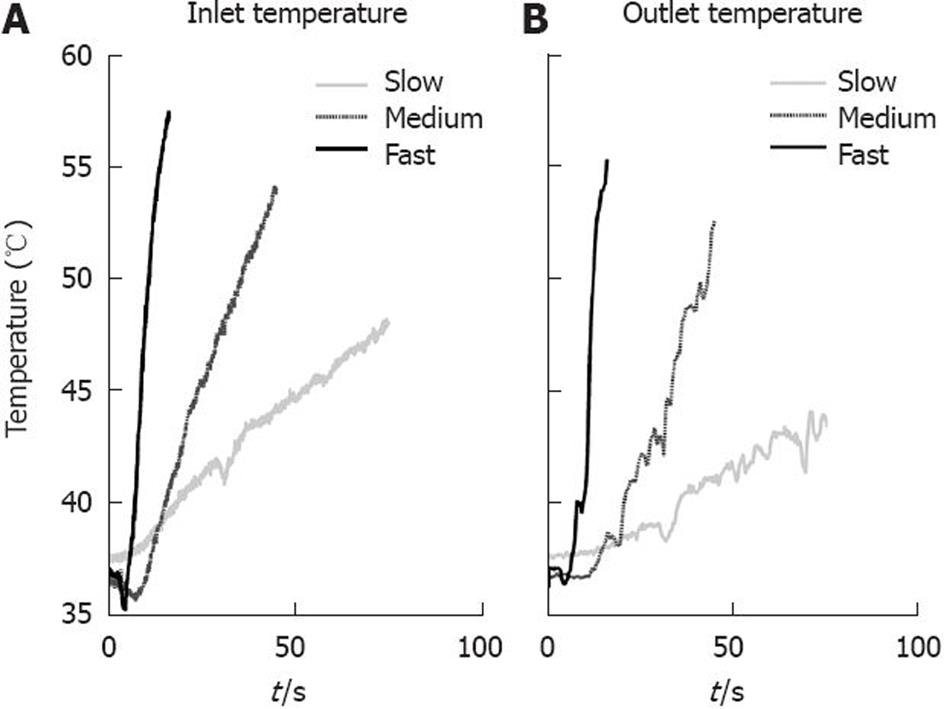
Rectal heat pain stimulation has been performed using a Peltier device[61]. To improve thermal stimulation in the gut, we have developed the multimodal esophageal probe - for details see Drewes and Gregersen[24]. Thermal stimulation is based on recirculation of cooled and/or heated water in the bag with a temperature sensor placed inside the bag. The method has also been integrated onto a multimodal rectal probe. In both cases, the most reliable proxy of the thermal energy applied is the area under the temperature curve[4364]. In the esophagus, the method aims at having a constant high or low temperature in the bag until the pain threshold is reached. This method has been shown to be reliable and robust in drug experiments[54]. In studies of healthy subjects, it has shown some limitations, as not all subjects reach a pain threshold during the 2 min stimulation that were empirically found to be safe. To improve control over the stimulation intensity and duration, the method has recently been changed in order to obtain a linear increase in temperature, with an adjustable temperature ramp. Such stimulation is shown in Figure 5. In these experiments, the stimulation intensity can continue until the subject reaches the pain threshold, an improvement that is expected to result in better validity and reliability of the method.
Figure 5 Temperature stimuli with three different incline rates.
A: Left graph shows the temperature measured at the inlet of the bag; B: Right graph shows the temperature measured at the outlet of the bag.
CHEMICAL STIMULATION
Chemical stimulation of the GI tract resembles clinical inflammation and approaches the ideal experimental visceral pain stimulus[7]. Such stimuli have successfully been applied to the skin[141765] and muscles[66], but are also widely used in the gut. As an example, esophageal acidification is commonly used as a method to sensitize the gut evoking allodynia/hyperalgesia[6768], but the model may also be used for direct stimulation[69]. The major relevance of the model may be induction of sensitization of visceral afferents to subsequent experimental stimulation. Chemical stimulation has been used to study, for example, basic pain mechanisms[4243484950627071] and functional gut disorders[2250]. However, drawbacks of chemical stimuli include a relative long latency time to onset of effects, and that the effects are often not reproducible[7]. Other stimuli such as glycerol, alcohol, bradykinin and other chemicals[72–75] have been used in uncontrolled studies, but their applicability has yet to be established. Recently, capsaicin has been used to evoke pain in the small and large intestine[76–78]. Chemical stimulation has also been used to explore basic functions such as autonomic changes in referred pain[79]. Hammer et al[77] have shown that activation of chemosensitive pathways induces symptoms that differ from those induced by mechanical activation, although animal data do not allow such a strict separation[8081]. As a result of the relative inconsistency of the effects of acid perfusion in the esophagus[82], we recently used perfusion of a combination of acid and capsaicin in the human esophagus[83]. It is believed that capsaicin has an additional effect on acid because of synergistic mechanisms on the transient receptor potential vanilloid type 1 (TRPV1) channels[83]. The perfusion induced locally reproducible hyperalgesia to subsequent heat and electrical stimulation, and an expansion of referred pain in all subjects. The increased referred pain reflects convergence mechanisms on second-order neurons in the spinal cord, and can be used to elucidate the central component of hyperalgesia. A further step is achieved by the demonstration of viscero-visceral hyperalgesia in the rectum following esophageal perfusion with acid and capsaicin[84]. Hence, the model may be more robust than acid perfusion alone, but further studies are needed.
SPATIAL AND TEMPORAL SUMMATION
Lewis[85] has found that distension of the gut is most painful when long, continuous segments of the gut are distended simultaneously. Even greater pressures within smaller segments of the gut are not as efficacious in producing painful sensations. Hence, spatial summation is clearly an important contributor to visceral pain mechanisms. An experimental design to achieve spatial summation is mounting either multiple inflatable bags or one long bag on a probe, and then assessing the distension volume at pain detection threshold (PDT) derived from each bag, compared to the distension volume at PDT during simultaneous distension of multiple bags or the long bag.
Also, integration over time-temporal summation-is important. If electrical stimuli are repeated over time, both pain and the area of referred pain increase progressively[47]. The same phenomenon is seen following repeated distensions[7].
ACTIVATION OF INHIBITORY MECHANISMS
Pain inhibits pain, and impairment of descending control mechanisms is believed to be an important part of the pathogenesis of chronic pain[86]. Descending inhibition involves a spinal-supraspinal-spinal feedback mechanism, which results in direct or indirect inhibition of spinal neuronal responses[8788]. Supraspinal sites can, however, also facilitate nociception, and the measured net output is either predominantly facilitation or inhibition[89]. Hence, experimental studies assessing human inhibitory processes measure the balance between these phenomena.
The most common method to provoke the noxious inhibitory system is the cold pressor test that is performed by immersing a hand or foot in 2.0 ± 0.3°C ice-cooled water for at least 2 min. Efficacy of the descending control can then be investigated by comparing two stimuli separated by the cold pressor test. The cold pressor test has been used to study basic pain mechanisms and functional gut disorders[90].
MULTIMODAL APPROACH
Five to ten years ago, the available probes did not possess the ability to produce more than a few of the above-mentioned stimuli. Some authors have combined mechanical and electrical stimuli[9192], and others have used electrical stimuli combined with sensitization to acid[50]. The esophageal multimodal probe and its use in basic, pharmacological and clinical studies has been reviewed recently, and the reader is referred to Drewes et al[18]. Recently, the model has been used in basic science, including other gut segments such as the duodenum[62] and rectum[43]. We are currently using the multimodal rectal approach in pharmacological and clinical studies of functional and organic disorders.
One limitation of multimodal pain stimulation in the gut is that it is done without visualization of the inside of the intestine. Hence, diseases such as esophageal erosions cannot be excluded. Recently, we have made an attempt to combine the multimodal probe with endoscopy, as illustrated in Figure 6, by passing a small (2.8 mm) video-endoscope into a multimodal probe, which allows mechanical, thermal and chemical stimulation. A schematic drawing of the probe is shown in Figure 6. As electrical stimulation can be done with electrodes attached at the biopsy forceps for the endoscope[48], the probe allows full multimodal stimulation including mucosal inspection and biopsies (albeit small) for histology and specific immunohistochemical staining. In diseases such as esophagitis, the TRPV1 receptor has been shown to be important[71] and the receptor also seems to play a role in sensation to heat and acid[93]. Hence, combined information about the sensory profile and histological findings may be important in evaluation of the pathogenesis in diseases. Theoretically, the endoscope can be replaced with an ultrasound probe that allows assessment of the gut wall and, therefore, can be used for advanced mechanical modeling[94].
Figure 6 Newly de-veloped multimodal endoscopic probe that allows comprehensive sensory information combined with visual mucosal inspection and biopsy specimens.
CONCLUSION
Over the last few years, the technical limitations of sensory testing in the GI tract have been increasingly surmounted. Multimodal esophageal, duodenal and rectal probes have been developed, which allow the investigator to use different stimulus modalities in the gut. The probes have proved to be robust across sessions, and have shown high reproducibility in all modalities. Future experiments using experimental testing will undoubtedly shed light on the pathogenesis of GI disorders, as well as assisting in finding new treatment modalities.
Supported by Det Obelske Familie fond and Spar Nord Fonden
Peer reviewer: Yvette Taché, PhD, Digestive Diseases Research Center and Center for Neurovisceral Sciences and Women’s Health, Division of Digestive Diseases, Department of Medicine, David Geffen School of Medicine at UCLA, University of California, Los Angeles and VA Greater Los Angeles Healthcare System, 11301 Wilshire Boulevard, CURE Building 115, Room 117, Los Angeles, CA, 90073, United States
S- Editor Li LF L- Editor Kerr C E- Editor Ma WH